- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The present study reports a new nanocomposite design using surface modified silver nanowires decorated on the surface of polyethyleneimine (PEI), a cationic polymer acting as glue
for anchoring nanowires and reduced graphene oxide (rGO). The synthesized nanocomposite was employed as a promising electrode material for immobilization of biomolecules and effective
transportation of electron, in enzymatic biofuel cell (EBFCs) application. The synthesized nanocomposite was confirmed by analytical techniques, for instance, Fourier transform infrared
spectroscopy (FTIR), scanning electron microscopy (SEM), transmission electron microscopy (TEM). The electrochemical behaviour of the nanobioelectrocatalysts rGO-PEI/Frt/GOx,
rGO-PEI/AgNWs/Frt/GOx, and rGO-PEI/Naph-SH/AgNWs/Frt/GOx was determined by cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and linear sweep voltammetry (LSV). The
maximum current density obtained by the modified bioanode was found to be 19.9 mA cm−2 at the limiting glucose concentration of 50 mM in PBS (pH 7.0) as supporting electrolyte at a scan rate
of 100 mVs−1. SIMILAR CONTENT BEING VIEWED BY OTHERS IMPROVING THE POWER PRODUCTION EFFICIENCY OF MICROBIAL FUEL CELL BY USING BIOSYNTHESIZED POLYANALINE COATED FE3O4 AS PENCIL GRAPHITE
ANODE MODIFIER Article Open access 02 January 2025 FABRICATION AND CHARACTERIZATION OF ELECTRICALLY CONDUCTING ELECTROCHEMICALLY SYNTHESIZED POLYPYRROLE-BASED ENZYMATIC BIOFUEL CELL ANODE
WITH BIOCOMPATIBLE REDOX MEDIATOR VITAMIN K3 Article Open access 09 February 2024 APPLICATIONS OF CHITOSAN (CHI)-REDUCED GRAPHENE OXIDE (RGO)-POLYANILINE (PANI) CONDUCTING COMPOSITE
ELECTRODE FOR ENERGY GENERATION IN GLUCOSE BIOFUEL CELL Article Open access 26 June 2020 INTRODUCTION Nanoscience and nanotechnology are gaining increasing interest due to their ability to
tailor diverse nanoscale materials. Interestingly, nanomaterials possess excellent properties such as large surface to volume ratio, high surface reactivity, and different morphologies1,2,3.
These dynamic properties of nanomaterials make them advantageous in any conceivable domain like in the food industry4, electronics5, pharmaceuticals6 and agriculture7. Nanoscale materials
have also been utilized in enzymatic biofuel cells (EBFCs) to improve their performance8. EBFC is one of the reliable energy generation technologies for powering miniaturized bioelectronic
systems. Enzymatic biofuel cells relay on two separate redox reactions coupled with the enzyme functionalized electrode materials linked through an external circuit9. The potential chemical
energy stored in biomolecules is converted into electrical energy by a suitable biocatalyst. The merits of the generation of electrical power via EBFCs are their eco-friendly and feasible
operational conditions, use of renewable physiological fluids (e.g., glucose10, fructose11, etc.) and specificity of biocatalysts. For example, the glucose oxidase (GOx)12 is a
bioelectrocatalyst that specifically catalyzes the most exploited glucose fuel, which is ubiquitous in the living systems. However, the redox-active centre of GOx is deeply seated inside its
protein shell which makes the electron transfer difficult through it13. Two main strategies such as the use of redox active mediator and orientated immobilization of enzymes have been used
to mitigate this problem14,15,16. Furthermore, the capability of nanomaterials such as carbon nanotubes or metal nanoparticles to link directly to the electroactive enzyme is of immense
interest to facilitate the direct electron transfer (DET)17 while in the case of mediated electron transfer (MET), a mediator is employed in between the electrode surface and the enzyme that
acts as an electron relay center by reducing the path length for electron transfer14. Reportedly, a 14-fold increase in catalytic current was obtained by using a free naphthoquinone to a
glucose oxidase (GOx)/multi-walled carbon nanotube (MWCNT)-based anode16. Nowadays, ferritin (Frt) has been employed as an active redox mediator that assists the electron shuttling between
redox active site of GOx and the electrode surface18. The biocompatible nature of Frt makes it more favourable than the other toxic mediators which enhances its utilization in a
glucose-based biofuel cell19. The specificity of the GOx towards the oxidation of glucose leads to the development of the miniaturized membrane-less system20 which makes EBFCs an essential
tool in the field of implantable power sources, self-powered sensors21, and portable electronic devices22. However, despite all the promises, still, there are specific roadblocks such as low
power output and short life span which limit their real-world applications. Over the past few decades, the development of advanced supporting materials is the intellectual strategy in
material research to achieve efficient immobilization of enzymes23. Recent progress in nanoscience and a detailed understanding of the chemistry of nanomaterials ensure cost-efficient
development of nanobiocatalysts24. To date, carbon nanomaterials (e.g., carbon nanotubes, graphene, etc.) have been exploited as a versatile supporting material for the enzyme wiring25.
Currently, researchers are showing great interest in exploiting the intrinsic properties of 2D graphene and its derivatives (graphene oxide, reduced graphene oxide) in various fields such as
nanoelectronics26, drug delivery27, environmental remediation28 and designing electrode materials29, on account of their high mechanical strength, good electrical conductivity, free
electron mobility, high aspect ratio, flexible tunability, and lightweight. The high surface to volume ratio of rGO offers the large accessible surface area for enzyme loading30. To date,
various combinations of rGO with conducting polymers such as PANI-rGO31, polypyrrole-rGO32 have been reported as potential electrode materials. However, very few articles have been published
on polyethyleneimine (PEI) with carbon nanomaterials33 in biofuel cells. However, some applications have been explored in biosensors34, filtration membrane35 and drug delivery36. PEI is a
biocompatible porous cationic polymer that is easily prepared and economically feasible. It is also renowned for its excellent enzyme immobilizing ability. Under normal conditions, it
possesses a positive charge which maintains strong interaction with GOx. Although PEI possesses all these fascinating attributes, still its conductivity is lowe as compared to that of others
conducting polymers such as polypyrrole, polythiophene, and polyaniline which limits its direct application as electrode materials37. To address the above issues, the use of metal
nanoparticles (MNPs) can be a promising strategy to improve the conductivity of PEI. The utilization of surface modified MNPs overcomes the problem of their aggregation and simultaneously
promotes their interaction with the polymer matrix. A variety of MNPs such as gold38, magnetite39 and silver nanoparticles40 have been explored for electrodes modification due to their high
catalytic activity. Similarly, the capability of electron transfer surges by introducing the thiol group on the surface of MNPs41. Furthermore, the morphology of MNPs, their shape, size, and
concentration influence the properties of the nanocomposite. Besides, one-dimensional nanowire has high surface activity due to the very high specific surface area which tremendously
enhances the loading capability of the nanocomposite. Herein, a nanocomposite composed of silver nanowires (AgNWs) modified with naphtalenethiol coupling agent decorated on the surface of
cationic polymer polyethyleneimine (PEI) and the rGO is prepared. Here, PEI acts as glue for anchoring nanowires assisted by thiol linkage of naphthalene thiol. Apart from it,
PEI/AgNWs@Naph-SH acts as a sandwich between rGO sheet and GOx in the developed biocatalyst rGO/PEI/AgNWs@Naph-SH/Frt/GOx. Moreover, modification of AgNWs with a napthalenethiol stabilizing
agent makes a protecting covering around the NWs that prevents them from further ionization. The contribution of each component of the synthesized nanocomposite rGO/PEI/AgNWs@Naph-SH plays a
significant role in improving the stability of the bioanode by establishing (i) hydrophobic interaction between GOx and the PEI/AgNWs@Naph-SH nanocomposite and (ii) π conjugation between
GOx and naphthalenethiol@AgNWs that assist the electron transfer process. In this study, three different types of electrodes; rGO/PEI/Frt/GOx, rGO/PEI/AgNWs/Frt/GOx, and
rGO/PEI/AgNWs@Napth-SH/Frt/GOx have been developed and compared to confirm the contribution of each component in enzyme wiring. MATERIALS AND METHODS The polyethyleneimine (PEI) (average
molecular weight 800 by light scattering), reduced graphene oxide (rGO), napthalenethiol (Naph-SH,99%), silver nitrate (AgNO3), ferritin (10 mg mL−1 in 0.15 M NaCl), glucose oxidase (GOx)
from Aspergillus niger and 2% glutaraldehyde aqueous solution were purchased from the Sigma-Aldrich, India. Sodium chloride (NaCl), potassium bromide (KBr), phosphate buffer saline (PBS, pH
7.0), polyvinylpyrrolidone (PVP, molecular weight 1,300,000) and ethylene glycol (EG) were procured from Alfa Aesar, India. INSTRUMENTS USED The Fourier transform infrared spectroscopy
(FTIR) was carried out with the help of Nicolet iS50 FT-IR instrument operating in the range of 4000–400 cm−1, and X-ray diffraction (XRD) analysis was performed by using Rigaku Smart Lab
X-ray diffractometer, to elucidate the functional groups and the crystalline structure of the nanocomposite, respectively. The morphologies of the synthesized nanocomposites were examined
using scanning electron microscopy (SEM) (JSM, 6510 LV, JEOL, Japan) and transmission electron microscopy (TEM) (TEM 2100, JEOL, Japan) operated at 200 kV on a carbon-coated copper grid.
Moreover, the electrochemical behaviour of the fabricated bioanode was investigated by a three-electrode assembly having glassy carbon electrode as working, a Pt wire as a counter and
Ag/AgCl (3 M KCl) as a reference electrode, coupled with the potentiostat/galvanostat (PGSTAT 302 Autolab, Switzerland). SYNTHESIS OF SILVER NANOWIRES (AGNWS) The synthesis of silver
nanowires (AgNWs) through controlled morphology can be obtained by keeping the molar ratio of [PVP] to [AgNO3] as mentioned elsewhere42. Briefly, PVP (75 mg) was dissolved in 10 mL of EG
with continuous heating and magnetic stirring (550 revolutions per minute) of the solution in an oil bath for 60 minutes at 160 °C temperature. After that, 20 µL of KBr (1.8 mM) and 40 µL of
NaCl (3.5 mM) solutions prepared in EG were added into the above solution followed by stirring and heating of the mixture for 30 minutes. Then, 5.2 ml AgNO3 solution was pipetted out and
continuously added to the above mixture while maintaining the flow rate of 0.2 mL min−1. Following this, the solution was refluxed at 160 °C upto 3 h and the noticeable change in the colour
was observed from yellow to turbid grey which indicates the formation of AgNWs. After cooling, the solution was agitated for 10 minutes in an ultra-sonication bath and centrifuged at 7000
rpm. Lastly, final pure AgNWs as shown in the SEM micrograph were obtained by decanting the supernatant liquid. SYNTHESIS OF RGO/PEI/AGNWS@NAPH-SH NANOCOMPOSITE Firstly, the 100 mg of rGO
was dispersed in 5 mL double distilled water (DDW) for the proper dispersion of rGO sheets and then 20 mL PEI polymer was added into it; the negatively charged rGO sheets interact with
positively charged PEI resulting in the homogenous suspension. Simultaneously, in a separate beaker, 1 mL of already synthesized AgNWs were ultrasonicated with 2 mg naphthalenthiol coupling
agent and centrifuged for 7 minutes. This resulting mixture was added into above mentioned rGO/PEI homogenous suspension and diluted it with distilled water so that the final concentration
of the solution reached 2.5 mg mL−1. The mixture was ultrasonicated for 10 minutes allowing the reaction to complete. The supernatant was dumped followed by 7 minutes centrifugation and this
step was repeated three times by washing with DDW to ensure the removal of excessive PEI. The same procedure was followed for the synthesis of rGO/PEI/AgNWs without the modification of
AgNWs41. PREPARATION OF RGO/PEI/AGNWS@NAPH-SH NANOCOMPOSITE DISPERSION The consistency of the synthesized (rGO/PEI/AgNWs@Naph-SH) nanocomposite was adjusted by adding double-distilled water
into it and the resultant dispersion was used as catalytic ink. FABRICATION OF BIOANODE The bioanodes rGO/PEI/Frt/GOx, rGO/PEI/AgNWs/Frt/GOx, and rGO/PEI/AgNWs@Naph-SH /Frt/GOx were
developed by the drop-casting method. Before using, a 3 mm diameter glassy carbon electrode (GCE) was cleaned as described elsewhere43. For designing the bioanode, firstly 6 µL of the
synthesized nanocomposite (rGO/PEI/AgNWs@Naph-SH) dispersion was drop-cast on the top of GCE by spreading evenly using micro-pipette and letting it dry for 5–6 hours at room temperature.
After that, 5 µL of Frt solution was applied on the dried GCE and allowed to dry for 30 minutes. Next, 6 µL of GOx solution was loaded on the modified GCE. Thereafter, 1.5 µL of 2% aqueous
solution of glutaraldehyde was coated on the bioanode to crosslink the enzyme. After that, the modified electrode was dipped in DDW for 2 minutes to wipe off the unloaded enzyme and kept the
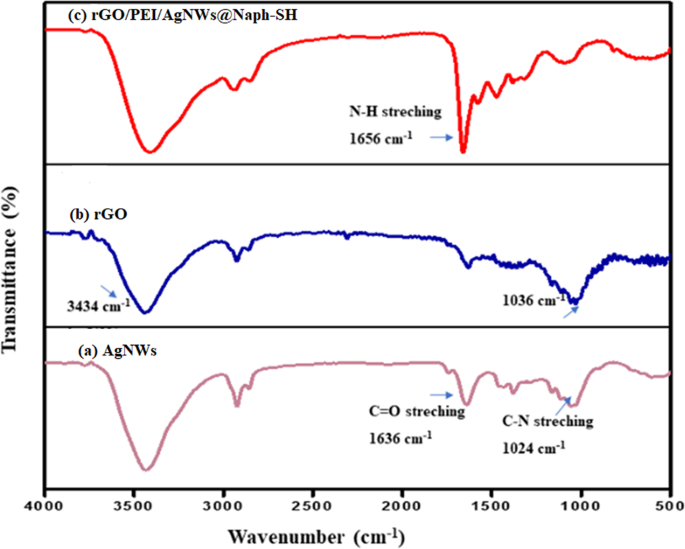
modified electrode in the refrigerator (4 °C) before conducting the experiment. The remaining electrodes were fabricated similarly. RESULTS AND DISCUSSION FTIR ANALYSIS Figure 1 represents
the FTIR spectra of the AgNWs, rGO and the synthesized rGO/PEI/AgNWs nanocomposite. In Fig. 1(a), the band at 1024 cm−1 attributes to the C-N stretching vibration of PVP whereas the
vibrations around 1636 cm−1 indicate the presence of the C = O group in PVP44. The bands (Fig. 1(b)) at around 1036 and 3434 cm−1 can be assigned to the C-C and O-H stretching vibrations of
rGO, respectively45. The FTIR spectrum of rGO/PEI/AgNWs nanocomposite as shown in Fig. 1(c) displays almost the same vibration peaks as found in the individual spectrum of its components
including a peak at 1656 cm−1 due to N-H bending vibrations of PEI46,47. XRD ANALYSIS The recorded X-ray diffraction patterns of the rGO and AgNWs were compared with as-prepared
rGO/PEI/AgNWs nanocomposite as shown in Fig. 2. The vibrations corresponding to 26° can be attributed to the hexagonal plane (002) of rGO displayed in Fig. 2(a)48. The four sharp diffracting
peaks of as-synthesized AgNWs in Fig. 2(b) were located at 2Ѳ angle of diffraction, for instance, 38.2°, 44.4°, 64.6° and 77.6° which are in agreement with the (111), (200), (220) and (311)
diffractions of face-centered cubic (FCC)42. The diffraction peaks found in the synthesized rGO/PEI/AgNWs nanocomposite (Fig. 2c) represent almost all the diffraction peaks as found in each
component and the amorphous nature appears due to the presence of PEI49. Hence, the XRD pattern also confirms the successful synthesis of the nanocomposite as well as of AgNWs. SEM AND TEM
ANALYSES The morphology of the synthesized AgNWs and the nanocomposite (rGO/PEI/AgNWs@Naph-SH) are represented in the SEM micrographs. Figure 3(a) displays the rod shape, smooth texture and
highly purified uniform nanowires of silver. From this image, it can be concluded that the AgNWs have been synthesized successfully. The SEM micrograph of nanocomposite illustrated in Fig.
3(b) clearly showed that the rGO sheets are wrapped around the PEI surface. The uniform dispersion of rGO sheets in the polymer matrix occurs due to the covalent interaction between rGO and
PEI. Also, it can be seen in Fig. 3(c) that the rough surface ensures the wiring of GOx over the rGO/PEI/AgNWs@Naph-SH nanocomposite. Moreover, the TEM micrographs at different
magnifications as shown in Fig. 4(a,b) revealed the non-uniform parallel arrays of AgNWs clinging to the surface of PEI and rGO sheets which further display the microenvironment of the
nanocomposite. It could be concluded that AgNWs and rGO sheets act as fillers in a polymer matrix that provides a continuous path for electron transfer. ELECTROCHEMICAL STUDIES The
electrocatalytic performance of the modified electrodes namely, rGO/PEI/Frt/GOx, rGO/PEI/AgNWs/Frt/GOx, and rGO/PEI/AgNWs@Naph-SH/Frt/GOx were examined by cyclic voltammetry (CV). Before the
examination, N2 gas was bubbled into the PBS (pH 7.0) to provide an inert environment. The electrochemical testing of the fabricated electrodes was conducted at the ambient temperature in
0.1 M PBS (pH 7.0) containing 50 mM glucose. In this study, the contribution of pure AgNWs and AgNWs@Naph-SH on the catalytic activity of biocatalyst was evaluated by comparing the CV curves
of fabricated electrodes as shown in Fig. 5. The outcomes that appeared in CV curves illustrate that the current density obtained from the redox reaction of FAD (Flavin adenine
dinucleotide) on the rGO/PEI/AgNWs/Frt/GOx bioanode was considerably higher than the rGO/PEI and rGO/PEI/Frt/GOx. It means that AgNWs favour the shuttling of the electron between the GOx and
electrode surface, whereas the voltammogram of rGO/PEI/AgNWs@Naph-SH/Frt/GOx bioanode displayed further enhancement in the intensity of the catalytic current. This enhancement in the
current density of rGO/PEI/AgNWs@Naph-SH/Frt/GOx bioanode can be attributed to the involvement of Naph-SH, which assists the electron shuttling by forming the Ag-thiol linkage. This linkage
creates a hydrophobic interaction with the GOx that accelerating the electron kinetics of the reaction. Additionally, Naph-SH improved the electron transfer capability of PEI by promoting
the electrical communication between the GOx and rGO sheets via π-π stacking50. Moreover, it can be seen from the figure that no catalytic activity was delivered by voltammogram of GOx due
to leakage or poor immobilization of enzyme over the bare GCE. Furthermore, the biocatalytic activity of the fabricated electrode rGO/PEI/AgNWs@Naph-SH/Frt/GOx was also studied as a function
of scan rate ranging from 20 to 100 mVs−1 as shown in Fig. 6(a). From the figure, it is evident that by increasing the scan rate, the current density of the specified electrode increases
linearly. The noticeable increment in current density is because of the high voltage applying that reduces the diffusion layer formed at the interface and facilitates electron transfer. The
calibration plot of redox peak current Vs scan rate is given in Fig. 6(b). The curve demonstrates that the magnitude of peaks current (anodic and cathodic) rises as the potential scan rate
increases linearly from 20 to 100 m Vs−1. So, it can be concluded that the catalytic reactions follow surface controlled processes51. One of the valuable information which gives insight
about the kinetics of the modified electrodes is the evaluation of rate constant (ks) using Laviron’s equation52. The ks values of the three developed electrodes were evaluated as, 8.7 s−1,
9.8 s−1, 11.2 s−1 at 100 mVs−1 scan rate. More interestingly, the ks values indicates that the use of AgNWs and AgNWs@Naph-SH contribute to enhancing the electron transfer rate. These
outcomes show a good response as compared to the reported catalyst as shown in Table 153,54,55,56,57,58. Also, the surface concentration of the enzyme immobilized on
rGO/PEI/AgNWs@Naph-SH/Frt/GOx was estimated to be 3.4 × 10−7 mol cm−2 by using Brown-Anson model as given in Eq. (1)43. $${I}_{p}=\frac{{n}^{2}{F}^{2}{I}^{\ast }AV}{4RT}$$ (1) where Ip =
anodic peak current at 100 mVs−1 scan rate, I* = surface concentration of rGO/PEI/AgNWs@Naph-SH/Frt/GOx to be determined, A = surface area of GC electrode (0.07 cm−2). R (gas constants;
8.314JK−1), T (temperature, 298 K), F (Faraday’s constant; 96485 Cmol−1), n = 2 (number of electron transfer). EIS STUDY Electrical impedance spectroscopy (EIS) was used to characterize the
developed electrodes as shown in Fig. 7. By using Nyquist plots, the charge transfer resistance (Rct) of three fabricated electrodes was measured. Fig. 7 showed that the electrolyte
resistance (Rs) remained the same for all the three developed catalysts. The rGO/PEI/Frt/GOx displays a well-defined semi-circle in the high-frequency region with the interface electron
transfer resistance (Rct) of 430 Ω (curve c). After incorporating the AgNWs, the diameter of the semi-circle reduced corresponding to Rct 410 Ω (curve b). The decrease in Rct ascribes the
highly conducting nanowires of silver, which assist the electron shuttling by forming a continuous phase in the nanocomposite. Subsequently, the decrease in Rct to 260 Ω (curve a) was
obtained for rGO/PEI/AgNWs@Naph-SH/Frt/GOx. It can be due to Ag-thiol linkage which further enhances the flow of electron50. LSV ANALYSIS Linear sweep voltammetry (LSV) was carried out to
optimize the current density by varying the glucose concentration of rGO/PEI/AgNWs@Naph-SH/Frt/GOx bioanode in PBS (pH 7.0). It is clear from Fig. 8(a) that the electrocatalytic current
density enhances dramatically with the rise in glucose concentration from 10 to 50 mM. Upon addition of 60 mM glucose, there is a decrease in the anodic current, suggesting its saturation at
50 mM glucose. From this result, it can be concluded that the rise in current occurred due to the availability of vacant sites on the catalyst surface that are active to bind with substrate
molecules and once all the active sites get filled, the saturation was reached leading to further decrease in current density. Moreover, the respective calibration curve of synthesized
bioanode displays a maximum current density of 19.9 mAcm−2 at the 50 mM glucose concentration in Fig. 8(b). The proposed work is compared with the reported work as given in Table
218,56,57,58,59. The current density of the proposed electrode is comparable with the electrodes already available. Moreover, the fabricated electrode can be used upto 45 days while keeping
it into the refrigerator at 4 °C when not in use. CONCLUSION In this work, three nano-scaffold rGO/PEI, rGO/PEI/AgNWs, and rGO/PEI/AgNWs@Naph-SH were synthesized. The catalytic activity of
these nano-scaffolds was tested by immobilizing enzyme (GOx) and mediator (Frt). The catalytic performance of the immobilized enzyme was examined by comparing the rGO/PEI/Frt/GOx,
rGO/PEI/AgNWs/Frt/GOx and rGO/PEI/AgNWs@Naph-SH/Frt/GOx bioanodes. The maximum electrocatalytic current density, higher ks 11.2 s−1 and lower charge transfer resistance (260 Ω) were found to
be in the case of rGO/PEI/AgNWs@Naph-SH/Frt/GOx. Thus, our outcomes will likely open the doors for the development of more efficient EBFCs by using PEI-rGO films as the electrode supports.
REFERENCES * Laura Soriano, M., Zougagh, M., Valcárcel, M. & Ríos, Á. Analytical Nanoscience and Nanotechnology: Where we are and where we are heading. _Talanta_ 177, 104–121 (2018).
Article CAS PubMed Google Scholar * Samal, S. S. & Manohara, S. R. Nanoscience and Nanotechnology in India: A broad perspective. _Mater. Today Proc._ 10, 151–158 (2019). Article
Google Scholar * Kouhi, A. Integrating Nanotechnology Into Engineered Products. _In Reference Module in Biomedical Sciences_, https://doi.org/10.1016/B978-0-12-801238-3.65571-X (Elsevier,
2018). Google Scholar * Hamad, A. F., Han, J.-H., Kim, B.-C. & Rather, I. A. The intertwine of nanotechnology with the food industry. _Saudi J. Biol. Sci._ 25, 27–30 (2018). Article
CAS PubMed Google Scholar * Akujuobi, C. M. Nanotechnology Safety in the Electronics and Telecommunications Industries. _In Nanotechnology Safety_ 141–159,
https://doi.org/10.1016/B978-0-444-59438-9.00011-4 (Elsevier, 2013). Chapter Google Scholar * Holban, A. M. _Series Preface: Pharmaceutical Nanotechnology_. _In Design of Nanostructures
for Theranostics Applications xxi–xxii_, https://doi.org/10.1016/B978-0-12-813669-0.00024-5 (Elsevier, 2018). Google Scholar * Chhipa, H. _Applications of nanotechnology in agriculture.
In_, https://doi.org/10.1016/bs.mim.2019.01.002 (2019). Google Scholar * Sekoai, P. T. _et al_. Application of nanoparticles in biofuels: An overview. _Fuel_ 237, 380–397 (2019). Article
CAS Google Scholar * Huang, X. _et al_. Wearable biofuel cells based on the classification of enzyme for high power outputs and lifetimes. _Biosens. Bioelectron._ 124–125, 40–52 (2019).
Article PubMed CAS Google Scholar * Kang, Z., Job Zhang, Y.-H. P. & Zhu, Z. A shriveled rectangular carbon tube with the concave surface for high-performance enzymatic glucose/O2
biofuel cells. _Biosens. Bioelectron._ 132, 76–83 (2019). Article CAS PubMed Google Scholar * Kuwahara, T. _et al_. Bioelectrocatalytic fructose oxidation with fructose
dehydrogenase-bearing conducting polymer films for biofuel cell application. _React. Funct. Polym._ 116, 87–91 (2017). Article CAS Google Scholar * Abreu, C. _et al_. Glucose oxidase
bioanodes for glucose conversion and H2O2 production for horseradish peroxidase biocathodes in a flow through glucose biofuel cell design. _J. Power Sources_ 392, 176–180 (2018). Article
ADS CAS Google Scholar * Janati-Fard, F., Housaindokht, M. R. & Monhemi, H. Investigation of structural stability and enzymatic activity of glucose oxidase and its subunits. _J. Mol.
Catal. B Enzym._ 134, 16–24 (2016). Article CAS Google Scholar * Kavanagh, P. & Leech, D. Mediated electron transfer in glucose oxidising enzyme electrodes for application to biofuel
cells: recent progress and perspectives. _Phys. Chem. Chem. Phys._ 15, 4859 (2013). Article CAS PubMed Google Scholar * Milton, R. D. _et al_. Rational design of quinones for high power
density biofuel cells. _Chem. Sci._ 6, 4867–4875 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Reuillard, B. _et al_. High power enzymatic biofuel cell based on
naphthoquinone-mediated oxidation of glucose by glucose oxidase in a carbon nanotube 3D matrix. _Phys. Chem. Chem. Phys._ 15, 4892 (2013). Article CAS PubMed Google Scholar * Baghayeri,
M. Glucose sensing by a glassy carbon electrode modified with glucose oxidase and a magnetic polymeric nanocomposite. _RSC Adv._ 5, 18267–18274 (2015). Article CAS Google Scholar * Haque,
S. U., Nasar, A., Inamuddin & Asiri, A. M. Preparation and characterization of a bioanode (GC/MnO2/PSS/Gph/Frt/GOx) for biofuel cell application. _Int. J. Hydrogen Energy_ 44, 7308–7319
(2019). Article CAS Google Scholar * Inamuddin, Haque, S. & Naushad, M. Electrochemical studies of biocatalytic anode of sulfonated graphene/ferritin/glucose oxidase layer-by-layer
biocomposite films for mediated electron transfer. _Enzyme Microb. Technol._ 87–88, 29–36 (2016). Article PubMed CAS Google Scholar * Mano, N., Mao, F. & Heller, A. A Miniature
Membrane-less Biofuel Cell Operating at +0.60 V under Physiological Conditions. _ChemBioChem_ 5, 1703–1705 (2004). Article CAS PubMed Google Scholar * Majdecka, D., Draminska, S.,
Janusek, D., Krysinski, P. & Bilewicz, R. A self-powered biosensing device with an integrated hybrid biofuel cell for intermittent monitoring of analytes. _Biosens. Bioelectron._ 102,
383–388 (2018). Article CAS PubMed Google Scholar * Wu, X. E., Guo, Y. Z., Chen, M. Y. & Chen, X. D. Fabrication of flexible and disposable enzymatic biofuel cells. _Electrochim.
Acta_ 98, 20–24 (2013). Article CAS Google Scholar * Verma, M. L., Puri, M. & Barrow, C. J. Recent trends in nanomaterials immobilised enzymes for biofuel production. _Crit. Rev.
Biotechnol._ 36, 108–119 (2016). Article CAS PubMed Google Scholar * Dev, A., Srivastava, A. K. & Karmakar, S. New Generation Hybrid Nanobiocatalysts. _In Handbook of Nanomaterials
for Industrial Applications_ 217–231, https://doi.org/10.1016/B978-0-12-813351-4.00013-4 (Elsevier, 2018). Chapter Google Scholar * Kumar, A., Sharma, S., Pandey, L. M. & Chandra, P.
Nanoengineered material based biosensing electrodes for enzymatic biofuel cells applications. _Mater. Sci. Energy Technol._ 1, 38–48 (2018). Google Scholar * Park, C. S., Yoon, H. &
Kwon, O. S. Graphene-based nanoelectronic biosensors. _J. Ind. Eng. Chem._ 38, 13–22 (2016). Article CAS Google Scholar * Gong, P. _et al_. Multifunctional fluorescent PEGylated
fluorinated graphene for targeted drug delivery: An experiment and DFT study. _Dye. Pigment._ 162, 573–582 (2019). Article CAS Google Scholar * Ali, I. _et al_. Graphene based adsorbents
for remediation of noxious pollutants from wastewater. _Environ. Int._ 127, 160–180 (2019). Article CAS PubMed Google Scholar * Zhang, J. _et al_. The graphene/lanthanum oxide
nanocomposites as electrode materials of supercapacitors. _J. Power Sources_ 419, 99–105 (2019). Article ADS CAS Google Scholar * Lai, Y. _et al_. Graphene oxide as nanocarrier for
sensitive electrochemical immunoassay of clenbuterol based on labeling amplification strategy. _Talanta_ 107, 176–182 (2013). Article CAS PubMed Google Scholar * Peng, C., Yu, J., Chen,
S. & Wang, L. High-performance supercapacitor based on ultralight and elastic three-dimensional carbon foam/reduced graphene/polyaniline nanocomposites. _Chinese Chem. Lett_. 30,
1137–1140 (2019). * Cai, Z. _et al_. Electrochemical synthesis of graphene/polypyrrole nanotube composites for multifunctional applications. _Synth. Met._ 227, 100–105 (2017). Article CAS
Google Scholar * Lee, S. _et al_. Layer-by-layer assembled carbon nanotube-polyethyleneimine coatings inside copper-sintered heat pipes for enhanced thermal performance. _Carbon N. Y._ 140,
521–532 (2018). Article CAS Google Scholar * Braiek, M. _et al_. A conductometric creatinine biosensor prepared through contact printing of polyvinyl alcohol/polyethyleneimine based
enzymatic membrane. _Microelectron. Eng._ 187–188, 43–49 (2018). Article CAS Google Scholar * Tekinalp, Ö. & Alsoy Altinkaya, S. Development of high flux nanofiltration membranes
through single bilayer polyethyleneimine/alginate deposition. _J. Colloid Interface Sci._ 537, 215–227 (2019). Article ADS CAS PubMed Google Scholar * Kordalivand, N. _et al_.
Polyethyleneimine coated nanogels for the intracellular delivery of RNase A for cancer therapy. _Chem. Eng. J._ 340, 32–41 (2018). Article CAS Google Scholar * Lee, H. L. _et al_. Thermal
conductivity improvement of surface-enhanced polyetherimide (PEI) composites using polyimide-coated h-BN particles. _Phys. Chem. Chem. Phys._ 16, 20041 (2014). Article CAS PubMed Google
Scholar * Hassani, S. _et al_. Novel label-free electrochemical aptasensor for determination of Diazinon using gold nanoparticles-modified screen-printed gold electrode. _Biosens.
Bioelectron._ 120, 122–128 (2018). Article CAS PubMed Google Scholar * Nurlilasari, P. _et al_. High-throughput production of magnetite nanoparticles prepared by the monopolar
arrangement of iron electrodes in water. _Chem. Eng. Sci._ 201, 112–120 (2019). Article CAS Google Scholar * Bunga, Y. & Kataky, R. Silver nanoparticle impacts on gold electrode
surfaces in flow-injection configuration. _Sensors Actuators B Chem._ 290, 140–146 (2019). Article CAS Google Scholar * Christwardana, M., Kim, D. H., Chung, Y. & Kwon, Y. A hybrid
biocatalyst consisting of silver nanoparticle and naphthalenethiol self-assembled monolayer prepared for anchoring glucose oxidase and its use for an enzymatic biofuel cell. _Appl. Surf.
Sci._ 429, 180–186 (2018). Article ADS CAS Google Scholar * Gebeyehu, M. B., Chala, T. F., Chang, S.-Y., Wu, C.-M. & Lee, J.-Y. Synthesis and highly effective purification of silver
nanowires to enhance transmittance at low sheet resistance with simple polyol and scalable selective precipitation method. _RSC Adv._ 7, 16139–16148 (2017). Article CAS Google Scholar *
Perveen, R., Nasar, A., Inamuddin, Asiri, A. M. & Mishra, A. K. Optimization of MnO2-Graphene/polythioaniline (MnO2-G/PTA) hybrid nanocomposite for the application of biofuel cell
bioanode. _Int. J. Hydrogen Energy_ 43, 15144–15154 (2018). Article CAS Google Scholar * Kumar, D., Kavita, Singh, K., Verma, V. & Bhatti, H. S. Microwave-assisted synthesis and
characterization of silver nanowires by polyol process. _Appl. Nanosci._ 5, 881–890 (2015). Article ADS CAS Google Scholar * Gong, Y., Li, D., Fu, Q. & Pan, C. Influence of graphene
microstructures on electrochemical performance for supercapacitors. _Prog. Nat. Sci. Mater. Int._ 25, 379–385 (2015). Article CAS Google Scholar * Filip, Z., Hermann, S. & Demnerová,
K. FT-IR spectroscopic characteristics of differently cultivated Escherichia coli. _Czech J. Food Sci._ 26, 458–463 (2008). Article Google Scholar * XING, H.-B. _et al_. Construction of a
tumor cell-targeting non-viral gene delivery vector with polyethylenimine modified with RGD sequence-containing peptide. _Oncol. Lett._ 7, 487–492 (2014). Article PubMed Google Scholar *
Zhang, Y. _et al_. Three-Dimensional Graphene Networks as a New Substrate for Immobilization of Laccase and Dopamine and Its Application in Glucose/O 2 Biofuel Cell. _ACS Appl. Mater.
Interfaces_ 6, 12808–12814 (2014). Article CAS PubMed Google Scholar * Sumisha, A., Arthanareeswaran, G., Ismail, A. F., Kumar, D. P. & Shankar, M. V. Functionalized titanate
nanotube–polyetherimide nanocomposite membrane for improved salt rejection under low pressure nanofiltration. _RSC Adv._ 5, 39464–39473 (2015). Article CAS Google Scholar * Chung, Y.,
Ahn, Y., Kim, D. H. & Kwon, Y. Amide group anchored glucose oxidase based anodic catalysts for high performance enzymatic biofuel cell. _J. Power Sources_ 337, 152–158 (2017). Article
ADS CAS Google Scholar * Cao, M. _et al_. New directions for diffusion-based network prediction of protein function: incorporating pathways with confidence. _Bioinformatics_ 30, i219–i227
(2014). Article CAS PubMed PubMed Central Google Scholar * Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical
systems. _J. Electroanal. Chem. Interfacial Electrochem._ 101, 19–28 (1979). Article CAS Google Scholar * Palanisamy, S., Karuppiah, C. & Chen, S.-M. Direct electrochemistry and
electrocatalysis of glucose oxidase immobilized on reduced graphene oxide and silver nanoparticles nanocomposite modified electrode. _Colloids Surfaces B Biointerfaces_ 114, 164–169 (2014).
Article CAS PubMed Google Scholar * Luo, Z. _et al_. Reduced graphene oxide/PAMAM–silver nanoparticles nanocomposite modified electrode for direct electrochemistry of glucose oxidase and
glucose sensing. _Biosens. Bioelectron._ 36, 179–185 (2012). Article CAS PubMed Google Scholar * Choi, B. G., Im, J., Kim, H. S. & Park, H. Flow-injection amperometric glucose
biosensors based on graphene/Nafion hybrid electrodes. _Electrochim. Acta_ 56, 9721–9726 (2011). Article CAS Google Scholar * Shakeel, N., Ahmad, A., Ahamed, M. I., Inamuddin & Asiri,
A. M. Kraton based polymeric nanocomposites bioanode for the application in a biofuel cell. _Enzyme Microb. Technol_. 127, 43-49 (2019). * Shakeel, N. _et al_. Functionalized magnetic
nanoparticle-reduced graphene oxide nanocomposite for enzymatic biofuel cell applications. _Int. J. Hydrogen Energy_ 44, 28294–28304 (2019). Article CAS Google Scholar * Inamuddin,
Shakeel. N., Ahamed, M.I., Kanchi, S. & Abbas Kashmery, H. Green synthesis of ZnO nanoparticles decorated on polyindole functionalized-MCNTs and used as anode material for enzymatic
biofuel cell applications. _Sci. Rep._ 10, 5052 (2020). * Perveen, R., Inamuddin, Nasar, A., Beenish & Asiri, A. M. Synthesis and characterization of a novel electron conducting
biocomposite as biofuel cell anode. Int. _J. Biol. Macromol._ 106, 755–762 (2018). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Deanship
of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (D-195-130-1440). The authors, therefore, gratefully acknowledge the DSR technical and financial support.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589, Saudi Arabia Inamuddin * Advanced Functional Materials
Laboratory, Department of Applied Chemistry, Faculty of Engineering and Technology, Aligarh Muslim University, Aligarh, 202 002, India Inamuddin * Department of Chemistry, Faculty of
Science, Aligarh Muslim University, Aligarh, 202002, India Nimra Shakeel Authors * Inamuddin View author publications You can also search for this author inPubMed Google Scholar * Nimra
Shakeel View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS I. and N.S. contributed to ideas, executed all the experiments, analysed and
interpreted the data. Both the authors contributed to the discussion and agreed to submit the manuscript. CORRESPONDING AUTHOR Correspondence to Inamuddin. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Inamuddin, Shakeel, N. Optimization of rGO-PEI/Naph-SH/AgNWs/Frt/GOx nanocomposite anode for biofuel cell applications. _Sci Rep_ 10, 8919 (2020).
https://doi.org/10.1038/s41598-020-65712-8 Download citation * Received: 30 March 2020 * Accepted: 08 May 2020 * Published: 02 June 2020 * DOI: https://doi.org/10.1038/s41598-020-65712-8
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative