- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Prokineticin receptors (PROKR1 and PROKR2) are G protein-coupled receptors which control human central and peripheral reproductive processes. Importantly, allelic variants of
_PROKR2_ in humans are associated with altered migration of GnRH neurons, resulting in congenital hypogonadotropic hypogonadism (CHH), a heterogeneous disease characterized by delayed/absent
puberty and/or infertility. Although this association is established in humans, murine models failed to fully recapitulate the reproductive and olfactory phenotypes observed in patients
harboring _PROKR2_ mutations. Here, taking advantage of zebrafish model we investigated the role of _prokr1b_ (ortholog of human _PROKR2_) during early stages of GnRH neuronal migration.
Real-Time PCR and whole mount _in situ_ hybridization assays indicate that _prokr1b_ spatial-temporal expression is consistent with _gnrh3_. Moreover, knockdown and knockout of _prokr1b_
altered the correct development of GnRH3 fibers, a phenotype that is rescued by injection of _prokr1b_ mRNA. These results suggest that _prokr1b_ regulates the development of the GnRH3
system in zebrafish. Analysis of gonads development and mating experiments indicate that _prokr1b_ is not required for fertility in zebrafish, although its loss determine changes also at the
testis level. Altogether, our results support the thesis of a divergent evolution in the control of vertebrate reproduction and provide a useful _in vivo_ model for deciphering the
mechanisms underlying the effect of _PROKR2_ allelic variants on CHH. SIMILAR CONTENT BEING VIEWED BY OTHERS DISRUPTING AMH AND ANDROGEN SIGNALING REVEALS THEIR DISTINCT ROLES IN ZEBRAFISH
GONADAL DIFFERENTIATION AND GAMETOGENESIS Article Open access 05 March 2025 LOSS-OF-FUNCTION VARIANTS IN _SEMA3F_ AND _PLXNA3_ ENCODING SEMAPHORIN-3F AND ITS RECEPTOR PLEXIN-A3 RESPECTIVELY
CAUSE IDIOPATHIC HYPOGONADOTROPIC HYPOGONADISM Article 25 January 2021 COMMON AND FEMALE-SPECIFIC ROLES OF PROTEIN TYROSINE PHOSPHATASE RECEPTORS N AND N2 IN MICE REPRODUCTION Article Open
access 07 January 2023 INTRODUCTION The hypothalamus-pituitary-gonadal (HPG) axis controls reproduction in vertebrates1,2,3,4,5 through the pulsatile release of gonadotropin-releasing
hormone (GnRH) hormone by GnRH neurons. During development, these neurons differentiate from neural crest cells and ectodermal progenitors in a niche at the border between the respiratory
epithelium and the vomeronasal/olfactory epithelium. From these regions these neurons migrate caudally to reach the medio-basal hypothalamus, where they complete their differentiation6.
Several studies in different animal models have shown that specific evolutionarily conserved genes regulate this migratory process and the functions of these neurons. The role of these genes
in human fertility is supported by the existence of variants in these genes associated with congenital hypogonadotropic hypogonadism (CHH)7, a rare and clinically heterogeneous disorder
characterized by abnormal pubertal development and/or infertility, a normal (nCHH) or defective sense of smell (Kallmann syndrome, KS) and other reproductive and non-reproductive
anomaliess8,9,10. More than 25 genes have been associated with CHH, although variants in these genes account only for 40–50% of reported cases. Several evidences indicate that CHH is a
complex genetic disease characterized by variable expressivity and penetrance of the associated genetic defects, which can be partially explained by an oligogenicinheritance model9,11. Among
the genes involved in CHH, prokineticin receptor 2 (_PROKR2)_ has an important role in GnRH neuron migration. PROKR2, as a member of the GPCR family, has an extracellular amino-terminal
end, an intracellular carboxy-terminal domain and a central core formed by seven transmembrane α-helical segments (TM1–TM7)12. Prokineticins have been previously demonstrated to be involved
in several physiological functions in neurogenesis, regulation of circadian rhythms, metabolism, angiogenesis, pain perception, muscle contractility, hematopoiesis, immune response,
thermoregulation and energy expenditure13,14,15. Matsumoto and colleagues in 2006 reported the first observation that _Prokr2_ knock-out mice display hypoplastic gonads and olfactory
structures, a phenotype reminiscent of KS, to link this gene to CHH16. Furthermore, _Prokr2_ knock-out mice show decreased plasma levels of testosterone and follicle-stimulating hormone, but
not luteinizing hormone. In humans, genetic screening of CHH cohorts has revealed mutations in _PROKR2_ in KS and nCHH patients, but mostly in the heterozygous state17,18,19,20. Moreover,
studies on transfected cells demonstrated that the missense _PROKR2_ variants observed in KS and nCHH patients have a deleterious effect on _PROKR2_ signaling18,20,21 although such variants
are also present in apparently unaffected individuals22,23,24. Thus, despite several _in vitro_ and _in vivo_ studies, the role of Prokr2 in GnRH neuron function and in CHH pathogenesis
remains incompletely understood. Here, we take advantage of the zebrafish to generate an _in vivo_ model to investigate _PROKR2_ function during GnRH neuron ontogeny. Using both transient
knockdown and germline knockout of _prokr1b_, the zebrafish ortholog of human _PROKR2_, we find that _prokr1b_ has an important role in the migration of GnRH axons, but not for fertility, in
this animal model. RESULTS Mammals possess two prokineticin receptors named _PROKR1_ and _PROKR2_25. The zebrafish genome contains two _prokr_ paralogs named _prokr1a_ and _prokr1b_ (or
_prokr1l_), and previous evidence suggests that _prokr1a_ and _prokr1b_ correspond to mammalian _PROKR1_ and _PROKR2_, respectively26. To determine which of these genes may be involved in
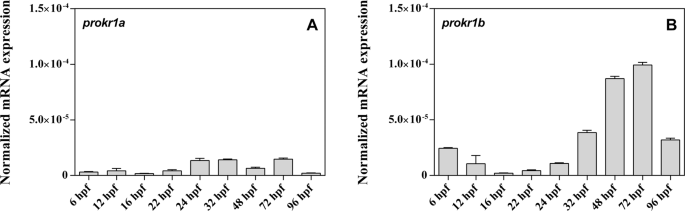
GnRH neuronal migration, we evaluated their expression during zebrafish embryo development. Real-Time PCR analysis on RNA extracts from whole embryos revealed that _prokr1b_ is expressed at
higher levels compared to _prokr1a_ and exhibits an increase from 24 hours post-fertilization (hpf) to 72 hpf (Fig. 1A,B). Whole-mount _in situ_ hybridization (WISH) performed at the same
developmental stages (Fig. 2) revealed that _prokr1b_, but not _prokr1a_, is expressed in cells adjacent to the olfactory placodes (Fig. 2G-L), similar to the pattern observed for _gnrh3_
(Fig. 2M-R). Given the role of _PROKR2_ in the migration of GnRH neurons in humans, these results suggest that _prokr1b_ may similarly be involved in GnRH3 neuron development in zebrafish.
Next we used morpholino anti-sense oligonucleotides (MOs) to knock-down expression of _prokr1a_ or _prokr1b_ in _tg (gnrh3:EGFP)_ embryos, which express EGFP in GnRH3 cells27 (Supplementary
Fig. S1A–C). Images collected at 48 hpf (Fig. 3) revealed that _prokr1a_ knock-down (Fig. 3C) did not affect the development of GnRH3 fibers that appeared similar to those of uninjected
wild-type animals (WT, Fig. 3A) or injected with a control MO(_ctrl-MO)_ (Fig. 3B). In contrast, knock-down of _prokr1b_ led to evident alterations in the architecture of the GnRH3 network
(Fig. 3D). In these embryos, GnRH3 fibers appeared disorganized, especially at the level of the anterior commissure (AC) and in the anterior fibers (dotted square in Fig. 3D; Supplementary
Fig. S1D). These results suggest that _prokr1b_ is required for normal GnRH neuron development in zebrafish. To confirm the defects observed for _prokr1b_ knock-down using MOs, a technique
that is prone to non-specific artefacts, we next analyzed GnRH3 neuron development in animals containing a germline mutation in _prokr1b_26. To do so, we compared EGFP-expressing GnRH3
neuron fibers in _prokr1b_ homozygous mutant, heterozygous mutant, and homozygous WT siblings at 48 hpf and 72 hpf. No differences were observed between _prokr1b_+_/−_ and WT siblings at
these two time points (Fig. 4A,B,E,F), while _prokr1b_−/− embryos (Fig. 4C,G) showed defects in GnRH3 neuron fibers in the same anatomical region as in the knockdown experiments (Fig. 3D).
This phenotype appears to be specific to the absence of _prokr1b_, as injection of WT _prokr1b_, but not _prokr1a_ mRNA into _prokr1b_−/− animals at the 1-cell stage rescued the phenotype
(Fig. 4D–H; Supplementary Fig. S2E,F). These observations were supported by quantitative analysis of GnRH3 neuron fibers(Fig. 5A,B), providing an evidence for a role of _prokr1b_ in GnRH
neuron development in zebrafish. In order to establish whether _prokr1b_ is required for the development and function of the reproductive system in zebrafish, as it is in humans and mice, we
compared the reproductive organs and fecundity of _prokr1b_−/−, _prokr1_+_/_- and _prokr1b_+/+ siblings. Histological sections of testes and ovaries of 3-months-old male and female animals
did not reveal obvious differences among the three genotypes, suggesting that _prokr1b_ is not required for gonadal maturation in both sexes and, by consequence, for puberty in zebrafish
(Fig. 6A-L). No differences were also observed among the three genotypes in the number of fertile eggs generated (Fig. 6M), neither in the GSI index of mutated male or female compared to WT
indicating that _prokr1b_ is not fundamental for fertility in zebrafish (Fig. 6N). Nevertheless, Real-Time qPCR conducted on tissue of adult fish, revealed for mutated males higher
expression of _lhβ_ and _fshβ_ in the brain (Fig. 6O) together with a strong decrease of the gonadotropic receptors _lhr_ and _fshr_ in the gonads (Fig. 6P). DISCUSSION Several recent
studies have demonstrated a remarkable evolutionary conservation of the developmental migration of GnRH neurons and of several genes involved in GnRH ontogeny28,29,30,31. In zebrafish, like
in mammals, GnRH secreting neurons starts their development at 24 hpf from cells located in the olfactory epithelium that send dorsal extensions that ultimately innervate the hypothalamus
and pituitary. An important gene involved in the development of the GnRH system in humans is _PROKR2_. The zebrafish genome contains two _prokineticin receptor_ paralogues, _prokr1a_ and
_prokr1b_26. WISH analyses reveal that _prokr1b_ expressionstarts in the brain at 24 hpf close to the olfactory bulbs and appears similar to that of _gnrh3_ at 48 and 72 hpf. Accordingly,
Real-Time PCR data show that during this time window _prokr1b_ expression increases and then drastically decreases at 96 hpf. At 72 hpf, the development of GnRH3 fibers is complete and is
followed by the migration of GnRH3 somata from the olfactory region to the hypothalamus27. Consistent with the possibility that zebrafish _prokr1b_ is an ortholog of human _PROKR2_,
knockdown of _prokr1b_, but not _prokr1a_, affected the formation of rostral GnRH3 fibers, similar to humans with _PROKR2_ mutations. Importantly, similar defects were also present in
homozygous _prokr1b_ mutant embryos at 48 hpf, and this phenotype was rescued by injection of WT _prokr1b_ mRNA. Taken together, these results demonstrate that _prokr1b_ is important for the
correct migration of GnRH3 neuron fibers. Although _prokr1b_ appears to be the zebrafish ortholog of human and murine _PROKR2_, the zebrafish _prokr1b_ mutant phenotype does not fully
recapitulate the clinical features of CHH. Indeed, _prokr1b_ mutation does not affect gonadal maturation or fertility in zebrafish, as demonstrated by fecundity testing and histological
analysis of testis and ovaries at 3 months of age. Moreover, dorsoventral projections of GnRH3 neurons, despite reduced, are present in zebrafish _prokr1b_ mutants at 72 hpf, in contrast to
murine _Prokr2_ mutants in which there is an early arrest in GnRH neuronal migration16. Two recent studies conducted in zebrafish have highlighted differences in the role of the GnRH system
during puberty and fertility. Liu and colleagues showed that triple mutants lacking _gnrh3_ and the 2 kisspeptin ligands undergo normal puberty and gonad maturation32. These results are
surprising, because GnRH3 has been considered the most important stimulator of gonadotropin release in fish and its expression, together with _kiss1_ and _kiss2_, have been found to be
higher during puberty and gonadal maturation in zebrafish33. Moreover Marvel and colleagues demonstrated that zebrafish _gnrh2_/_gnrh3_ double mutants show normal fertility, demonstrating
that neither GnRH2, nor Kiss1 and Kiss2, compensate for loss of GnRH3 in zebrafish34. Nevertheless, comparison between WT and double or triple mutants revealed in both studies different
expression patterns of neuropeptides known to be important in mammal control of reproduction, such as _tachykinin 3_, _secretogranin II_ and _neuropeptide Y_. These results suggest that, in
contrast with mammals, multiple factors act in parallel with GnRH to stimulate the reproductive axis in zebrafish32,34,35. Our results in the knockout male fish might further confirm this
hypothesis. Indeed, we reported a _lhr_ and _fshr_ lower expression in the testis that could firstly indicate a role of _prokr1b_ in this organ similar to what already observed in mice,
where absence of _Prokr2_ lead to a variable degree of compromised vasculature, even in the absence of evident structural gonadal modification36. Moreover, this could also be related to
primary testes defect that has been described, in association to those in the hypothalamus and pituitary, also in human male with CHH (Sykiotis _et al_. JCEM 2010). Secondly, this reduced
receptor expression might lead to a relative resistance to Lh and Fsh action which, in turn, might activates, through the negative feedback mechanism, the central compartment of the HPG
axis. Higher _lhβ_ and _fshβ_ expression levels in our male knockout fish, seem to confirm this stimulation, nevertheless they are not consequence of level modification in _gnrh3_ expression
levels. Thus, other factors from GnRH3 system might be implicated in the stimulation of the pituitary as previously suggested32,34,35. In conclusion, even if mechanisms controlling the HPG
and, by consequence, fertility have slightly diverged along evolution, these studies together demonstrate that genes regulating GnRH ontogeny present a certain degree of conservation among
humans, mice and zebrafish37,38. Indeed, despite the variable phenotypic features, the _Tg(gnrh3:EGFP);prokr1b__ct814/ct814_ line presented here suggests that _prokr1b_ is the orthologue of
human _PROKR2_, and demonstrates that its loss affects the development of GnRH neuronal fibers in zebrafish, asin humans, but also the expression of the _lhr_ and _fshr_ at the testes level,
thus indicating a complex implication of the prokineticin pathway in the HPG functionality. Moreover, this mutant lineis a useful _in vivo_ tool that, combined with mutant lines for other
GnRH related genes, could contribute to our understanding of the development of the GnRH system and the complex mechanisms underlying CHH and related diseases. METHODS ZEBRAFISH LINES AND
MAINTENANCE Zebrafish (_Daniorerio_) embryos obtained from natural spawning were raised and maintained according to established techniques39. All experiments with live animals were performed
at the University of Milan. All experimental protocols and methods were carried out in accordance with relevant guidelines and regulations of Good Animal Practice approved by the
institutional and licensing committee IACUC (Institutional Animal Care and Use Committee) and University of Milan by the Italian Decree of March 4th, 2014, n.26. Embryos were staged
according to morphological criteria40. Beginning from 24 hpf, embryos were cultured in fish water containing 0.003% PTU (1-phenyl-2-thiourea; Sigma–Aldrich, Saint Louis, MO) to prevent
pigmentation and 0.01% methylene blue to prevent fungal growth39. Wild-type (WT) zebrafish of the AB strain were obtained from the Wilson lab (University College London, London, United
Kingdom). The _tg (gnrh3:EGFP)_27 and _prokr1b_ct814/ct814 26 zebrafish lines have been previously described. REAL-TIME PCR Reverse Transcription-Polymerase Chain Reaction (RT-PCR) was
performed on total RNA prepared from 20 zebrafish oocytes and embryos for each different developmental stages using the Total RNA Isolation Kit (Ambion, Thermo Fisher, Waltham MA) or the
RNAgents Total RNA Isolation System (Promega, Madison, WI), treated with DNase I RNase free (Roche, Basel, Switzerland) to avoid possible contamination from genomic DNA. RNA concentrations
and quality were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, USA). Total RNA (1 ug) was reverse transcribed to produce cDNA using
Superscript III reverse transcriptase (Invitrogen) primed with random hexamers, as described previously41. In all cases, a reverse transcriptase negative control was used to test for genomic
DNA contamination. The primers used for quantitative Real-Time PCRare listed in the Supplementary Table S1. _IN SITU_ HYBRIDIZATION Whole-mount _in situ_ hybridization (WISH) was performed
as described42. PCR products were cloned into the pGEM-T Easy vector (Promega, Table S2). The cDNA-containing plasmids were linearized and transcribed with T7 and SP6 RNA polymerase (Roche)
for antisense and sense riboprobe synthesis. Images of stained embryos were taken with a Leica MZFLIII epifluorescence stereomicroscope equipped with a DFC 480-R2 digital camera. KNOCKDOWN
EXPERIMENTS We tested one antisense morpholino oligonucleotide (MO) each for _prork1a_ and _prokr1b_ (Supplementary Table S3). Both were splice-blocking MOs synthesised by Gene Tools LLC
(Oregon, USA). Morpholinos were dissolved in Danieau’s solution (58 mMNaCl; 0,7 mMKCl; 0,4 mM MgSO4. H2O; 0,6 mMCa(NO3)2; 5 mMHepes pH 7.2) at 2 mM and stored at −80 °C. Embryos were
microinjected at the 1–4 cell stage with rhodamine dextran (Molecular Probes) co-injected as a tracer. As a control for non-specific effects, a standard control morpholino (_ctrl-_MO) was
injected, which targets the human _β-globin_ gene. Morpholinos were tested for efficacy and toxicity by injecting different doses in _tg(gnrh3:EGFP)_ embryos and evaluating them for
morphological defects (Supplementary Fig. S1A-C). After injection, embryos were raised in fish water at 28 °C and observed until the developmental stage of interest. Embryos that were to be
imaged after 24 hpf were treated with PTU. For imaging, embryos were anaesthetized using tricaine (ethyl 3-aminobenzoate methanesulfonate salt, Sigma; 25x stock = 0.08 g in 20 ml of
distilled H2O) in fish water. Injected embryos (morphants) were embedded at 48 hpf in UltraPure Low Melting Point Agarose (Thermo Fisher Scientific) and photographed using a confocal laser
scanning microscope (Nikon C2) with a 20x objective. GENERATION OF _TG(GNRH3:EGFP)_; _PROKR1B_ CT814/CT814 LINE AND RESCUE EXPERIMENTS We crossed _tg(gnrh3:EGFP)_27 animals to
_prokr1b_ct814/ct814 animals26 to generate the _tg(gnrh3:EGFP)_; _prokr1b_ct814/ct814 line. Fin clipping was performed to isolate genetic material from individual fish for genotyping
accordingly to what previous published in Chen and colleague26 (Table S4). The _prokr1b_ct814/ct814 fish contain a 1 bp deletion (nucleotide 12 of the open reading frame: 5’-C-3’), which
results in a change in reading frame after amino acid 4 and a premature stop codon after amino acid 13 compared to 396 amino acids for the wild-type (WT) protein. Rescue experiments were
performed by injecting 400 pg _prokr1b_ mRNA diluted in the Danieau’s solution into 1-cell stage embryos. LIVE-IMAGING OF GNRH3 FIBERS IN _PROKR1B_ KO EMBRYOS To assess the role of _prokr1b_
during GnRH3 fiber development, _prokr1b_ KO animals were embedded at 48 or 72 hpf in UltraPure Low Melting Point Agarose (Thermo Fisher Scientific) and analysed using a confocal laser
scanning microscope (Nikon C2+) with a 20x objective. GnRH3 fiber structure was assessed and 3D reconstructed using Fiji43. Due to the complexity of GnRH3 fibers, a specific region of
interest (ROI) was selected and analyzed at each developmental stage (Supplementary Fig. S2), with background fluorescence subtracted from each image (Supplementary Fig. S2B–D). The number
of green pixels within each ROI was used as a proxy for the amount of GnRH3 fibers. GONADS HISTOLOGY, FECUNDITY/FERTILIZATION RATESAND GSI For histological analysis, gonads from 3-months-old
fish were fixed in 4% paraformaldehyde (PFA), dehydrated, wax-embedded, cut into 8 µm sections using a microtome (Leitz 1516), and stained with eosin. Samples were imaged using a Leica
DM6000 B microscope equipped with a Leica 480 digital camera using the Leica Application Suite (LAS version 4.7.0). The assessment of fecundity (number of eggs released) and fertilization
rate (fraction of eggs that developed into an embryo), WT and mutant females and males at 3 months-old were paired in a spawning tray. After one hour, eggs were collected in 30% Danieau’s
solution and counted. The number of fertilized and unfertilized eggs was discerned using a dissecting microscope at 6 hpf. Twelve-months-old male and female fish were then dissected to
collect ovaries and testicles for gonadosomatic index (GSI) measurement and Real-time PCR. The GSI was calculated according to the formula (organosomatic index = organ weight × 100/body
weight)44. STATISTICAL ANALYSIS Statistical analyses in Fig. 5 was performed using one-way ANOVA with Dunnett’s post-hoc test using GraphPad PRISM version 6.0 (GraphPad, San Diego, CA). In
the graphs, *_P_ < 0.05, **_P_ < 0.01, ***_P_ < 0.001. CHANGE HISTORY * _ 15 MAY 2020 An amendment to this paper has been published and can be accessed via a link at the top of the
paper. _ REFERENCES * Calvin, J. L., Slater, C. H., Bolduc, T. G., Laudano, A. P. & Sower, S. A. Multiple molecular forms of gonadotropin-releasing hormone in the brain of an
elasmobranch: evidence for IR-lamprey GnRH. _Peptides_ 14, 725–729 (1993). Article CAS Google Scholar * Uchida, K. _et al_. Evolutionary origin of a functional gonadotropin in the
pituitary of the most primitive vertebrate, hagfish. _Proc Natl Acad Sci USA_ 107, 15832–15837 (2010). Article ADS CAS Google Scholar * Takahashi, A., Kanda, S., Abe, T. & Oka, Y.
Evolution of the hypothalamic-pituitary-gonadal axis regulation in vertebrates revealed by knockout medaka. _Endocrinology_ 157, 3994–4002 (2016). Article CAS Google Scholar *
Lethimonier, C., Madigou, T., Munoz-Cueto, J. A., Lareyre, J. J. & Kah, O. Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish. _Gen Comp Endocrinol_
135, 1–16 (2004). Article CAS Google Scholar * Kavanaugh, S. I., Nozaki, M. & Sower, S. A. Origins of gonadotropin-releasing hormone (GnRH) in vertebrates: identification of a novel
GnRH in a basal vertebrate, the sea lamprey. _Endocrinology_ 149, 3860–3869 (2008). Article CAS Google Scholar * Forni, P. E. & Wray, S. GnRH, anosmia and hypogonadotropic
hypogonadism–where are we? _Front Neuroendocr._ 36, 165–177 (2015). Article CAS Google Scholar * Vezzoli, V. _et al_. The complex genetic basis of congenital hypogonadotropic
hypogonadism. _Minerva Endocrinol_ 41, 223–239 (2016). PubMed Google Scholar * Seminara, S. B., Hayes, F. J. & Crowley, W. F. Gonadotropin-Releasing Hormone Deficiency in the Human
(Idiopathic Hypogonadotropic Hypogonadism Genetic Considerations. _Endocr. Rev._ 19, 521–539 (1998). CAS PubMed Google Scholar * Boehm, U. _et al_. Expert consensus document: European
Consensus Statement on congenital hypogonadotropic hypogonadism-pathogenesis, diagnosis and treatment. _Nat Rev Endocrinol_ 11, 547–564 (2015). Article Google Scholar * Bonomi, M. _et al_.
Characteristics of a nationwide cohort of patients presenting with isolated hypogonadotropic hypogonadism (IHH). _Eur J Endocrinol_ 178, 23–32 (2018). Article CAS Google Scholar *
Sykiotis, G. P. _et al_. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. _Proc Natl Acad Sci USA_ 107, 15140–15144 (2010). Article ADS CAS Google Scholar * Soga,
T. _et al_. Molecular cloning and characterization of prokineticin receptors. _Biochim Biophys Acta_ 1579, 173–179 (2002). Article CAS Google Scholar * Negri, L. _et al_.
Bv8/Prokineticins and their Receptors A New Pronociceptive System. _Int. Rev. Neurobiol._ 85, 145–157 (2009). Article CAS Google Scholar * Zhou, W., Li, J.D, Hu, W. P., Cheng, M. Y. &
Zhou, Q. Y. Prokineticin 2 is involved in the thermoregulation and energy expenditure. _Regul. Pept_. https://doi.org/10.1016/j.regpep.2012.08.003 (2012). * Shojaei, F. _et al_.Bv8
regulates myeloid-cell-dependent tumour angiogenesis. _Nature_ https://doi.org/10.1038/nature06348 (2007). * Matsumoto, S. _et al_. Abnormal development of the olfactory bulb and
reproductive system in mice lacking prokineticin receptor PKR2. _Proc Natl Acad Sci USA_ 103, 4140–4145 (2006). Article ADS CAS Google Scholar * Abreu, A. P., Kaiser, U. B. &
Latronico, A. C. The role of prokineticins in the pathogenesis of hypogonadotropic hypogonadism. _Neuroendocrinology_ 91, 283–290 (2010). Article CAS Google Scholar * Libri, D. V. _et
al_. Germline prokineticin receptor 2 (PROKR2) variants associated with central hypogonadism cause differental modulation of distinct intracellular pathways. _J Clin Endocrinol Metab_ 99,
E458–63 (2014). Article CAS Google Scholar * Dode, C. _et al_. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. _PLoS Genet_ 2,, e175 (2006).
Article Google Scholar * Cole, L. W. _et al_. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and
clinical spectrum. _J Clin Endocrinol Metab_ 93, 3551–3559 (2008). Article CAS Google Scholar * Abreu, A. P. _et al_. Loss-of-function mutations in the genes encoding prokineticin-2 or
prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. _J Clin Endocrinol Metab_ 93, 4113–4118 (2008). Article CAS Google Scholar * Dode, C. & Rondard, P. PROK2/PROKR2
Signaling and Kallmann Syndrome. _Front Endocrinol_ 4, 19 (2013). Article Google Scholar * Martin, C. _et al_. The role of the prokineticin 2 pathway in human reproduction: evidence from
the study of human and murine gene mutations. _Endocr Rev_ 32, 225–246 (2011). Article CAS Google Scholar * Pitteloud, N. _et al_. Digenic mutations account for variable phenotypes in
idiopathic hypogonadotropic hypogonadism. _J Clin Invest_ 117, 457–463 (2007). Article CAS Google Scholar * Lin, D. C. _et al_. Identification and molecular characterization of two
closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. _J Biol Chem_ 277, 19276–19280 (2002). Article CAS Google Scholar
* Chen, S. _et al_. Light-Dependent Regulation of Sleep and Wake States by Prokineticin 2 in Zebrafish. _Neuron_ 95, 153–168.e6 (2017). Article CAS Google Scholar * Abraham, E. _et al_.
Early development of forebrain gonadotrophin-releasing hormone (GnRH) neurones and the role of GnRH as an autocrine migration factor. _J Neuroendocr._ 20, 394–405 (2008). Article CAS
Google Scholar * Palevitch, O. _et al_. Nasal embryonic LHRH factor plays a role in the developmental migration and projection of gonadotropin-releasing hormone 3 neurons in zebrafish. _Dev
Dyn_ 238, 66–75 (2009). Article CAS Google Scholar * Biran, J., Palevitch, O., Ben-Dor, S. & Levavi-Sivan, B. Neurokinin Bs and neurokinin B receptors in zebrafish-potential role in
controlling fish reproduction. _Proc Natl Acad Sci USA_ 109, 10269–10274 (2012). Article ADS CAS Google Scholar * Yanicostas, C., Herbomel, E., Dipietromaria, A. & Soussi-Yanicostas,
N. Anosmin-1a is required for fasciculation and terminal targeting of olfactory sensory neuron axons in the zebrafish olfactory system. _Mol Cell Endocrinol_ 312, 53–60 (2009). Article CAS
Google Scholar * Kim, H. G. _et al_. WDR11, a WD protein that interacts with transcription factor EMX1, is mutated in idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. _Am J
Hum Genet_ 87, 465–479 (2010). Article CAS Google Scholar * Liu, Y. _et al_. Genetic evidence for multifactorial control of the reproductive axis in zebrafish. _Endocrinology_ 158,
604–611 (2017). Article CAS Google Scholar * Kitahashi, T., Ogawa, S. & Parhar, I. S. Cloning and expression of kiss2 in the zebrafish and medaka. _Endocrinology_ 150, 821–831 (2009).
Article CAS Google Scholar * Marvel, M., Spicer, O. S., Wong, T.-T., Zmora, N. & Zohar, Y. Knockout of the Gnrh genes in zebrafish: effect on reproduction and potential compensation
by reproductive and feeding-related neuropeptides. _Biol. Reprod._ 0, 1–13 (2018). Google Scholar * Tang, H. _et al_. The kiss/kissr systems are dispensable for zebrafish reproduction:
evidence from gene knockout studies. _Endocrinology_ 156, 589–599 (2015). Article Google Scholar * Svingen, T. _et al_. Prokr2-deficient mice display vascular dysmorphology of the fetal
testes: Potential implications for Kallmann syndrome aetiology. _Sex. Dev_. https://doi.org/10.1159/000335160 (2012). * Zhao, Y., Lin, M. C., Mock, A., Yang, M. & Wayne, N. L.
Kisspeptins modulate the biology of multiple populations of gonadotropin-releasing hormone neurons during embryogenesis and adulthood in zebrafish (Danio rerio). _PLoS One_ 9, e104330
(2014). Article ADS Google Scholar * Palevitch, O. _et al_. Cxcl12a-Cxcr4b signaling is important for proper development of the forebrain GnRH system in zebrafish. _Gen Comp Endocrinol_
165, 262–268 (2010). Article CAS Google Scholar * Westerfield, M. The Zebrafish Book.A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th Edition. _Univ. Oregon Press. Eugene_
(2007). * Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. _Dev Dyn_ 203, 253–310 (1995). Article CAS
Google Scholar * Tang, R., Dodd, A., Lai, D., McNabb, W. C. & Love, D. R. Validation of Zebrafish (Danio rerio) Reference Genes for Quantitative Real-time RT-PCR Normalization. _Acta
Biochim. Biophys. Sin. (Shanghai)._ 39, 384–390 (2007). Article CAS Google Scholar * Thisse, C., Thisse, B., Halpern, M. E. & Postlethwait, J. H. goosecoid Expression in neurectoderm
and mesendoderm is disrupted in zebrafish cyclops gastrulas. _Dev. Biol._ 164, 420–429 (1994). Article CAS Google Scholar * Schindelin, J. _et al_. Fiji: An open-source platform for
biological-image analysis. _Nature Methods_ https://doi.org/10.1038/nmeth.2019 (2012). * Gonzales, J. M. & Law, S. H. W. Feed and feeding regime affect growth rate and gonadosomatic
index of adult zebrafish (Danio Rerio). _Zebrafish_ https://doi.org/10.1089/zeb.2013.0891 (2013). Download references ACKNOWLEDGEMENTS The study was supported by funds from IRCCS Istituto
Auxologico Italiano (Ricerca Corrente funds: 05C623_2016) and funds from University of Milan – Dept. of Clinical Sciences and Community Health (Piano di sostegno alla ricerca - Linea 2
Azione B). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * IRCCS Istituto Auxologico Italiano, Division of Endocrine and Metabolic Diseases & Lab. of Endocrine and Metabolic Research,
Milan, Italy Ivan Bassi, Francesca Luzzani, Federica Marelli, Valeria Vezzoli, Ludovica Cotellessa & Luca Persani * Department of Clinical Sciences and Community Health, University of
Milan, Milan, Italy Federica Marelli, Ludovica Cotellessa, Luca Persani & Marco Bonomi * Division of Biology and Biological Engineering, California Institute of Technology, Pasadena, CA,
USA David A. Prober * Department of Neurobiology, The George S. Wise Faculty of Life Sciences and Sagol School of Neuroscience, Tel-Aviv University, Tel-Aviv, Israel Yoav Gothilf Authors *
Ivan Bassi View author publications You can also search for this author inPubMed Google Scholar * Francesca Luzzani View author publications You can also search for this author inPubMed
Google Scholar * Federica Marelli View author publications You can also search for this author inPubMed Google Scholar * Valeria Vezzoli View author publications You can also search for this
author inPubMed Google Scholar * Ludovica Cotellessa View author publications You can also search for this author inPubMed Google Scholar * David A. Prober View author publications You can
also search for this author inPubMed Google Scholar * Luca Persani View author publications You can also search for this author inPubMed Google Scholar * Yoav Gothilf View author
publications You can also search for this author inPubMed Google Scholar * Marco Bonomi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
I.B. designed, performed and interpreted the experiments and wrote the manuscript. F.L., F.M., V.V. and L.C. performed and assisted in the experiments. D.P. provided the _prokr1b_ct814/ct814
mutant line and interpreted the experiments. M.B., Y.G., and L.P. conceived the study, supervised the publication, interpreted the experiments and wrote the manuscript. CORRESPONDING AUTHOR
Correspondence to Marco Bonomi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. SUPPLEMENTARY INFORMATION2. SUPPLEMENTARY
INFORMATION3. SUPPLEMENTARY INFORMATION4. SUPPLEMENTARY INFORMATION5. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bassi, I., Luzzani, F., Marelli, F. _et al._ Prokineticin receptor 2 affects GnRH3
neuron ontogeny but not fertility in zebrafish. _Sci Rep_ 10, 7632 (2020). https://doi.org/10.1038/s41598-020-64077-2 Download citation * Received: 19 October 2019 * Accepted: 07 April 2020
* Published: 06 May 2020 * DOI: https://doi.org/10.1038/s41598-020-64077-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative


