- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Industrial hemp (_Cannabis sativa_ L.) is a high-yielding annual crop primarily grown for fiber, seeds, and oil. Due to the phytochemical composition of hemp, there has been an
increased interest in the market for nutraceuticals and dietary supplements for human health. Recent omics analysis has led to the elucidation of hemp candidate genes involved in the
syntheses of specialized metabolites. However, a detailed study of these genes has not been undertaken due to the lack of a stable transformation system. We report for the first time an
agroinfiltration system in hemp utilizing vacuum infiltration, which is an alternative method to stable transformation. A combination of 0.015% Silwett L-77, 5 mM ascorbic acid, and thirty
second sonication followed by a 10-minute vacuum treatment resulted in the highest β-glucuronidase expression in the leaf, male and female flowers, stem, and root tissues. The phytoene
desaturase gene was silenced with a transient hairpin RNA expression, resulting in an albino phenotype in the leaves and the male and female flowers. This agroinfiltration system would be
useful for overexpression and silencing studies of target genes to regulate the yield of specialized metabolites in hemp. SIMILAR CONTENT BEING VIEWED BY OTHERS NANOTECHNOLOGY TO ADVANCE
CRISPR–CAS GENETIC ENGINEERING OF PLANTS Article 12 March 2021 THE EMERGING ROLE OF NANOTECHNOLOGY IN PLANT GENETIC ENGINEERING Article 22 February 2023 DIRECT DELIVERY AND FAST-TREATED
_AGROBACTERIUM_ CO-CULTURE (FAST-TRACC) PLANT TRANSFORMATION METHODS FOR _NICOTIANA BENTHAMIANA_ Article 17 October 2022 INTRODUCTION Industrial hemp (_Cannabis. sativa_ L.) is a diploid (2n
= 20) and typically dioecious plant primarily grown for fiber, seeds, and oil. Recently, this crop has been exploited for medicinal compounds as it produces 565 secondary metabolites, such
as cannabinoids, terpenes and other phenolic compounds1. Unlike drug-type _Cannabis_ (marijuana) that produces high levels of the psychoactive component Δ9-tetrahydrocannabinol (THC), hemp
preferentially produces cannabidiol (CBD) and low amounts of THC (0.3% compared with marijuana). In addition, a total of 25,000 hemp products are currently produced and sold on the market,
for both scientific and industrial purposes2. Cannabinoids are terpenophenolic compounds that include more than 100 chemical compounds, such as THC and CBD2. These molecules have
pharmacological properties in the treatment of pain, mood disorders, diabetes, cancer, and neurodegenerative and inflammatory diseases3,4. The biosynthesis of these molecules occurs in the
glandular trichomes located predominantly on the flowers and leaves of _C. sativa_1. The cannabinoid pathway is initiated by the synthesis of olivetolic acid (OA) originating from the
primary metabolite precursor via hexanoyl-CoA by tetraketide synthase (type III polyketide synthase) and olivetolic acid cyclase5. Together with geranyldiphosphate (GPP) from the plastidal
2-C- methyl-D-erythritol-4-phosphate pathway, OA is converted into cannabigerolic acid (CBGA) by CBGA synthase. This cannabinoid pathway concludes with the syntheses of
tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA) by THCA synthase (THCAS) and CBDA synthase (CBDAS), respectively6. THCAS and CBDAS are converted to THC and CBD,
respectively, by nonenzymatic reactions. Terpenes have anxiolytic, antibacterial, anti-inflammatory, and sedative effects on human diseases7. For example, the sesquiterpene β-caryophyllene
interacts with mammalian cannabinoid receptors to reduce inflammation8. The terpene biosynthesis pathway in _C. sativa_ includes two pathways: the plastidial methylerythritol phosphate (MEP)
pathways, which leads to the production of monoterpenes, and the cytosolic mevalonate (MEV) pathway, which leads to the production of sesquiterpenes1. Seven enzymes have been shown to
produce two terpene precursors: GPP and farnesyl diphosphate (FPP). Furthermore, the nine terpene synthase genes that catalyze terpene production from GPP and FPP have been identified9.
Knowledge of these secondary metabolite synthesis genes will help facilitate genetic improvements to obtain desirable metabolite profiles. However, these candidate genes were proposed based
on genomic, transcriptomic and proteomic analyses and remain to be studied. Towards this goal, stable transformation is ideal; however, a hemp transformation protocol has not yet been
developed due to the low shoot regeneration rate10,11. Agroinfiltration is a prominent methodology for transiently expressing or silencing genes of interest in an efficient manner. This
method can be used to produce vaccines and enzymes for industrial use as an alternative to stable transformation12. Agroinfiltration studies have also provided insight into various
biological processes, such as promoter function, gene expression, subcellular protein localization, protein-protein interactions, and metabolism13. To date, this agroinfiltration system has
been developed and utilized in both 1) model plant species such as tobacco, _Nicotiana benthamiana_, _Arabidopsis_, tomato and 2) agronomic crops such as rice, soybean, onion, flax, cowpea,
grapevine, rose, and cacao14. Depending on the plant species, the agroinfiltration efficiency varies, resulting in high expression in plants such as _N. benthamiana_ and tobacco or low
expression in a wide range of plant species, including hemp. For infection of the plant leaf cells, _Agrobacterium_ suspension is injected into the leaf parenchyma, but in many cases,
agroinfiltration is not successful because the morphology of the leaf and particularly the structure of the thick epidermis do not allow the bacterial culture to enter the parenchyma,
consequently resulting in a low gene expression efficiency15. In hemp, a hairy root culture system for transient gene expression assays was developed and resulted in slight β-glucuronidase
(GUS) positive staining using _Agrobacterium tumefaciens_ and _A. rhizogenes_16. However, pharmaceutical components are not abundant in the _C. sativa_ root17, and therefore, this system is
not appropriate for analyses of the genes encoding enzymes that synthesize specialized metabolites. To our knowledge, this article constitutes the first report of _C. sativa_
agroinfiltration with a high efficacy in the aerial parts of the plant where pharmaceutical components are highly synthesized, transported and accumulated. The agroinfiltration method was
optimized by a combination of physical and chemical treatments with different _Agrobacterium_ strains and hemp cultivars. This optimized system had a high efficiency for exogeneous gene
expression and silencing of endogenous genes in different hemp tissue/organs. RESULTS OPTIMIZATION OF AGROINFILTRATION The _GUS_ gene (_uidA_), which encodes the enzyme GUS, is one of the
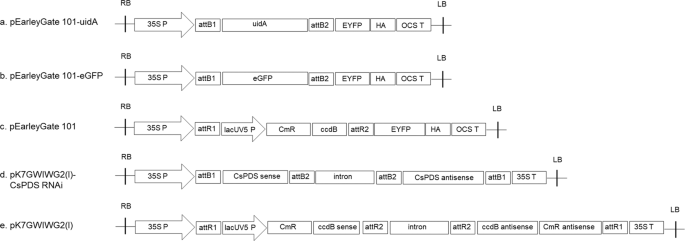
most widely used reporter genes in plant molecular biology18. For analysis of the efficiency of agroinfiltration, the _uidA_ gene was cloned into the binary vector pEarleyGate 101 and
expressed under the control of the cauliflower mosaic virus (CaMV) 35 S promoter (Fig. 1a). This cassette was transformed into _Agrobacterium_ GV3101. Further agroinfiltration was carried
out, and the tissues underwent histochemical GUS staining. Agroinfiltration was carried out via vacuum infiltration to test the different tissues of the plant. The mature leaf, male flower,
female flower, stem and root tissues were excised from the hemp plants two months after seed germination. In a histochemical GUS assay, only the male flower in all the tissues tested showed
weak GUS staining. Then, various parameters such as chemical additives, sonication, different vacuum time, hemp cultivars and _Agrobacterium_ strains were optimized using the male flower to
create an optimal agroinfiltration protocol. EFFECT OF CHEMICAL ADDITIVES The effect of four chemical additives including Silwett L-77, Pluronic F-68, L-Ascorbic acid and
polyvinylpyrrolidone (PVP) was evaluated at different concentrations. Silwett L-77 and Pluronic F-68 are surfactants that can reduce the surface tension and enhance the entry of bacteria
into plant tissues19. The addition of Silwett L-77 at 0.015% drastically increased the GUS expression to approximately three times that of the negative control plant (0% Silwett L-77) (Fig.
2a). The percentage of the GUS-stained male flowers was higher in the 0.015% Silwett L-77 group (11.1%) than the negative control group (2.2%). This effect was significant for both relative
GUS expression and the percentage of GUS-stained female flowers with 0.015% Silwett L-77. Therefore, Silwett L-77 at a final concentration of 0.015% was added to the agroinfiltration media
for all subsequent agroinfiltration experiments. Addition of Pluronic F-68 led to a 25% increase in the relative GUS expression and a 2.5-fold increase in the percentage of GUS-stained
female flowers (24.4%) at a concentration of 0.05%, while 0.5% did not display any positive effect (Fig. 2b), presumably due to tissue damage by the high concentration of this surfactant
(Fig. 2b). Ascorbic acid and PVP were tested because of their roles as antioxidants that scavenge the excess reactive oxygen species (ROS) produced by _Agrobacterium_ infection. Ascorbic
acid concentrations correlated with increased relative GUS expression (1.3-fold increase compared to the negative control) and a higher percentage of GUS-stained female flowers (18.9%)
compared with the negative control (10%), with a final concentration of 5 mM (Fig. 2c). No significant increases in the GUS level were observed in the plants treated with PVP (Fig. 2d).
VACUUM AND SONICATION TIME Vacuum infiltration for 5 minutes resulted in a higher relative GUS expression (5.5-fold) and percentage of GUS-stained flower buds (17.7%) than the non-vacuum
treatment (Fig. 3a). Increasing the vacuum time to 10 minutes and 15 minutes and three iterations of 5 minutes resulted in a higher relative GUS expression than that with 5 minutes of vacuum
treatment, but there was no significant effect on the percentage of GUS-stained flower buds (Fig. 3a). Exposure of the plant to sonication in the presence of _Agrobacterium_ produces many
microwounds across the tissue, which allows the _Agrobacterium_ to penetrate deeper and more completely throughout the tissue15. Sonication for 30 seconds before vacuum infiltration had a
significant effect on the nonsonicated male flowers for both relative GUS expression (1.2-fold) and the percentage of GUS-stained male flowers (22.2%; Fig. 3b). A combination of different
vacuum and sonication times with ascorbic acid was tested. Ten minutes of vacuum infiltration after 30 seconds of sonication together with the addition of 0.05% Pluronic F-68 and 5 mM of
ascorbic acid resulted in GUS staining of more than 50% of the male flowers and the highest relative GUS expression (3-fold) and _GUS_ transcript level (Fig. 3c,d). _AGROBACTERIUM_ STRAIN
AND HEMP CULTIVARS Three disarmed _Agrobacterium_ strains, EHA105, LBA4404 and GV3101 containing the pEarlyGate 101-_uidA_ binary vector, were used to perform the histochemical GUS assays.
Among the three _Agrobacterium_ strains, GV3101 demonstrated an approximately 1.7-fold increase in GUS expression compared to the other two strains (Fig. 4a); therefore, GV3101 was used to
evaluate the effect of hemp cultivars on the transient GUS expression. Since the structures of the male flowers are different between monoecious and dioecious cultivars, it is not possible
to compare the transient GUS expression in male flowers. Therefore, mature leaves were used to evaluate the effect of the hemp cultivars on the transient GUS expression. The dioecious CRS-1
cultivar had the highest relative GUS expression (Fig. 4b). Santhica 27, CFX-2 and Futura 75 exhibited approximately 80% of the activity compared to CRS-1. Fedora17, USO31 and Ferimon showed
approximately 60% relative GUS expression compared to CRS-1. Felina 32 showed the lowest GUS expression among all eight cultivars tested (Fig. 4b). TRANSIENT GENE EXPRESSION IN DIVERSE HEMP
TISSUES/ORGANS The optimized hemp agroinfiltration protocol was applied to a wide range of hemp tissues/organs. Successful GUS staining was observed in the mature leaf discs (Fig. 5b),
mature leaves (Fig. 5d), pollen sacs (Fig. 5f), anthers and sepals (Fig. 5g), pollen sac clusters (Fig. 5i), filaments (Fig. 5j), pollen grains (Fig. 5k), nonglandular trichomes (Fig. 5l),
female flowers (Fig. 5m) and pistils (Fig. 5n). The empty pEarleyGate 101 vector did not result in GUS staining in the mature leaf discs (Fig. 5a), mature leaves (Fig. 5c), pollen sacs (Fig.
5e) and pollen sac clusters (Fig. 5h). The pEarleyGate 101-_eGFP_ vector (Fig. 1b) was also transiently expressed via a GV3101 strain of _Agrobacterium_. GFP fluorescence was detected in
the hemp leaf discs (Fig. 6d), pollen sac clusters (Fig. 6f), anthers and sepals (Fig. 6h), filaments (Fig. 6j), capitate-stalked trichomes (Fig. 6l) and roots in the hemp seedlings (Fig.
6n). The hemp leaf discs filtrated by the empty pEarley vector did not show any GFP fluorescence (Fig. 6b). The bright field image of a leaf disc that was treated with the empty vector
pEarleyGate 101 is shown in Fig. 6a. The bright field images of the leaf discs, pollen sacs, anthers and sepals, filaments, capitate-stalked trichomes and roots that were treated with
pEarleyGate 101-_eGFP_ are shown in Fig. 6c,e,g,i,k,m, respectively. SILENCING OF THE PHYTOENE DESATURASE (PDS) GENE BY AN RNAI VECTOR We chose the CsPDS gene that encodes the phytoene
desaturase for _Agrobacterium_-mediated gene silencing because of its visible mutant phenotype caused by the lack of carotenoids20,21. The 200 bp fragment of the hemp PDS gene (accession no.
PK24508.1, nucleotide no. 601–800) was amplified from the hemp leaf cDNA library and used to hairpin-based RNAi vectors. The _PDS_ silencing vector was designed to include both sense and
antisense strands of the _CsPDS_ fragment to form the hairpin RNA. This silencing vector was transformed into the GV3101 strain and used for hemp agroinfiltration. Four days after
inoculation, an albino phenotype was observed in the mature leaves, male flowers and female flowers (Fig. 7a). qPCR analysis demonstrated that the CsPDS gene expression was sharply decreased
to less than 20% compared with that of the control tissues that were inoculated by GV3101 harboring the empty vector pK7GWIWG2(I) (Fig. 7b). DISCUSSION Vacuum agroinfiltration was attempted
in mature leaves, male flowers, and female flowers, but only the male flowers exhibited a low transient GUS expression. Then, we used male flowers to optimize the hemp agroinfiltration. A
concentration of 0.015% Silwett L-77 drastically increased the transient GUS expression and resulted in three times higher relative GUS expression than that without Silwett L-77,
demonstrating that Silwett L-77 is a critical factor for successful hemp vacuum agroinfiltration (Fig. 2a). Thus, this concentration was used for all subsequent agroinfiltration experiments.
Pluronic F-68 at 0.05% also increased the GUS activity by approximately 20% (Fig. 2b). The efficiency of both surfactants on the GUS expression in the hemp male flower (Fig. 2a,b) proved
that the addition of these surfactants effectively increased the agroinfiltration efficiency in hemp as well as various crops22,23. In plant cells, agroinfiltration triggers an oxidative
burst response and induces the accumulation of ROS that injures plant cells and leads to necrosis24. Excess levels of ROS were reported to reduce the ability for _Agrobacterium_ to colonize
plant cells and transfer T-DNA into the nuclei25. For scavenging of the excess ROS, two antioxidant compounds were tested: ascorbic acid and PVP26,27. A 5 mM concentration of ascorbic acid
had a significant and positive effect on transient GUS activity and increased the relative GUS expression by 50% (Fig. 2c), whereas PVP did not significantly improve the efficiency of
agroinfiltration (Fig. 2d). These results are consistent with a _N. benthamiana_ infiltration study previously reported by Norkunas _et al_. (2018)22. For vacuum agroinfiltration, plant
tissue was submerged into the suspension of _Agrobacterium_ harboring a binary vector. Application of a vacuum results in evacuation of the air in the interstitial space from the submerged
plant tissue through the stomata. The suspension of _Agrobacterium_ enters the plant tissue to replace the evacuated gases once the vacuum is broken and the internal pressure is increased.
Long periods of vacuum time lead to damaged plant tissues and short vacuum times result in insufficient air evacuation and low agroinfiltration efficiency14. In this study, both 10 minutes
and 15 minutes as well as 3 iterations of 5 minutes of the vacuum treatment resulted in approximately seven times higher relative GUS expression than that of the control (non-vacuum) male
flower. In statistical tests, these values were significantly higher than those after shorter periods of vacuum application for both relative GUS expression and the percentage of GUS-stained
flower buds (Fig. 3a). Sonication leads to the formation of microwounds within the plant tissue, allowing _Agrobacterium_ to effectively infect the plant cells28. Since this treatment
produces more entry points for _Agrobacterium_ to enter into the plant cell, sonication facilitates agroinfiltration in recalcitrant plants. We performed tests to determine the best
sonication time for hemp and found that 30 seconds of sonication before vacuum infiltration led to 20% higher relative GUS expression than that without sonication (Fig. 3b). Thus, Silwett
L-77, Pluronic F-68, ascorbic acid, vacuum and sonication all proved to be effective for hemp agroinfiltration. To further optimize the agroinfiltration efficiency, we tested a combination
of Silwett L-77, Pluronic F-68, ascorbic acid, vacuum and sonication. As a consequence, ten minutes of vacuum infiltration after 30 seconds of sonication together with the addition of 0.015%
Silwett L-77, 0.05% Pluronic F-68 and 5 mM of ascorbic acid resulted in 2.5-fold higher _uidA_ gene expression and staining of more than 50% of the male flowers than those of the Silwett
L-77 only group (Fig. 3c). This result indicates the synergistic effect of Silwett L-77, Pluronic F-68, ascorbic acid and acetosyringone. The genetic background of the _Agrobacterium_ can
influence the T-DNA transfer into plant cellular nuclei, and some _Agrobacterium_ strains are more virulent than others based on the target species29,30. In this study, different
transformation rates were observed for three avirulent strains. To the best of our knowledge, there are few reports about appropriate _Agrobacterium_ strains for hemp due to the limited
tissue culture and transformation studies. The GV3101 strain demonstrated significantly higher GUS expression (1.7-fold) than the other two strains (Fig. 4a), and it therefore should be the
optimal choice for _C. sativa_. Ti-plasmids are classified into several types based on the opines; EHA105, LBA4404 and GV3101 are grouped into the succinamopine, octopine and nopaline types,
respectively25. Since GV3101 showed high transient GUS expression, octopine-type strains might have better compatibility with _C. sativa_, and other _Agrobacterium_ strains belonging to
this type, such as C58C1, GV3100, GV3850, A136, and EHA 101, should be tested for hemp agroinfiltration. _C. sativa_ is a dioecious plant, but to overcome the unsynchronized maturity of male
and female plants and the problem with mechanized harvesting, genetically monoecious diploid (2n = 20) hemp cultivars have been developed31. The dioecious cultivar CRS-1 exhibited
significantly higher relative GUS expression than seven other varieties. Among the five monoecious cultivars, Santhica 27 presented the highest relative GUS expression, and therefore this
cultivar will be used to optimize the agroinfiltration protocol for future studies due to the agronomical advantages of monoecious cultivars. Interestingly, this optimized agroinfiltration
protocol was applied to various hemp tissue/organs, including hemp female flowers, and resulted in high GUS and GFP expression (Figs. 5 and 6). Recently, the PDS gene has been used to
examine transient gene silencing systems in both monocots and dicots32,33. A mutant of this gene, which is a single copy gene in most plants, induces an albino phenotype and is easily
detected through visual observations due to the reduction of chlorophyll content34. Transient expression of the _PDS_ silencing vector decreased the PDS mRNA level to 20% compared with that
of the mock treatment and led to an albino phenotype in the leaves, male flowers and female flowers (Fig. 7). These results revealed that the optimized hemp agroinfiltration system reported
in this study was effective for gene silencing via the transient expression of hairpin RNA. In conclusion, we report an efficient protocol for agroinfiltration of various hemp tissues,
including female flowers that contain glandular trichomes where hemp phytochemicals are produced and accumulated at high concentrations. This method is therefore a promising technique for
functional genomics of hemp genes, particularly for the synthesis of cannabinoids, terpenoids, flavonoids and alkaloids. Since there are several reports regarding the production of proteins
related to vaccines, pigmentation, photosynthesis and pharmaceutical components via agroinfiltration13,35,36, this technique can also play an important role in the metabolic engineering of
hemp, for example by overexpression of the _CBDAS_ gene and/or silencing of the THCAS gene. MATERIALS AND METHODS PLANT MATERIALS AND GROWTH CONDITIONS The study was performed and the hemp
plants were grown in accordance with the approved guidelines for Industrial Hemp provided by the Pennsylvania Department of Agriculture - Bureau of Plant Industry under regulated permits
IH-16-P-2017 and IH-17-P-2017. Six monoecious industrial hemp cultivars: Fedora 17, Felina 32, Ferimon, Futura 75, Santhica 27 and USO31 were provided by the Pennsylvania Department of
Agriculture, and two dioecious cultivars: CRS-1 and CFX-2 were purchased by Hemp Genetics International (Saskatoon, SK, Canada). They were grown in the greenhouse at 21 °C with a 14-hour
light photoperiod at 25–40 μEm−2s−1. For _in vitro_ growth, the hemp seeds were sterilized and transferred to germination media consisting of Murashige and Skoog (1962)37 (MS) with vitamins
supplemented with 2% sucrose and 0.8% agar. The seeds were placed under fluorescent light with a 16/8-hour cycle for one week to allow germination and growth prior to _Agrobacterium_
inoculation. EFFECT OF PLASMID, _AGROBACTERIUM_ STRAINS AND CULTURE CONDITIONS For overexpression, a pEarleyGate 101 vector (Invitrogen, Carlsbad, CA, USA) harboring the _uidA_ gene and
_eGFP_ gene under the control of a cauliflower mosaic virus (CaMV) _35 S_ promoter and _OCS_ terminator was used for GUS staining and GFP fluorescence assays (Fig. 1a,b). An empty
pEarleyGate 101 vector was used as a negative control (Fig. 1c). The pEarleyGate 101-_uidA_ was transformed into _A. tumefaciens_ GV3101, LBA4404 and EHA105 strains14,15 and the pEarleyGate
101-_eGFP_ vector was transformed into a GV3101 strain. For gene silencing, the hairpin RNA-expressing RNAi construct was prepared by inserting sense and antisense partial _CsPDS_ fragments
into the pK7GWIWG2 gateway vector (Invitrogen; Fig. 1d). A 200 bp _CsPDS_ partial sequence (_PDS_ 601–800) was amplified in the sense orientation from a cDNA library that was prepared using
total RNA from hemp leaves. For cloning of the gene fragments into the entry vector Gateway pDONR/Zeo (Thermo Fisher Scientific, Waltham, MA, USA), primers were synthesized to include the
recombination sequences _attB1_ and _attB2_. Targeted gene fragments in the entry clones were then transferred to the binary silencing vector pK7GWIWG2(I)38 to form the hairpin RNA, which is
under the control of a CaMV 35 S promotor and a CaMV _35_ S terminator (Fig. 1e). The construct was evaluated by sequencing and subsequently transformed into _A. tumefaciens_ GV3101 cells.
The full-length _CsPDS_ (PK24508) sequence information was obtained from the hemp genome gateway browser (http://genome.ccbr.utoronto.ca/cgi-bin/hgGateway). A single colony of recombinant
_Agrobacterium_ was inoculated and grown in LB buffer. The culture was then harvested and centrifuged at 4,000 g for 10 minutes and resuspended to an OD600 of 0.5 in agroinfiltration media
containing 10 mM MES, 1x MS, 2% glucose, and 200 µM acetosyringone at a pH 5.6. The chemical additives including Silwett L-77 (Lehle seeds, Round Rock, USA), Pluronic F-68 (Gibco, NY, USA),
L-Ascorbic acid (Sigma-Aldrich, St Louis, MO, USA) and polyvinylpyrrolidone (PVP) (Sigma-Aldrich) were added before vacuum infiltration. VACUUM INFILTRATION Hemp tissues/organs were excised
from the plants two months after seed germination, placed on a 100 ×15 mm Petri dish filled with _Agrobacterium_ solution and placed in a vacuum chamber. The vacuum pump was turned on to
decrease the pressure, and the agroinfiltration time was calculated after the vacuum reached 80 mbar. Successful infiltration required bubbles to be flowing up from the hemp
tissues/cultures. After 5–15 minutes at low pressure, the release valve was opened slowly to allow entrance of _Agrobacterium_ into the interstitial spaces of the plant tissues. The plant
tissues were then washed with distilled water, transferred onto a moist filter paper in a Petri dish and placed in a growth room at 21 °C. Sonication was carried out using a 22.5 kHz XL2000
Qsonica sonicator (Qsonica LLC, Newtown, CT, USA). ANALYSIS OF TRANSIENT GUS EXPRESSION Four days after agroinfiltration, the plants were analyzed by histochemical staining for GUS activity
in the hemp tissues/organs as described by Bakshi _et al_. (2011) with some modifications24. The infiltrated plant tissues/organs were washed for 30 minutes in ice-cold 100 mM phosphate
buffer (pH 7.0) and then vacuum infiltrated in 5-bromo-4-chloro-3-indolyl-beta-D-glucuronide (X-Glu) substrate solution [0.0005% X-Glu (w/v), 100 mM phosphate buffer pH 7.0, 1 mM potassium
ferrocyanide, 1 mM potassium ferricyanide, 0.05% Triton X-100 (w/v)]. The GUS-stained tissues/organs were then submerged in 95% ethanol overnight and washed with sterilized water for
decolorization. The GUS-stained hemp tissues/organs were placed in a Petri dish and observed using a Nikon SMZ1500 microscope (Nikon, Tokyo, Japan) at 2x magnification with Imaging Software
NIS Elements F Package (Nikon) and equipped with a DS-Ril1 camera (Nikon). The relative GUS expression was calculated with 30 pollen sacs per treatment to obtain the total expression value
(stained area x color intensity) with ImageJ (Rasband 1997–2011) as previously reported15. Photos of the strongest GUS-stained regions in the pollen sacs were taken using bright field
illumination. Prior to quantification, the color mode of the light microscope images was converted from RGB to HSB using ImageJ. A rectangular region of interest (ROI) was defined by using
the “Rectangular selection” drawing tool from the ImageJ toolbar. The intensity of the GUS staining was measured in the saturation channel. The mean gray values were measured and compared
among different treatments. All analyzed tissues were thoroughly cleared to correlate the color information with the degree of blue staining. In addition, the number of stained pollen sacs
was counted from 10 pollen sacs to calculate the percentage of GUS staining. This calculation included three repetitions. Statistical analysis was performed using a 1-way ANOVA with Tukey’s
multiple comparison test (a = 0.05). IMAGE COLLECTION AND ANALYSIS OF GFP FLUORESCENCE Four days after vacuum agroinfiltration, the hemp tissues/organs were subject to GFP fluorescence
observation using a Nikon SMZ1500 microscope with Imaging Software NIS Elements F Package (Nikon) equipped with a DS-Ril1 camera (Nikon), an Intensilight C-HGFI Precentered Fiber Illuminator
(Nikon) and a GFP2 filter set (Ex. 480 ± 40 nm; Em. 510 nm LP). QUANTITATIVE REAL-TIME PCR (QPCR) ANALYSIS OF THE AGROINFILTRATED HEMP PLANTS Real-time PCR was performed with 5 μL of SYBR
Select Master Mix (Applied Biosystems, Waltham, MA, USA) in a 10 μL total reaction mixture containing 400 nM of the gene-specific primers and 1 μL of cDNA. The following thermal program was
used: 95 °C for 10 m, 40 cycles at 95 °C for 10 seconds, and 60 °C for 1 minute carried out with a CFX-96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The gene
expression level was calculated from the cycle threshold according to the 2−∆∆Ct method39. Statistical analysis was performed using a paired t-test (a = 0.05). The PDS gene primers were
designed to amplify a region upstream of the siRNA sequence (_PDS_ 466–555). The _F-box_ gene was used as an internal reference for hemp, and three independent biological replications were
conducted. All primer sequences are listed in Supplemental Table S1. REFERENCES * Andre, C. M., Hausman, J. F. & Guerriero, G. _Cannabis sativa_: The plant of the thousand and one
molecules. _Frontiers in Plant Science._ 7, 1–19 (2016). Article Google Scholar * Chandra, S., Lata, H., Khan, I., & ElSohly, M. A. _Cannabis Sativa_ L.: _Botany and Horticulture in
Cannabis sativa L_.: _Botany and Biotechnology_. Cham. Switzerland: _Springer_. 79–100 (2017). * Izzo, A. A., Borrelli, F., Capasso, R., Di Marzo, V. & Mechoulam, R. Non-psychotropic
plant cannabinoids: new therapeutic opportunities from an ancient herb. _Trends Pharmacological Science._ 30, 515–527 (2009). Article CAS Google Scholar * Gonçalves, J. _et al_.
_Cannabis_ and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. _Medicines._ 6, 1 (2019). Article Google Scholar * Gagne, S.
J. _et al_. Identification of olivetolic acid cyclase from _Cannabis sativa_ reveals a unique catalytic route to plant polyketides. _Proceedings of the National Academy of Sciences USA_ 109,
12811–12816 (2012). Article ADS CAS Google Scholar * van Bakel, H. _et al_. The draft genome and transcriptome of _Cannabis sativa_. _Genome Biology._ 12, R102 (2011). Article Google
Scholar * Russo, E. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. _British Journal of Pharmacology._ 163, 7 (2011). Article Google Scholar *
Gertsch, J. _et al_. Beta-Caryophyllene Is a Dietary Cannabinoid. _Proceedings of the National Academy of Sciences._ 105, 26 (2008). Article Google Scholar * Booth, J. K., Page, J. E.
& Bohlmann, J. Terpene synthases from _Cannabis sativa_. _PLoS One_. 12, (2017). * Feeney, M. & Punja, Z. K. Tissue culture and _Agrobacterium_-mediated transformation of hemp
(_Cannabis sativa_ L.). _Vitro Cellular & Developmental Biology – Plant._ 39, 578–585 (2003). Article CAS Google Scholar * Slusarkiewicz-Jarzina, A., Ponitka, A. & Kaczmarek, Z.
Influence of cultivar, explant source and plant growth regulator on callus induction and plant regeneration of _Cannabis sativa_ L. _Acta Biologica Cracoviensia Series Botanica._ 47, 145–151
(2005). Google Scholar * Chen, Q. and Lai, H. Gene delivery into plant cells for recombinant protein production. _BioMed research international_. 2015, (2015). * Chen, Q. _et al_.
Agroinfiltration as an Effective and Scalable Strategy of Gene Delivery for Production of Pharmaceutical Proteins. _Advanced Techniques in Biology & Medicine._ 1, 103 (2013). Article
CAS Google Scholar * Shamloul, M., Trusa, J., Mett, V. & Yusibov, V. Optimization and utilization of _Agrobacterium_-mediated transient protein production in _Nicotiana_. _Journal of
Visualized Experiments._ 86, 51204 (2014). Google Scholar * King, J. L., Finer, J. J. & McHale, L. K. Development and optimization of agroinfiltration for soybean. _Plant Cell Reports._
34, 133–140 (2015). Article CAS Google Scholar * Wahby, I., Caba, J. & Ligero, F. _Agrobacterium_ infection of hemp (_Cannabis sativa_ L.): establishment of hairy root cultures.
_Journal of Plant Interactions._ 8, 312–320 (2013). Article CAS Google Scholar * Farag, S. & Kayser, O. Cannabinoids production by hairy root cultures of _Cannabis sativa_ L.
_American Journal of Plant Sciences._ 6, 1874–1884 (2015). Article CAS Google Scholar * Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. GUS fusions: _β_ -glucuronidase as a sensitive
and versatile gene fusion marker in higher plants. _The EMBO Journal._ 6, 3901–3907 (1987). Article CAS Google Scholar * Sheikh, A. H. _et al_. Agroinfiltration by cytokinin-producing
_Agrobacterium_ sp. strain GV3101 primes defense responses in _Nicotiana tabacum_. _Molecular Plant-Microbe Interactions._ 27, 1175–1185 (2014). Article Google Scholar * Andrieu _et al_.
An _in planta_, _Agrobacterium_-mediated transient gene expression method for inducing gene silencing in rice (_Oryza sativa_ L.) leaves. _Rice._ 5, 23 (2012). Article Google Scholar *
Miki, D. & Shimamoto, K. Simple RNAi vectors for stable and transient suppression of gene function in rice. _Plant Cell Physiology._ 45, 445–450 (2004). Article Google Scholar *
Norkunas, K., Harding, R., Dale, J. & Dugdale, B. Improving agroinfiltration-based transient gene expression in _Nicotiana benthamiana_. _Plant Methods._ 14, 71 (2018). Article Google
Scholar * Fister, A. S. _et al_. Protocol: Transient expression system for functional genomics in the tropical tree _Theobroma cacao_ L. _Plant Methods._ 12, 19 (2016). Article Google
Scholar * Bakshi, S., Sadhukhan, A., Mishra, S. & Sahoo, L. Improved _Agrobacterium_-mediated transformation of cowpea via sonication and vacuum infiltration. _Plant Cell Reports._ 30,
2281–2292 (2011). Article CAS Google Scholar * Dan, Y. _et al_. Lipoic acid—An unique plant transformation enhancer. In _Vitro Cellular & Developmental Biology – Plant_. 45, 630–638
(2009). Article CAS Google Scholar * Dutt, M., Vasconcellos, M. & Grosser, J. W. Effects of antioxidants on _Agrobacterium_-mediated transformation and accelerated production of
transgenic plants of Mexican lime (_Citrus aurantifolia_ Swingle). _Plant Cell, Tissue and Organ Culture._ 107, 79–89 (2011). Article CAS Google Scholar * Subramanyam, K., Subramanyam,
K., Sailaja, K. V., Srinivasulu, M. & Lakshmidevi, K. Highly efficient _Agrobacterium_-mediated transformation of banana cv. Rasthali (AAB) via sonication and vacuum infiltration. _Plant
Cell Reports._ 30, 425–436 (2011). Article CAS Google Scholar * Hwang, H.H., Yu, M., Lai, E.M. _Agrobacterium_-mediated plant transformation: biology and applications. _Arabidopsis_
Book. 15, (2017). Article Google Scholar * Kim, M. J., Baek, K. & Park, C. M. Optimization of conditions for transient _Agrobacterium_-mediated gene expression assays in _Arabidopsis_.
_Plant Cell Reports._ 28, 1159–1167 (2009). Article CAS Google Scholar * Han, Z. F., Hunter, D. M., Sibbald, S., Zhang, J. S. & Tian, L. Biological activity of the tzs gene of
nopaline _Agrobacterium tumefaciens_ GV3101 in plant regeneration and genetic transformation. _Molecular Plant Microbe Interactions._ 26, 1359–1365 (2013). Article CAS Google Scholar *
Razumova, O. V., Alexandrov, O. S., Divashuk, M. G., Sukhorada, T. I. & Karlov, G. I. Molecular cytogenetic analysis of monoecious hemp (_Cannabis sativa_ L.) cultivars reveals its
karyotype variations and sex chromosomes constitution. _Protoplasma._ 253, 895–901 (2016). Article Google Scholar * Fernandez, A. I. _et al_. Flexible tools for gene expression and
silencing in tomato. _Plant Physiology._ 151, 1729–1740 (2009). Article CAS Google Scholar * Jia, H. & Wang, N. Targeted genome editing of sweet orange using Cas9/sgRNA. _PLoS One_.
9, (2014). * Leonelli, L., Erickson, E., Lyska, D. & Niyogi, K. K. Transient expression in _Nicotiana benthamiana_ for rapid functional analysis of genes involved in non-photochemical
quenching and carotenoid biosynthesis. _The Plant Journal._ 88, 375–386 (2016). Article CAS Google Scholar * Leuzinger, K. _et al_. Efficient agroinfiltration of plants for high-level
transient expression of recombinant proteins. _Jove of Visualized Experiments_. 77, (2013). * Zeinipour, M. _et al_. Agroinfiltration: a rapid and reliable method to select suitable rose
cultivars for blue flower production. _Physiology and Molecular Biology of Plants._ 24, 503–511 (2018). Article CAS Google Scholar * Murashige & Skoog A revised medium for rapid
growth and bio-assays with tobacco tissue cultures. _Physiol. Plant._ 15, 473–497 (1962). Article CAS Google Scholar * Karimi, M., Inze, D. & Depicker, A. GATEWAY vectors for
_Agrobacterium_-mediated plant transformation. _Trends in Plant Science._ 7, 193–195 (2002). Article CAS Google Scholar * Livak, K. J. & Schmittgen, T. D. Analysis of relative gene
expression data using real-time quantitative PCR and the 2−∆∆C(T) Method. _Methods._ 25(4), 402–408 (2001). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors
would like to acknowledge support from the PA Options for Wellness and Penn State Harrisburg School of Science, Engineering, and Technology. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Penn State Harrisburg, 777 West Harrisburg Pike, Middletown, Pennsylvania, USA Michihito Deguchi, Daniel Bogush, Hannah Weeden, Zachary Spuhler, Shobha Potlakayala & Sairam Rudrabhatla *
AGROSAVIA, Centro de Investigación Palmira, Calle 23, Carrera 37, Continuo al Penal Palmira, Valle, Colombia Takumasa Kondo * Plant Biotechnology Innovation Laboratory, Division of Plant
Sciences, University of Missouri, Columbia, Missouri, USA Zhanyuan J. Zhang Authors * Michihito Deguchi View author publications You can also search for this author inPubMed Google Scholar *
Daniel Bogush View author publications You can also search for this author inPubMed Google Scholar * Hannah Weeden View author publications You can also search for this author inPubMed
Google Scholar * Zachary Spuhler View author publications You can also search for this author inPubMed Google Scholar * Shobha Potlakayala View author publications You can also search for
this author inPubMed Google Scholar * Takumasa Kondo View author publications You can also search for this author inPubMed Google Scholar * Zhanyuan J. Zhang View author publications You can
also search for this author inPubMed Google Scholar * Sairam Rudrabhatla View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.D. and S.R.
contributed to the experimental design. M.D., D.B. and S.Z. performed the experiments. M.D. collected and analyzed the data, prepared the original figures and wrote the original draft of the
paper. M.D., D.B., H.W. and S.R. were responsible for the plant cultivation. T.K., S.P., Z.J.Z. and S.R. contributed to the data interpretation. All authors reviewed the original manuscript
and read and approved the final one. S.R. was responsible for project administration, funding acquisition, resources, supervision and validation of this study. CORRESPONDING AUTHOR
Correspondence to Sairam Rudrabhatla. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s
Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Deguchi, M., Bogush, D., Weeden, H. _et al._
Establishment and optimization of a hemp (_Cannabis sativa_ L.) agroinfiltration system for gene expression and silencing studies. _Sci Rep_ 10, 3504 (2020).
https://doi.org/10.1038/s41598-020-60323-9 Download citation * Received: 31 July 2019 * Accepted: 29 January 2020 * Published: 26 February 2020 * DOI:
https://doi.org/10.1038/s41598-020-60323-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








