- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Several seamounts have been identified as hotspots of marine life in the Azores, acting as feeding stations for top predators, including cetaceans. Passive acoustic monitoring is an
efficient tool to study temporal variations in the occurrence and behaviour of vocalizing cetacean species. We deployed bottom-moored Ecological Acoustic Recorders (EARs) to investigate the
temporal patterns in acoustic presence and foraging activity of oceanic dolphins at two seamounts (Condor and Gigante) in the Azores. Data were collected in March–May 2008 and April
2010–February 2011. Dolphins were present year round and nearly every day at both seamounts. Foraging signals (buzzes and bray calls) were recorded in >87% of the days dolphin were
present. There was a strong diel pattern in dolphin acoustic occurrence and behaviour, with higher detections of foraging and echolocation vocalizations during the night and of social
signals during daylight hours. Acoustic data demonstrate that small dolphins consistently use Condor and Gigante seamounts to forage at night. These results suggest that these seamounts
likely are important feeding areas for dolphins. This study contributes to a better understanding of the feeding ecology of oceanic dolphins and provides new insights into the role of
seamount habitats for top predators. SIMILAR CONTENT BEING VIEWED BY OTHERS ACOUSTIC AND VISUAL CETACEAN SURVEYS REVEAL YEAR-ROUND SPATIAL AND TEMPORAL DISTRIBUTIONS FOR MULTIPLE SPECIES IN
NORTHERN BRITISH COLUMBIA, CANADA Article Open access 10 November 2022 EFFECTS OF INTENSE STORM EVENTS ON DOLPHIN OCCURRENCE AND FORAGING BEHAVIOR Article Open access 06 November 2020
PASSIVE ACOUSTIC MONITORING OF KILLER WHALES (_ORCINUS ORCA_) REVEALS YEAR-ROUND DISTRIBUTION AND RESIDENCY PATTERNS IN THE GULF OF ALASKA Article Open access 13 October 2021 INTRODUCTION
Animals should make optimal decisions about where and when to forage to maximize energy intake1 and it is expected that predators preferentially associate with areas where prey density is
high2. Foraging activity of air-breathing diving predators like cetaceans is also severely constrained by the vertical distribution of their prey3. In the pelagic realm, where prey
patchiness is usually high and topography deep, biophysical coupling at bathymetric and oceanographic features can aggregate prey at accessible diving depths. This “prey aggregator” effect
has often been invoked to explain the association of cetaceans to static and dynamic features4,5,6. Seamounts represent important discontinuity structures in the open ocean that may promote
a range of physical processes that can serve to concentrate prey7. Upwelling in the vicinity of these structures can stimulate local productivity by bringing cool, nutrient-rich water into
the photic zone. Enhanced water column mixing may also push weakly swimming zooplankton and larval/juvenile fish close to the surface. Seamounts may also be responsible for entrapping
vertically migrating species within the depth range of predators7,8,9. Acoustic monitoring at Cross seamount (350–450 m depth) near Hawaii, and at a seamount chain in the central equatorial
Pacific (1300 m depth), detected echolocation signals from beaked whales on most recording days10,11. The authors hypothesized that seamounts increase foraging opportunities for beaked
whales, by enhancing local prey concentration and facilitating prey capture10. In contrast, sperm whales (_Physeter macrocephalus_) were rarely detected at the equatorial Pacific seamount
chain11 and their seasonal presence at Kelvin seamount (~1600 m) appears to be related to regional variations in primary productivity12. Satellite telemetry studies showed that humpback
whales (_Megaptera novaeangliae_) spent several days around the Antigonia seamount (60 m) and Torche Bank (30 m), off New Caledonia13, while North Atlantic blue whales (_Balaenoptera
musculus_) occasionally engaged in area-restricted search (ARS) behaviours in the deep waters (>5000 m) around New England Seamounts14. In a similar study also based on satellite
telemetry data, blue and fin (_B. physalus_) whales also engaged in ARS behaviour along a chain of shallow seamounts off the Azores15. One of the first studies to specifically investigate
the effect of seamounts on the distribution of cetaceans was conducted in the Azores. This work demonstrated that common dolphins (_Delphinus delphis_) were significantly more abundant in
the vicinity of some shallow water seamounts, while the relative abundance of spotted dolphins (_Stenella frontalis_), bottlenose dolphins (_Tursiops truncatus_) and sperm whales was not
associated with the presence of seamounts8. More recently, Tobeña _et al_.16 developed habitat suitability models for 16 cetacean species in the same area. While presence and depth of
seamounts had no effect on the distribution of any cetacean species, the density of seamounts (number of seamounts/km2) was a significant predictor of the distribution of sperm whales,
killer whales (_Orcinus orca_), common and spotted dolphins. Hence, the relationship between cetaceans and seamount habitats remains elusive. This is not surprising given that most research
on cetacean usage of seamount habitats has relied on short-term observations and has often focused on seamounts for which there is little knowledge about the distribution and abundance of
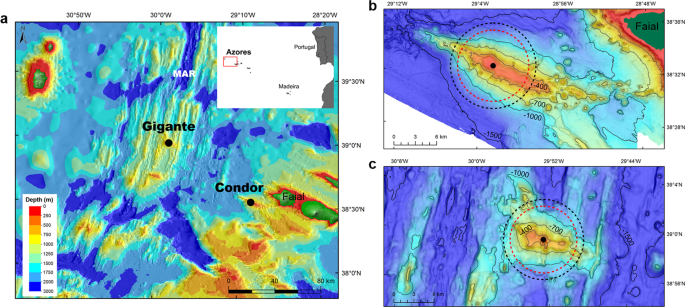
pelagic communities. Here we use a long-term dataset from passive acoustic recorders deployed at two seamounts in the Azores, Condor and Gigante (Fig. 1), to investigate the relationship of
small delphinids to these habitats. Condor and Gigante are two relatively small, shallow-intermediate seamounts (summits at ~190 m depth) that rise more than 1000 m from the surrounding
seafloor. Active acoustic surveys conducted at these seamounts found very high densities of micronekton (i.e., assemblages of small (<20 cm) pelagic fish, cephalopods and crustaceans17)
over the plateau of both seamounts, throughout the day, seasons and years18. Such high densities are believed to be caused by the presence of a seamount-associated micronekton community and
by the retention of vertically migrating micronekton at the summits19. Five species of small dolphins occur in the Azores. Common and spotted dolphins are by far the most frequent and
abundant, comprising over 45% of all cetacean sightings, followed by bottlenose and Risso’s dolphins (_Grampus griseus_); striped dolphins (_S. coeruleoalba_) are sighted only
sporadically20. These dolphin species forage opportunistically on a variety of epipelagic and mesopelagic schooling fishes and cephalopods, key constituents of the micronekton21,22,23,24.
They produce a variety of high-intensity acoustic signals making them ideal for monitoring with passive acoustics25,26. Detection of acoustic signals mainly used by dolphins when foraging
(such as buzzes and bray calls) can offer unique insights into the foraging activity of dolphins27,28,29,30 at seamount habitats. This study investigates the temporal dynamics of dolphin
acoustic detections and behaviour in Condor and Gigante seamounts to understand (i) how dolphins use seamount habitats, and (ii) if and how seamounts affect dolphin foraging behaviour. Our
results indicate that dolphins use these seamounts intensively during the whole year to forage at night, possibly to take advantage of increased densities of micronekton prey within their
foraging depths. RESULTS The Ecological Acoustic Recorders (EARs) on Condor and Gigante seamounts produced 72,660 data files, corresponding to 689 unique days and 1,467 total hours of
recordings (Table 1). Dolphins were detected 16,945 times, which is equivalent to 11.5 detections/hour. Overall, 35% of recordings contained foraging signals (buzzes and/or bray calls), 36%
echolocation clicks with social signals (whistles and/or burst-pulsed sounds), 21% only social signals and 8% only echolocation clicks. DOLPHIN ACOUSTIC PRESENCE Dolphin vocalizations were
detected in all years, months and nearly every day at both seamounts. Detections occurred in 99.5% and 98.7% of the recording days at Condor and Gigante, respectively (Fig. 2). The
proportion of dolphin positive hours per day (DPH) did not differ between the two seamounts but varied significantly across sampling periods (Supplementary Table S1). When comparing the
sampling periods with recordings available for both sites, temporal variability in daily presence was similar in Condor and Gigante: mean DPH was highest in January and February 2011, and in
March 2008 (only in Condor), intermediate in November and December 2010, and lowest in July–October 2010 and in April–May 2008, although differences in Gigante were not always significant
(Fig. 3, Supplementary Table S3). In Condor, daily presence was higher in April and in May 2010 than in April and May 2008 (Fig. 3, Supplementary Table S2). Dolphins were detected both day
and night at Condor and Gigante seamounts, but the majority of detections occurred during the night (Fig. 2). Day-night differences in dolphin detections were especially pronounced in
June-December 2010, a pattern observed at both seamounts, despite data gaps Gigante. In January-February 2011 and in March 2008, there were more detections during the day, and diel
differences were not as evident. These results are supported by the Generalized Additive Mixed Model (GAMM), which revealed that dolphin detections were significantly related with hours
after sunset, but the relationship differed between the two periods analysed (Fig. 4, Supplementary Table S4). In January-March, dolphin detections increased linearly with increasing hours
after sunset (Fig. 4a). In the remaining sampling period detections exhibited a clear diel rhythm: they were lowest 7–4 h before sunset, increased sharply 2 h prior to sunset peaking at 5 h
after sunset, and decreased again 10–11 h after sunset (Fig. 4b). DOLPHIN FORAGING ACTIVITY Foraging activity was analysed using only the days and hours during which dolphins were detected.
Foraging signals were recorded in 87% and 91% of the days that dolphins were detected at Condor and Gigante seamounts, respectively (Fig. 2). The Generalized Linear Model (GLM) model showed
that the proportion of foraging positive hours per day (FPH) did not vary significantly between seamounts nor among sampling periods (Supplementary Table S1). On average, dolphins foraged
3.9 ± 2.7 (s.d.) hours per day at Condor and 4.8 ± 3.2 hours per day at Gigante, with a maximum of 19 and 16 hours at Condor and Gigante, respectively. To investigate if time of year also
influenced diel patterns in dolphin foraging activity and acoustic behaviour, we included in the GAMM models a different smoother for hours after sunset for each period (January-March and
April-December) (not shown). Because the shape of the smoothers for different periods was remarkably similar, we chose to fit the models again using a single smoother for all data pooled.
All classes of acoustic signals showed a very pronounced diel pattern (Fig. 5, Supplementary Table S4). Foraging signals and echolocation clicks were recorded predominantly at night (Fig.
5a,b). Foraging activity was low throughout the day, increased gradually during the afternoon and night until reaching highest values ~9 h after sunset (Fig. 5a). Echolocation activity was
lowest 3 h prior to sunset, increased rapidly towards sunset, peaked 4 h after sunset and decreased again 11 h after sunset (Fig. 5b). Social signals were recorded more often during daylight
hours than at night, with a clear peak in the afternoon (~4 h prior sunset) and subsequently decreasing (Fig. 5c). DISCUSSION This study shows that small dolphins consistently use Condor
and Gigante seamounts to forage at night. Dolphins were acoustically detected in all years, months and nearly every day for which data were available, and engaged in foraging activity in the
great majority of those days (Condor: 87%, Gigante: 91%). Because we lacked data from all the months in any given recording year, monthly patterns and inter-annual changes in acoustic
presence of dolphins at these seamounts could not be conclusively resolved. Nevertheless, our results show that the daily proportion of dolphin positive hours varied between months within
years (in Condor and Gigante), as well as between the same months in different years (in Condor). Some of this intra- and inter-annual variability was related to the diel pattern in dolphin
detections, which varied through the study period. In fact, DPH peaked in January–February 2011 and March 2008 (in Condor), when daytime detections were higher. The variation in daily
detection rates within and between years likely reflects changes in the number of dolphins and/or time spent at these seamounts. Changes in seamount use by dolphins could be driven by local
shifts in prey availability and foraging conditions or reflect changes in the distribution of dolphins at broader spatial scales. A better understanding of the temporal habitat use patterns
could provide further insights into the driving factors. Dolphin detections may also have been influenced by varying sound propagation conditions and ambient noise levels31. The diel pattern
in dolphin acoustic presence was evident at both seamounts, with higher rates of detections during nighttime periods. Although we cannot completely rule out the existence of periodic daily
movements outside the EARs detection range, analysis of the acoustic behaviour of dolphins indicates a diel cycle in the use of distinct vocalizations, which may explain observed day-night
differences in dolphin detections. All categories of dolphin signals were detected both during the day and night at both seamounts but some signals were more frequently recorded during the
day and others at night. Foraging signals (buzzes and/or bray calls) and echolocation clicks were lowest during daylight hours and increased around sunset, remaining high until much later in
the night. In contrast, social signals (whistles and/or burst-pulsed) were mainly recorded during daylight hours, with detections decreasing sharply after sunset and increasing gradually
throughout the night. Similar diel cycles in vocal behaviour have been reported in several dolphin species. In general, whistling used in social interactions is higher during daylight hours
or remain constant throughout the day, whereas echolocation clicks used primarily for foraging and navigating occur mainly at night32,33,34,35. In addition, concurrent observations of
behaviour and acoustic recordings indicate that dolphins echolocate at high rates while foraging, exhibit low vocal activity while resting, and moderate vocal activity while socializing and
travelling32,36,37. Increased vocal activity during nighttime foraging and lower vocal activity during socializing could explain the diel pattern in dolphin detections at seamounts. High
rates of nighttime foraging and echolocation in dolphins living in pelagic waters have been associated with feeding on vertical migrating micronekton organisms at night32,34,35,38. Most
micronektonic taxa undergo diel vertical migrations, residing in deeper waters during the day, swimming towards the surface around sunset to feed during night, and returning to deeper waters
at sunrise39. The depth distribution and density of prey, in addition to overall abundance, are critical to the foraging success of air-breathing diving predators because they determine
whether prey items are accessible, how many prey can be encountered during a foraging dive and at what cost40. In order to minimize oxygen use during diving and maximize feeding rates,
dolphins foraging over deep waters may benefit from concentrating their foraging activity at night, when suitable concentrations of vertically migrating prey are available within their
diving range. Hawaiian spinner dolphins (_Stenella longirostris_) have been shown to track the diel vertical migration of the deep scattering layer (DSL), and to forage mainly at night, when
the layer is shallower41,42. Melon-headed whales (_Peponocephala electra_) rest and socialize over shallower waters during the day, and move towards deep water to feed at night on
mesopelagic prey43, with a concurrent increase in echolocation click rates and less whistling34. Dolphins foraging at Condor and Gigante seamounts could benefit from increased foraging
opportunities during the day, compared to dolphins foraging over deep waters. Daytime micronekton densities over the plateaus of Condor and Gigante are significantly higher than densities
measured in open-waters, at any time of the day and across all seasons18. Micronekton layers are also strongly structured during the day, forming small patches less than 16 m thick, nested
into layers extending 111 m vertically. These layers tend to remain close to the seafloor throughout the day (mean population centred at ~190 m)19. Although the exact composition of these
micronekton layers is unknown, they presumably include small pelagic and benthopelagic fishes (_Anthias anthias_, _Callanthias ruber_, _Trachurus picturatus_, _Macrorhamphosus scolopax_,
_Capros aper_), as well as mesopelagic organisms from the DSL that became entrapped over the seamount plateaus19. Small dolphins are capable of diving to 200–300 m depth44 so the daytime
depth of the micronekton layer over seamount plateaus is within the diving range of these dolphins. Therefore, we expected dolphins would take advantage of these dense prey patches close to
the seamount plateau to forage throughout the day. If this were true, we would also expect the diel cycle in their foraging activity to have been less pronounced. Instead, our results
revealed a strong diel pattern in foraging activity, with increasing levels just before darkness, high levels through the night and the lowest levels during the day, similarly to what has
been reported in other habitats. This pattern coincided with an increase in acoustic density of prey in surface layers over Condor and Gigante seamount summits and slopes, resulting from the
vertical migration of part of the DSL19. Dolphins’ foraging activity increased when prey from the DSL begins moving towards the surface and reaches the peak when these prey disperse in the
upper water column. It is possible that the benefits of foraging during the day on high-density prey aggregations near the seafloor may be offset by the costs of diving deeper, so dolphins
choose to wait for the prey to be available at shallower depths. Even if the daytime depth of the micronekton layer is accessible to these dolphins, the longer and deeper dive may imply
greater energy expenditure45 and more time to recover oxygen stores at the surface46, which translates into lower feeding rates. It comes to a point when the energetic gains are offset by
the costs associated with acquiring energy, and foraging is no longer an optimal strategy47. This explanation is consistent with a recent study on humpback whales that showed that whales
increased their foraging effort at night when prey was shallowest but less densely distributed, presumably to minimize diving and searching costs48. Prey depth is also known to be a strong
predictor of the habitat use of seabirds and seals3. Alternatively, the diel cycle in detection of foraging signals could reflect a switch from acoustic to visual predation. Given the
daytime depth of micronekton (~190 m) is deeper than the lower limit of the photic zone (~150 m)49, it seems unlikely that dolphins would rely primarily on vision for detecting prey.
CONCLUSION Passive acoustic monitoring at two seamounts in the Azores shows that oceanic dolphins frequently foraged in these seamount habitats during the night. Dolphins were detected
nearly every day and foraging activity occurred on most recording days. Dolphin foraging activity at Condor and Gigante seamounts was significantly higher at night than during the day,
similarly to what was reported for dolphins foraging in coastal areas and in pelagic deep waters. This is somewhat surprising and suggests that dolphins did not exploit the high densities of
micronekton prey that are continuously available to them near the plateau (~190 m depth) of these seamounts. Instead, foraging activity increased shortly after sunset, coinciding with the
upward migration of organisms from the DSL. The benefits of exploiting higher prey densities may be offset by the costs of foraging at higher depths. The depth rating of our acoustic system
did not enable monitoring open-ocean areas to compare dolphin detections with seamount habitats. This would help understanding the ecological relevance of the high dolphin detection rates
reported for Condor and Gigante seamounts, and clarifying whether oceanic dolphins specifically target these seamounts to forage. Studies using other acoustic systems may provide these
answers in the future, contributing to a better understanding of the role of seamount ecosystems for these predators. Finally, this work emphasizes the bottom-up perspective but it will be
equally important to understand the top-down effect of dolphins on the structure and functioning of seamount systems. Daily presence and regular foraging of dolphins at seamounts suggests
these animals may play an important role in driving local dynamics of micronekton prey, regulating food web structure and composition, and in nutrient cycling50,51. METHODS DATA COLLECTION
Passive acoustic monitoring data were collected at two shallow-intermediate seamounts, Condor and Gigante, in the Azores (Fig. 1). Condor has a relatively flat summit with two major peaks at
182 m and 214 m depth and a total surface area of 11.6 km2. It is an elongated feature approximately 26 km long and 7.4 km wide at the 1000-m depth contour (Fig. 1b). Gigante reaches 161 m
depth, and has a small plateau of 0.7 km2. It is about 16 km long and 6–13 km wide at the 1000-m depth contour (Fig. 1c)18. At each seamount, an Ecological Acoustic Recorder (EAR) was moored
~10 m above the seafloor, at approximately 190 m depth. The EAR is a microprocessor-based autonomous recorder produced by Oceanwide Science Institute (Honolulu, HI)52 with a Sensor
Technology SQ26-01 hydrophone that has a flat frequency response (±1.5 dB) from 18 Hz to 28 kHz with a sensitivity between −193 and −194 dB re 1 V/μPa (Table 1). Acoustic recordings were
collected in March-May 2008 and April 2010-February 2011, but there were periods with no data available due to gaps in successive deployments and occasional instrument failure (Table 1).
Additionally, recorders had to be duty cycled. In the first deployment, the EARs were programmed to record 30 s every 10 min at a sampling rate of 50 kHz, providing an effective recording
bandwidth of 25 kHz at a 5% duty cycle (Table 1). To increase the likelihood of recording dolphin vocalizations, the duty cycle was subsequently changed to record 90 s every 15 min (10% duty
cycle). With this duty cycle, the system was capable of storing data for periods of 4 to 6 months. After this period, the EARs were recovered to download the data. DATA ANALYSIS DATA
PROCESSING Acoustic data were processed using Triton, a custom Matlab script53. Triton was used to create long-term spectral averages (LTSA) of the acoustic recordings, which provide a means
of quickly evaluating long-term data sets for acoustic events. Instead of inspecting short duration spectrograms for individual calls, successive spectra are calculated and averaged
together. These averaged-spectra are arranged sequentially to provide a time series of the spectra54. Using LTSAs, delphinid whistling and echolocation clicking bouts can easily be
distinguished from background noise and other biotic or abiotic sound sources (Fig. 6). LTSAs were calculated with 20 Hz frequency and 15 s time resolution. These LTSAs were manually
scrutinized to assign ones or zeros, representing presence or absence of dolphin signals, for every 1 h long LTSA segment. When dolphin signals were detected within a 1 h LTSA segment, 30%
of the files with the strongest signals (indicated by dB intensity) were selected for visual inspection of the spectrograms to classify dolphin vocalizations and detect potential foraging
activity. Delphinid echolocation clicks are short, broadband pulses with peak frequencies that vary from tens of kilohertz to well over 100 kHz. These clicks generally occur in trains
containing few to hundreds of clicks and are used for navigation and target detection and discrimination55. During the final stages of prey capture, these echolocation click trains become
more rapid with shorter inter-click intervals forming buzzes28,30. Burst-pulsed signals are short, discrete bursts of broadband sound pulses believed to function mainly in social
interactions56, although some signals may also play a role in foraging events, such as bray calls27,29. Bray calls consist of a single- or multi-unit sequences of sounds with burst-pulsed
signals alternating with short tonal sweeps, only recorded for bottlenose dolphins57,58,59. Whistles are continuous, narrow band, frequency modulated signals that range in duration from
several tenths of a second to several seconds. The fundamental frequency of most whistles ranges from 2 to 30 kHz. Whistles are believed to function in social contexts60. Based on the above,
detection of buzzes or bray calls was used as an indicator of dolphin foraging activity27,28,29,30, and detection of any signal attributed to dolphins was used to indicate dolphin presence.
Delphinid species identification from acoustic data is challenging, due to the strong similarity of most signals, especially echolocation clicks. Because the sampling rate used in this
study (50 kHz) did not allow us to record the full spectrum of echolocation clicks, we did not attempt to separate signals into probable species and pooled all acoustic data under a single
delphinid group. Nevertheless, of the five dolphin species that occur in our study area20, Risso’s dolphin clicks have peaks frequencies slightly higher than frequencies recorded by our
system61. Moreover, in the Azores, Risso’s dolphins show a preference for habitats close to the islands16 and striped dolphins are only occasional visitors20. Hence, we assume the large
majority of acoustic detections to be of common, spotted and bottlenose dolphins. We were unable to determine the detection range of the EAR, which may vary considerably, depending on
location of the recorders, environmental conditions, as well as vocalization type and behavioural context31,62,63. However, given that the maximum expected detection range of dolphin clicks
is around 5 km64, that the communication range of whistling bottlenose dolphins is about 6 km65 and that whistles propagate farther than echolocation clicks66, in this study we assume a
maximum detection range of about 6 km. This means that dolphins detected acoustically were over the plateau or slopes of Condor and Gigante seamounts (Fig. 1b,c). STATISTICAL ANALYSIS
Because most months were only monitored once during the study period, we could not assess monthly or inter-annual variation in acoustic detections or foraging activity of dolphins. Instead,
data were pooled by month of the year (hereafter called sampling periods) within each seamount, and sampling periods were treated. A GLM model, with a binomial distribution and a logit link
function, was used to investigate if dolphin detections varied between seamounts and sampling periods. The response variable was the proportion of dolphin positive hours per day (DPH),
calculated as the number of hours with at least one dolphin detection divided by the number of hours recorded on that day, to account for variation in acoustic effort. A non-parametric
pairwise Wilcoxon rank-sum test was used to detect differences in DPH between pairs of sampling periods. Dolphin foraging behaviour at seamounts was examined by calculating the proportion of
foraging positive hours per day (FPH), i.e., the number of hours with at least one foraging signal divided by the number of hours with dolphin detections on that day. A GLM, with a binomial
distribution and a logit link function, was used to explore the effect of seamount and sampling period in FPH. Diel patterns in dolphin detections were examined with a GAMM model, with a
binomial distribution and logit link function, using presence/absence of dolphin detections in each hourly record as the response variable. As the duration of day-night cycles varies
considerably between summer and winter months, hours after the sunset was used as an explanatory variable. The local times of sunrise and sunset were obtained from the U.S. Naval Observatory
Astronomical Applications Department database. Because dolphin detections in January-February 2011 and March 2008 showed a different day-night pattern from detections in the remaining
sampling periods (Fig. 2), we included a different smoother for hours after sunset for each period (January-March and April-December). To account for differences in sampling effort per day,
log of recording hours was used as an offset and an autoregressive moving average (ARMA) autocorrelation structure was included in the model to address the temporal dependence in the data67.
Similar models were used to investigate diel patterns in dolphin acoustic behaviour, using presence/absence of different classes of signals as the response variable. A separate model was
run for foraging (buzzes + bray calls), echolocation (clicks) and social (whistles + burst-pulsed) signals. A backward stepwise selection procedure was used to identify the best fitting
model based on the Akaike Information Criterion (AIC) value and analysis of deviance. All statistical analyses were done in R68 using the ‘_mgcv’_ R package69. DATA AVAILABILITY The datasets
generated and/or analysed during this study can be made available upon request. REFERENCES * Stephens, D. W. & Krebs, J. R. _Foraging Theory_ (Princeton University Press, Princeton, New
Jersey, 1986). * Curio, E. _The Ethology of Predation_ (Springer-Verlag, Berlin Heidelberg, New York, 1976). * Benoit-Bird, K. J. _et al_. Prey patch patterns predict habitat use by top
marine predators with diverse foraging strategies. _PLoS One_ 8(1), e53348 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Skov, H. _et al_. Small-scale spatial
variability of sperm and sei whales in relation to oceanographic and topographic features along the Mid-Atlantic Ridge. _Deep-Sea Res. II_ 55(1-2), 254–268 (2008). Article ADS Google
Scholar * Woodworth, P. A. _et al_. Eddies as offshore foraging grounds for melon-headed whales (Peponocephala electra). _Mar. Mamm. Sci._ 28(3), 638–647 (2012). Article Google Scholar *
Rogan, E. _et al_. Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. _Deep-Sea Res. II_ 141, 8–19 (2017). Article Google Scholar * Genin, A.
Bio-physical coupling in the formation of zooplankton and fish aggregations over abrupt topographies. _J. Mar. Syst._ 50, 3–20 (2004). Article Google Scholar * Morato, T. _et al_. Evidence
of a seamount effect on aggregating visitors. _Mar. Ecol. Prog. Ser._ 257, 23–32 (2008). Article ADS Google Scholar * Morato, T., Hoyle, S. D., Allain, V. & Nicol, S. J. Seamounts
are hotspots of pelagic biodiversity in the open ocean. _PNAS_ 107(21), 9707–9711 (2010). Article ADS CAS PubMed Google Scholar * Johnston, D. W. _et al_. Temporal patterns in the
acoustic signals of beaked whales at Cross Seamount. _Biol. Lett._ 4, 208–211 (2008). Article CAS PubMed PubMed Central Google Scholar * Baumann-Pickering, S., Trickey, J. S., Wiggins,
S. M. & Oleson, E. M. Odontocete occurrence in relation to changes in oceanography at a remote equatorial Pacific seamount. _Mar. Mamm. Sci._ 32(3), 805–825 (2016). Article Google
Scholar * Wong, S. N. P. & Whitehead, H. Seasonal occurrence of sperm whales (Physeter macrocephalus) around Kelvin Seamount in the Sargasso Sea in relation to oceanographic processes.
_Deep-Sea Res. I_ 91, 10–16 (2014). Article Google Scholar * Garrigue, C., Clapham, P. J., Geyer, Y., Kennedy, A. S. & Zerbini, A. N. Satellite tracking reveals novel migratory
patterns and the importance of seamounts for endangered South Pacific humpback whales. _R. Soc. Open Sci._ 2(11), 150489 (2015). Article ADS PubMed PubMed Central Google Scholar *
Lesage, V., Gavrilchuk, K., Andrews, R. D. & Sears, R. Foraging areas, migratory movements and winter destinations of blue whales from the western North Atlantic. _Endang. Species Res._
34, 27–43 (2017). Article Google Scholar * Silva, M. A., Prieto, R., Jonsen, I., Baumgartner, M. F. & Santos, R. S. North Atlantic blue and fin whales suspend their spring migration to
forage in middle latitudes: building up energy reserves for the journey? _PLoS One_ 8(10), e76507 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Tobeña, M., Prieto,
R., Machete, M. & Silva, M. A. Modeling the potential distribution and richness of cetaceans in the Azores from Fisheries Observer Program data. _Front. Mar. Sci._ 3, 202 (2016). Article
Google Scholar * Brodeur, R. D. & Yamamura, O. Micronekton of the North Pacific. PICES Scientific Report 30, Sydney, BC, Canada (2005). * Cascão, I. _et al_. Persistent enhancement of
micronekton backscatter at the summits of seamounts in the Azores. _Front. Mar. Sci._ 4, 25 (2017). Article Google Scholar * Cascão, I., Domokos, R., Lammers, M. O., Santos, R. S. &
Silva, M. A. Seamount effects on the diel vertical migration and spatial structure of micronekton. _Progr. Oceanogr._ 175, 1–13 (2019). Article ADS Google Scholar * Silva, M. A. _et al_.
Spatial and temporal distribution of cetaceans in the mid-Atlantic waters around the Azores. _Mar. Biol. Res._ 10(2), 123–137 (2014). Article Google Scholar * García-Godos, I., Van
Waerebeek, K., Reyes, J. C., Alfaro-Shigueto, J. & Arias-Schreiber, M. Prey occurrence in the stomach contents of four small cetacean species in Peru. _LAJAM_ 6(2), 171–183 (2007).
Article Google Scholar * Doksaeter, L., Olsen, E., Nøttestad, L. & Fernö, A. Distribution and feeding ecology of dolphins along the Mid-Atlantic Ridge between Iceland and the Azores.
_Deep-Sea Res. II_ 55, 243–253 (2008). Article ADS Google Scholar * Herzing, D. L. & Elliser, C. R. Nocturnal feeding of Atlantic spotted dolphins (Stenella frontalis) in the Bahamas.
_Mar. Mamm. Sci._ 30(1), 367–373 (2014). Article Google Scholar * Arranz, P. _et al_. Diving behavior and fine-scale kinematics of free-ranging Risso’s dolphins foraging in shallow and
deep-water habitats. Front. _Ecol. Evol._ 7, 53 (2019). Google Scholar * Van Parijs, S. M., Smith, J. & Corkeron, P. J. Using calls to estimate the abundance of inshore dolphins: a case
study with Pacific humpback dolphins Sousa chinensis. _J. Appl. Ecol._ 39, 853–864 (2002). Article Google Scholar * Hastie, G. D., Swift, R. J., Slesser, G., Thompson, P. M. &
Turrell, W. R. Environmental models for predicting oceanic dolphin habitat in the Northeast Atlantic. _ICES J. Mar. Sci._ 62, 760–770 (2005). Article Google Scholar * Janik, V. M.
Food-related bray calls in wild bottlenose dolphins (Tursiops truncatus). _Proc. Biol. Sci._ 267(1446), 923–927 (2000). Article CAS PubMed PubMed Central Google Scholar * Wisniewska, D.
M., Johnson, M., Nachtigall, P. E. & Madsen, P. T. Buzzing during biosonar-based interception of prey in the delphinids Tursiops truncatus and Pseudorca crassidens. _J. Exp. Biol._ 217,
4279–4282 (2014). Article PubMed Google Scholar * King, S. L. & Janik, V. M. Come dine with me: food-associated social signaling in wild bottlenose dolphins (Tursiops truncatus).
_Anim. Cogn._ 18, 969–974 (2015). Article PubMed Google Scholar * Ridgway, S., Dibble, D. S., Van Alstyne, K. & Price, D. On doing two things at once: dolphin brain and nose
coordinate sonar clicks, buzzes and emotional squeals with social sounds during fish capture. _J. Exp. Biol._ 218, 3987–3995 (2015). Article PubMed Google Scholar * Cato, D., McCauley,
R., Rogers, T. & Noad, M. Passive acoustics for monitoring marine animals - progress and challenges. _Proceedings of ACOUSTICS_, 453–460 (2006). * Norris, K. S., Würsig, B., Wells, R. S.
& Würsig, M. _The Hawaiian Spinner Dolphin_ (University of California Press, Berkeley, CA., 1994). * Soldevilla, M. S., Wiggins, S. M. & Hildebrand, J. A. Spatial and temporal
patterns of Risso’s dolphin echolocation in the Southern California Bight. _J. Acoust. Soc. Am._ 127(1), 124–132 (2010). Article ADS PubMed Google Scholar * Baumann-Pickering, S., Roch,
M. A., Wiggins, S. M., Schnitzler, H. U. & Hildebrand, J. A. Acoustic behavior of melon-headed whales varies on a diel cycle. _Behav. Ecol. Sociobiol._ 69, 1553–1563 (2015). Article
PubMed PubMed Central Google Scholar * Caruso, F. _et al_. Long-term monitoring of dolphin biosonar activity in deep pelagic waters of the Mediterranean Sea. _Sci. Rep._ 7, 4321 (2017).
Article ADS PubMed PubMed Central CAS Google Scholar * Van Parijs, S. M. & Corkeron, P. J. Vocalizations and behavior of Pacific humpback dolphins Sousa chinensis. _Ethology_ 107,
701–716 (2001). Article Google Scholar * Nowacek, D. P. Acoustic ecology of foraging bottlenose dolphins (Tursiops truncatus), habitat-specific use of three sound types. _Mar. Mamm. Sci._
21(4), 587–602 (2005). Article Google Scholar * Benoit-Bird, K. J. & Au, W. W. L. Phonation behavior of cooperatively foraging spinner dolphins. _J. Acoust. Soc. Am._ 125(1), 539–546
(2009). Article ADS PubMed Google Scholar * Sutton, T. T. Vertical ecology of the pelagic ocean: classical patterns and new perspectives. _J. Fish Biol._ 83, 1508–1527 (2013). Article
CAS PubMed Google Scholar * MacArthur, R. H. & Pianka, E. R. On optimal use of a patchy environment. _Am. Nat._ 100(916), 603–609 (1966). Article Google Scholar * Benoit-Bird, K. J.
& Au, W. W. L. Prey dynamics affect foraging by a pelagic predator (Stenella longirostris) over a range of spatial and temporal scales. _Behav. Ecol. Sociobiol._ 53, 364–373 (2003).
Article Google Scholar * Benoit-Bird, K. J., Würsig, B. & McFadden, C. J. Dusky dolphin (Lagenorhynchus obscurus) foraging in two different habitats: Active acoustic detection of
dolphins and their prey. _Mar. Mamm. Sci._ 20(2), 215–231 (2004). Article Google Scholar * Brownell, R. L. Jr., Ralls, K., Baumann-Pickering, S. & Poole, M. M. Behavior of melon-headed
whales, Peponocephala electra, near oceanic islands. _Mar. Mamm. Sci._ 25, 639–658 (2009). Article Google Scholar * Stewart, B. S. Diving behaviour. In Encyclopedia of Marine Mammals, 2nd
ed. (eds. Perrin, W. F., Würsig, B. & Thewissen, J. G. M.) 321-327 (Academic Press, Massachusetts, USA, 2009). * Costa, D. P. Diving physiology of marine vertebrates. In Encyclopedia of
Life Sciences (John Wiley & Sons Ltd, Chichester, 2007). * Ponganis, P. J. Diving Physiology of Marine Mammals and Seabirds (Cambridge University Press, UK, 2015). * Charnov, E. L.
Optimal foraging, the marginal value theorem. _Theor. Popul. Biol._ 9(2), 129–136 (1976). Article CAS PubMed MATH Google Scholar * Friedlaender, A. S. _et al_. Multiple-stage decisions
in a marine central-place forager. _R. Soc. Open Sci._ 3, 160043 (2016). Article ADS PubMed PubMed Central Google Scholar * Wisshak, M., Form, A., Jakobsen, J. & Freiwald, A.
Temperate carbonate cycling and water mass properties from intertidal to bathyal depths (Azores, N-Atlantic). _Biogeosci. Discuss_ 7, 3297–3333 (2010). Article ADS Google Scholar * Bowen,
W. Role of marine mammals in aquatic ecosystems. _Mar. Ecol. Progr. Series_ 158, 267–274 (1997). Article ADS Google Scholar * Doughty, C. E. _et al_. Global nutrient transport in a world
of giants. _PNAS_ 113(4), 868–873 (2016). Article ADS MathSciNet CAS PubMed Google Scholar * Lammers, M. O., Brainard, R. E., Au, W. W. L., Mooney, T. A. & Wong, K. B. An
ecological acoustic recorder (EAR) for long-term monitoring of biological and anthropogenic sounds on coral reefs and other marine habitats. _J. Acoust. Soc. Am._ 123(3), 1720–1728 (2008).
Article ADS PubMed Google Scholar * Wiggins, S. Autonomous acoustic recording packages (ARPs) for long-term monitoring of whale sounds. _Mar. Technol. Soc. J._ 37(2), 13–22 (2003).
Article Google Scholar * Wiggins, S. M. & Hildebrand, J. A. High-frequency acoustic recording package (HARP) for broad-band, long-term marine mammal monitoring. In _International
Symposium on Underwater Technology 2007 and International Workshop on Scientific Use of Submarine Cables and Related Technologies_ 2007. 551–557 pp. (IEEE, Tokyo, Japan, 2007). * Au, W. W.
L. The Sonar of Dolphins (Springer New York, New York, 1993). Chapter Google Scholar * Frankel, A. S. Sound production. In Encyclopedia of Marine Mammals, 2nd ed. (eds. Perrin, W. F.,
Würsig, B. & Thewissen, J. G. M.) 1056–1071 (Academic Press, Massachusetts, USA 2009). * Dos Santos, M. E., Caporin, G., Moreira, H. O., Ferreira, A. J. & Coelho, J. L. B. Acoustic
behavior in a local population of bottlenose dolphins. In Sensory Abilities of Cetaceans (eds. Thomas, J. A. & Kastelein, R. A.) vol. 196, 585–598 (NATO ASI Series, Series A: Life
Sciences, Springer, Boston, MA, 1990). * Dos Santos, M. E., Ferreira, A. J. & Harzen, S. Rhythmic sound sequences emitted by aroused bottlenose dolphins in the Sado estuary, Portugal.
_Sensory Systems of Aquatic Mammals_, 325–334 (1995). * Luís, A. R., Alves, I. S., Sobreira, F. V., Couchinho, M. N. & Dos Santos, M. E. Brays and bits: information theory applied to
acoustic communication sequences of bottlenose dolphins. _Bioacoustics_ 27, 1–11 (2018). Article Google Scholar * Dudzinski, K. M., Thomas, J. A. & Gregg, J. D. Communication in marine
mammals. In _Encyclopedia of Marine Mammals_, 2nd ed. (eds. Perrin, W. F., Würsig, B. & Thewissen, J. G. M.) 260–269 (Academic Press, Massachusetts, USA, 2009). * Soldevilla, M. S. _et
al_. Geographic variation in Risso’s dolphin echolocation click spectra. _J. Acoust. Soc. Am._ 142(2), 599–617 (2017). Article ADS PubMed Google Scholar * Mellinger, D. K., Stafford, K.
M., Moore, S. E., Dziak, R. P. & Matsumoto, H. An overview of fixed passive acoustic observation methods for cetaceans. _Oceanogr._ 20(4), 36–45 (2007). Article Google Scholar *
Zimmer, W. M. X., Harwood, J., Tyack, P. L., Johnson, M. P. & Madsen, P. T. Passive acoustic detection of deep-diving beaked whales. _J. Acoust. Soc. Am._ 124(5), 2823–2832 (2008).
Article ADS PubMed Google Scholar * Frasier, K. E. _et al_. Delphinid echolocation click detection probability on near-seafloor sensors. _J. Acoust. Soc. Am._ 140, 1918–1930 (2016).
Article ADS PubMed Google Scholar * Jensen, F. H., Beedholm, K., Wahlberg, M., Bejder, L. & Madsen, P. T. Estimated communication range and energetic cost of bottlenose dolphin
whistles in a tropical habitat. _J. Acoust. Soc. Am._ 131(1), 582–592 (2012). Article ADS PubMed Google Scholar * Frankel, A. S., Zeddies, D., Simard, P. & Mann, D. Whistle source
levels of free-ranging bottlenose dolphins and Atlantic spotted dolphins in the Gulf of Mexico. _J. Acoust. Soc. Am._ 135, 1624–1631 (2014). Article ADS PubMed Google Scholar * Zuur, A.
F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. _Mixed Effects Models and Extensions in Ecology with R_ (Springer New York, New York, 2009). * R Core Team. R: A language
and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2016). * Wood, S. N. _Generalized Additive Models: An Introduction with R_ (Chapman and
Hall/CRC, 2006). * Amorim, P. & Tempera, F. Portugal (Azores) - Report on the compilation of bathymetry information. In _Report on Collation of Historic Maps. Bathymetry, Substrate and
Habitats_ (eds. Dulce, M C. _et al_.) 98 pp. (MeshAtlantic Report, Spanish Institute of Oceanography, 2013). Download references ACKNOWLEDGEMENTS This research was supported by the Fundação
para a Ciência e a Tecnologia (FCT), Azores 2020 Operational Programme and the Fundo Regional da Ciência e Tecnologia (FRCT), through research projects TRACE (PTDC/MAR/74071/2006), MAPCET
(M2.1.2/F/012/2011), FCT-Exploratory (IF/00943/2013/CP1199/CT0001), WATCH IT (Acores-01-0145-FEDER-000057) and MISTIC SEAS II (GA11.0661/2017/750679/SUB/ENV.C2), co-funded by FEDER, COMPETE,
QREN, POPH, European Social Fund (ESF), the Portuguese Ministry for Science and Education, and EU-DG/ENV. The Azores 2020 Operational Programme is funded by the community structural funds
ERDF and ESF. Funds were also provided by FCT to MARE, through the strategic project UID/MAR/04292/2013. MAS was supported through a FCT Investigator contract funded by POPH, QREN, ESF and
the Portuguese Ministry for Science and Education (IF/00943/2013). IC was supported by a FCT doctoral grant (SFRH/BD/41192/2007) and RP by a FCT postdoctoral grant (SFRH/BPD/108007/2015). We
thank the field and crew teams for assisting with the many deployments and recoveries of the EARs. Special thanks to Norberto Serpa for helping with mooring design, Ken Sexton and Michael
Richlen for their roles in manufacturing the EARs, Sergio Gomes for building the battery packs, and Lisa Munger for adapting Triton for EAR data analysis. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Marine and Environmental Sciences Centre (MARE), Institute of Marine Research (IMAR) and Okeanos R&D Centre, University of the Azores, Rua Frederico Machado 4, 9901-862,
Horta, Portugal Irma Cascão, Rui Prieto, Ricardo S. Santos & Mónica A. Silva * Hawaiian Islands Humpback Whale National Marine Sanctuary, National Oceanic and Atmospheric Administration
(NOAA), Kihei, HI, 96753, USA Marc O. Lammers * Oceanwide Science Institute (OSI), PO Box 61692, Honolulu, HI, 96744, USA Marc O. Lammers * Biology Department, Woods Hole Oceanographic
Institution, Woods Hole, MA, 02543, USA Mónica A. Silva Authors * Irma Cascão View author publications You can also search for this author inPubMed Google Scholar * Marc O. Lammers View
author publications You can also search for this author inPubMed Google Scholar * Rui Prieto View author publications You can also search for this author inPubMed Google Scholar * Ricardo S.
Santos View author publications You can also search for this author inPubMed Google Scholar * Mónica A. Silva View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS Conceived and designed the data collection: I.C., M.O.L., M.A.S. Executed data collection: I.C. Performed data analyses: I.C., M.A.S. Wrote the paper: I.C., M.A.S.
Reviewed the manuscript and approved the final version: I.C., M.O.L., R.P., R.S.S., M.A.S. CORRESPONDING AUTHOR Correspondence to Irma Cascão. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a
link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license,
unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory
regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cascão, I., Lammers, M.O., Prieto, R. _et al._ Temporal patterns in acoustic
presence and foraging activity of oceanic dolphins at seamounts in the Azores. _Sci Rep_ 10, 3610 (2020). https://doi.org/10.1038/s41598-020-60441-4 Download citation * Received: 19
September 2019 * Accepted: 12 February 2020 * Published: 27 February 2020 * DOI: https://doi.org/10.1038/s41598-020-60441-4 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative







