- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Download PDF Article Open access Published: 21 February 2020 Equilibrium Thermodynamic Properties of Aqueous Solutions of Ionic Liquid 1-Ethyl-3-Methylimidazolium Methanesulfonate
[EMIM][MeSO3] Chaolun Zheng1, Jian Zhou1, Yong Pei1 & …Bao Yang ORCID: orcid.org/0000-0003-1971-21741 Show authors Scientific Reports volume 10, Article number: 3174 (2020) Cite this
article
3198 Accesses
7 Citations
1 Altmetric
Metrics details
Subjects Materials scienceMechanical engineering AbstractThe ionic liquid 1-ethyl-3-methylimidazolium methanesulfonate ([EMIM][MeSO3]) has been considered as a promising alternative desiccant to triethylene glycol and lithium bromide commonly used
in the industry. In this paper, the water activity coefficient of this binary system was measured from 303 K to 363 K with water concentration from 18% to 92%. The interaction energies
between the ionic liquid molecules (\({g}_{22}\)) and between the ionic liquid and water molecules (\({g}_{12}\)) for the [EMIM][MeSO3]/water binary system were determined from the water
activity coefficient data using the Non-Random Two-Liquid (NRTL) model. The magnitude of the interaction energy between the [EMIM][MeSO3] and water molecules (\({g}_{12}\)) was found to be
in the range of 45~49 kJ/mol, which was about 20% larger than that between the water molecules (\({g}_{11}\)) in the [EMIM][MeSO3]/water system. The large (\({g}_{12}\)) can explain many
observed macroscopic thermodynamic properties such as strong hygroscopicity in the ionic liquid [EMIM][MeSO3]. These interaction energies were used to determine the heat of desorption of the
[EMIM][MeSO3]/water system, and the obtained heat of desorption was in good agreement with that calculated from the conventional Clausius-Clapeyron Equation.
Similar content beingviewed by others Investigating the phase diagram-ionic conductivity isotherm relationship in aqueous solutions of common acids: hydrochloric, nitric, sulfuric and phosphoric acid Article
Open access 03 April 2024 A new method based on binary mixture concept for prediction of ionic liquids critical properties using molecular dynamics simulation Article Open access 04 March
2025 Thermodynamic studies of solute–solute and solute–solvent interactions in ternary aqueous systems containing {betaine + PEGDME250} and {betaine + K3PO4 or K2HPO4} at 298.15 K Article
Open access 18 October 2023 Introduction
Ionic liquids are compounds composed of organic cations and inorganic anions, and they show a negligible vapor pressure and good fluidity over a wide temperature range1,2. Some ionic liquids
have been found to be highly hygroscopic, which makes them promising desiccants for applications in gas dehydration and absorption cooling. In contrast to triethylene glycol currently used
in gas dehydration, losses by evaporation can be eliminated when the ionic liquids are used as desiccants. In addition, the use of ionic liquids can avoid the crystallization and corrosion
problems3,4, which are the two major concerns of the most commonly used halide salt liquid desiccants5,6.
The ionic liquid 1-ethyl-3-methylimidazolium methanesulfonate ([EMIM][MeSO3]) is among those that are highly hygroscopic, and is a promising candidate for next generation of desiccants7,8.
The determination of thermodynamic properties is important for performance evaluation for this special ionic liquid. Many researchers have conducted experiments to measure the important
macroscopic thermodynamic properties, such as specific heat9,10, density7,9,10,11,12, viscosity7,9,11,13, electrical conductivity9, surface tension11,14, reflective index11, diffusion
coefficient7, nuclear magnetic resonance (NMR) spectroscopy9,14,15, excess molar heats of mixing9,12,16, and water activity coefficient7,16.
Although the macroscopic thermodynamic properties of the [EMIM][MeSO3]/water binary system have been extensively investigated, the molecular thermodynamic properties of this binary system
are not comprehensive. For example, the interaction energy or bonding energy between the ionic liquid molecules, and between the ionic liquid and water molecules are extremely scarce for the
ionic liquid [EMIM][MeSO3]/water systems. These molecular interaction energy properties are related to many macroscopic thermodynamic properties such as heat of desorption, heat capacity,
hygroscopicity, and water vapor pressure.
In this work, the Non-Random Two-Liquid (NRTL) model was used to determine the interaction energies between different molecule pairs inside the [EMIM][MeSO3]/water binary system from the
measured water activity coefficients data. These interaction energies were used to determine the heat of desorption of the [EMIM][MeSO3]/water system, which was in good agreement with those
calculated from the Clausius-Clapeyron Equation. A formula to predict heat of desorption from the interaction energies was also developed for the binary systems.
TheoreticalBackgroundWater activity coefficient
The activity coefficient of water \({\gamma }_{{{\rm{H}}}_{2}{\rm{o}}}\) is a fundamental thermodynamic parameter that accounts for deviations from ideal behavior in non-ideal solutions,
such as the aqueous ionic liquid solutions, which is defined as7,8:
$${\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}=\frac{{p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{non}} \mbox{-}{\rm{ideal}}}}{{p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{ideal}}}}=\frac{{p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{non}} \mbox{-} {\rm{ideal}}}}{{x}_{{{\rm{H}}}_{2}{\rm{O}}}\cdot
{p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{sat}}}}$$ (1)
where \({p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{ideal}}}\) and \({p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{non}} \mbox{-} {\rm{ideal}}}\) are the partial pressure of water above the ideal and non-ideal
aqueous solutions, respectively, \(\,{p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{sat}}}\) is the saturation pressure of pure water, and \({x}_{{{\rm{H}}}_{2}{\rm{O}}}\) is the molar fraction of water
in the aqueous solutions. In Eq. (1), the ratio \({p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{non}} \mbox{-} {\rm{ideal}}}/{p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{sat}}}\) is the relative humidity (RH) of the
non-ideal aqueous solutions. In the ideal aqueous solutions, the partial pressure of water can be described by Raoult’s
law7:
$${p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{ideal}}}={x}_{{{\rm{H}}}_{2}{\rm{O}}}{p}_{{{\rm{H}}}_{2}{\rm{O}},{\rm{sat}}}$$ (2)However, the [EMIM][MeSO3]/water solution is a non-ideal solution, in which the interaction energy between [EMIM][MeSO3] and water is significantly stronger than those between water and
water. A small value of water activity coefficient \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\) indicates a large intermolecular force between water and ionic liquid molecules and strong
hygroscopicity for water absorption. The water activity coefficient at infinite dilution \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) is often used for the comparison of the hygroscopicity
or absorption strength of different desiccants7.
Non-random two-liquid (NRTL) modelThe NRTL model17,18,19,20 correlates the activity coefficients \({\gamma }_{i}\) of a compound i with its mole fractions \({x}_{i}\) in the liquid solutions:
$$\mathrm{ln}\,{\gamma}_{1}={x}_{2}^{2}(\frac{\Delta {g}_{12-11}}{RT}\frac{\exp (\,-\,2{\alpha }_{12}\Delta {g}_{12-11}/RT)}{{[{x}_{1}+{x}_{2}\exp (-{\alpha }_{12}\Delta {g}_{12-11}/RT)]}^{2}}+\frac{\Delta
{g}_{12-22}}{RT}\frac{\exp (\,-\,{\alpha }_{12}\Delta {g}_{12-22}/RT)}{{[{x}_{2}+{x}_{1}\exp (-{\alpha }_{12}\Delta {g}_{12-22}/RT)]}^{2}})$$ (3) $$\mathrm{ln}\,{\gamma
}_{2}={x}_{1}^{2}(\frac{\Delta {g}_{12-22}}{RT}\frac{\exp (\,-\,2{\alpha }_{12}\Delta {g}_{12-22}/RT)}{{[{x}_{2}+{x}_{1}\exp (-{\alpha }_{12}\Delta {g}_{12-22}/RT)]}^{2}}+\frac{\Delta
{g}_{12-11}}{RT}\frac{\exp (\,-\,{\alpha }_{12}\Delta {g}_{12-11}/RT)}{{[{x}_{1}+{x}_{2}\exp (-{\alpha }_{12}\Delta {g}_{12-11}/RT)]}^{2}})$$ (4)
where the subscripts 1 and 2 refer to component 1 and component 2 in the binary solution, respectively, \(\Delta {g}_{12-11}\) and \(\Delta {g}_{12-22}\,\)are the exchange in the interaction
energy between molecules, \({\alpha }_{12}\) is the non-randomness parameter, R is the molar gas constant, and T is the absolute temperature. In the case of infinite dilution, the NRTL
equations reduce to
$$\mathrm{ln}\,{\gamma }_{1,\infty }=\frac{\Delta {g}_{12-11}}{RT}+\frac{\Delta {g}_{12-22}}{RT}\exp (\,-\,{\alpha }_{12}\Delta {g}_{12-22}/RT)$$ (5)$$\mathrm{ln}\,{\gamma }_{2,\infty }=\frac{\Delta {g}_{12-22}}{RT}+\frac{\Delta {g}_{12-11}}{RT}\exp (\,-\,{\alpha }_{12}\Delta {g}_{12-11}/RT)$$ (6)
The water activity coefficient at infinite dilution \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) can be used to compare the hygroscopicity or absorption strength of different desiccants.
In the NRTL model, the exchange in the interaction energy \(\Delta {g}_{12-11}={g}_{12}-{g}_{11}\), which is the interaction energy change as a result of breaking a 1-1 interaction
\({g}_{11}\) and forming a 1–2 interaction \({g}_{12}\). In this study, components 1 and 2 refer to the water and the [EMIM][MeSO3], respectively. So \({g}_{11}\), \(\,{g}_{22}\), and
\({g}_{12}\) are the interaction energies between the water molecules, between the ionic liquid [EMIM][MeSO3] molecules, and between the water and [EMIM][MeSO3] molecules. The interaction
energy parameters \(\Delta {g}_{12-11}\) and \(\Delta {g}_{12-22}\) can be determined by data fitting using Eqs. (3) and (4) if the water activity coefficients \({\gamma
}_{{{\rm{H}}}_{2}{\rm{O}}}\) with its mole fraction \({x}_{{{\rm{H}}}_{2}{\rm{O}}}\) can be measured in the [EMIM][MeSO3]/water binary system. The interaction energy between the ionic liquid
and water molecules \({g}_{12}\) can be determined using the formula: \({g}_{12}=\Delta {g}_{12-11}+{g}_{11}\) if the interaction energy between water molecules \({g}_{11}\) is known.
Similarly, the interaction energy between the ionic liquid molecules \({g}_{22}\) can be determined by \({g}_{22}=\Delta {g}_{12-22}+{g}_{12}\). These molecular interaction energy properties
are related to many macroscopic thermodynamic properties such as heat of desorption, heat capacity, hygroscopicity, and water vapor pressure. One application of these molecular interaction
energies is that they can be used to predict the heat of desorption of the aqueous ionic liquid solutions with water concentration from 0% to 100%. In comparison, the Clausius-Clapeyron
Equation determines the heat of desorption at the water concentration where the vapor pressure and temperature are known.
Clausius-Clapeyron equationThe Clausius-Clapeyron Equation relates the vapor-liquid equilibrium (VLE) data (p and T) to the thermodynamic property, enthalpy of vaporization (\(\Delta {H}_{v}\)), which is given
by21
$$\mathrm{ln}(p)=-\,\frac{\Delta {H}_{v}}{RT}+{\rm{C}},$$ (7)where \(p\) is the vapor pressure at the temperature T, \(\Delta {H}_{v}\) is enthalpy of vaporization, R is the molar gas constant, and \({\rm{C}}\) is a constant. In Eq. (7), \(\Delta
{H}_{v}\) is assumed to be independent of T. However, the temperature dependence of \(\Delta {H}_{v}\) cannot be overlooked in the water and [EMIM][MeSO3] binary solutions due to the complex
interaction between water and [EMIM][MeSO3]21,22. In a moderate temperature range, \(\Delta {H}_{v}\) can be assumed to change linearly with T,
$$\Delta {H}_{v}=\Delta {H}_{v,0}+aT,$$(8)
where \(a\) is the temperature coefficient. So the modified Clausius–Clapeyron Equation for the ionic liquid solutions can be written as
follows:
$${\rm{l}}{\rm{n}}(\frac{p}{{p}_{0}})=-\,\frac{\Delta {H}_{v,0}}{R}(\frac{1}{T}-\frac{1}{{T}_{0}})+\frac{a}{R}\,{\rm{l}}{\rm{n}}(\frac{T}{{T}_{0}}).$$ (9)Therefore \(\Delta {H}_{v}\) can be determined from Eqs. (8) and (9) when the VLE data are measured.
Uncertainty calculationThe experimental uncertainty in this experiment is estimated using the root-sum-square method suggested by Moffat23:
$${\rm{\delta }}R={\{\mathop{\sum}\limits_{{\rm{i}}=1}^{{\rm{N}}}{(\frac{\partial R}{\partial {x}_{i}}{\rm{\delta }}{x}_{{\rm{i}}})}^{2}\}}^{1/2}$$ (10)
In particular, whenever the equation describing the result is a pure “product form”, as shown in Eq. (11):
$$R={X}_{1}^{a}{X}_{2}^{b}{X}_{3}^{c}\,\cdots \,{X}_{M}^{m}$$ (11)the relative uncertainty can be calculated by Eq. (12):
$$\frac{\delta R}{R}={\{{(a\frac{\delta {X}_{1}}{{X}_{1}})}^{2}+{(b\frac{\delta {X}_{2}}{{X}_{2}})}^{2}+\cdots +{(m\frac{\delta{X}_{M}}{{X}_{M}})}^{2}\}}^{1/2}$$ (12) Experimental MethodsMaterials
The 1-ethyl-3-methylimidazolium methanesulfonate ([EMIM][MeSO3]) was purchased from Sigma-Aldrich (purity higher than 95 wt. %). Deionized water was used in the experiment.
Experimentsetup
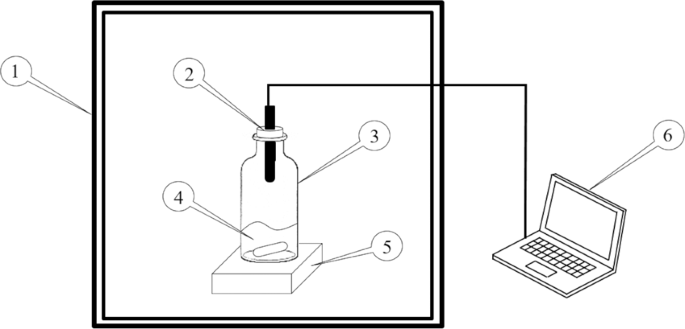
The experiment setup for the vapor-liquid equilibrium (VLE) measurement is shown in Fig. 1. The temperature during experiments was controlled using a temperature-controlled oven (Yamato,
DKN-402C). The [EMIM][MeSO3]/water solution was placed inside a reactant bottle, in which a humidity sensor (Rotronic HC2A-SM) was attached. The RH and temperature (T) of the gas phase
inside the reactant bottle were measured simultaneously using this humidity sensor with an uncertainty of T = ±0.1 K and RH = ±0.8% of the RH reading. The water concentration in
[EMIM][MeSO3]/water solutions was measured using the Karl Fisher Titrator (Mettler Toledo™ C20D) with a relative uncertainty less than 0.5%. A magnetic stirrer (Thermo Scientific Cimarec
Micro Stirrers) was used to stir the [EMIM][MeSO3]/water solution inside the reactant bottle during the VLE measurement. The data logging and the conversion from RH and T to water vapor
pressure were performed using the HW4-E software in a computer.
Figure 1Schematic of the experimental setup for the VLE Measurements. Legend: 1. temperature-controlled oven; 2. humidity sensor; 3. reagent bottle; 4. [EMIM][MeSO3]/water solution; 5. magnetic
stirrer; 6. DAQ/computer.
Full size imageVLE measurementsTwelve [EMIM][MeSO3]/water solutions with molar concentration of water from 18% to 92% were prepared in the VLE measurements. For each solution, its RH was measured at temperatures 303 K,
323 K, 343 K, and 363 K. The sample solution with an approximate volume of 100 ml was placed in the reactant bottle. The oven temperature was set to the desired temperature, and the sample
solution was heated and stirred vigorously with magnetic stirrers to get homogeneous mixing. At the same time, the data logging was started. After the system reached the equilibrium and the
RH stayed unchanged for 30 minutes, the equilibrium temperature and RH were recorded. A series of equilibrium temperature and water vapor pressure (or RH) data were obtained for the
[EMIM][MeSO3]/water solutions.
Results and DiscussionActivity coefficient of waterThe experimental VLE data for the [EMIM][MeSO3]/water binary solutions with water molar concentrations from 18% to 92% were measured and listed in Table 1. The vapor pressure
\({p}_{{{\rm{H}}}_{2}{\rm{O}}}\) were determined using the measured RH. The relative uncertainty of the measured mole fraction x(H2O) was found to be less than 5.02% using Eq. (12). The RH
and T were measured simultaneously using the humidity sensor with an uncertainty of T = ±0.1 K and RH = ±0.8% of the RH reading. Figure 2 shows the measured water vapor pressure in
[EMIM][MeSO3]/water binary solutions versus the molar fraction of water in a temperature range of 303 K to 363 K.
Table 1 Experimental VLE data of the[EMIM][MeSO3]/water binary solutionsat different temperatures.Full size tableFigure 2
Water vapor pressure in the [EMIM][MeSO3]/water binary solutions versus the molar fraction of water in the temperature range of 303 K to 363 K.
Full size imageThe effect of the ionic liquid on the non-ideality of the aqueous solutions can be expressed by the activity coefficients of water \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\), which was
calculated by Eq. (1). Table 2 shows the calculated activity coefficients of water \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\) in the [EMIM][MeSO3]/water binary solutions. The relative
uncertainty of the water activity coefficient \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\) was found to be 5.09%. The plot of the activity coefficient \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\) versus
the molar concentration of water is shown in Fig. 3. As shown in this figure, the water activity coefficient \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\) approaches a value of one when the water
concentration is close to 100%, as expected by Raoult’s law. For water concentrations below about 30 mol.%, \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\) approaches almost constant values between
0.10 and 0.15. A small value of \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\,\)indicates a large deviation from the ideal solution behavior or from Raoult’s law. A small value of \({\gamma
}_{{{\rm{H}}}_{2}{\rm{O}}}\,\)is desired for the application in gas dehydration. The influence of the temperature on \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\) is small in the temperature range
of test, especially for low water concentrations. For a given water concentration, \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\) increases slightly when the temperature increases, which is
consistent with the temperature dependence of the interaction energy \(\Delta {g}_{12-11}\) in the [EMIM][MeSO3]/water binary solutions.
Table 2 Water Activity Coefficient\({{\boldsymbol{\gamma }}}_{{{\rm{H}}}_{2}{\rm{O}}}\) of the [EMIM][MeSO3]/water binary solutions at different temperatures.Full size tableFigure 3
Activity coefficient of water in the [EMIM][MeSO3]/water binary solutions versus the molar fraction of water in the temperature range of 303 K to 363 K.
Full size imageThe water activity coefficients at infinite dilution \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) for the [EMIM][MeSO3]/water binary solutions are listed in Table 3. It is found that the
limiting water activity coefficients \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) is in the range of 0.102 to 0.151 at temperatures from 303 K to 363 K, more than 4 times lower than that
for triethylene glycol, which indicates that the ionic liquid [EMIM][MeSO3] possesses much stronger ability of absorbing water vapor than the commonly used triethylene glycol
desiccants.
Table 3 Water activity coefficient at infinite dilution \({{\boldsymbol{\gamma }}}_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) for the [EMIM][MeSO3]/water binary system at differenttemperatures.Full size table
There exist some discrepancies in \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) in literature. For example, Krannich et al.7 reported the \({{\rm{\gamma }}}_{{{\rm{H}}}_{2}{\rm{O}},\infty
}\) around 0.20 that was determined using the boiling point method. Domanska et al.16 reported the \({{\rm{\gamma }}}_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) in the range 0.071~0.088, which were
determined with the gas-liquid chromatography method. In this study, the limiting water activity coefficients \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) was found to be in the range of
0.102 to 0.151 at temperatures from 303 K to 363 K. The differences in the reported \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}},\infty }\) data might be due to the different measurement
techniques.
Interaction energy between molecules and non-randomness parametersThe exchange in interaction energy \(\Delta {g}_{12-11}\), and \(\Delta {g}_{12-22}\) and the non-randomness parameter \({\alpha }_{12}\) in the [EMIM][MeSO3]/water binary solutions can be
extracted by fitting the experimental data \({\gamma }_{{{\rm{H}}}_{2}{\rm{O}}}\,\)to the NRTL equations (Eqs. (3–4)). These parameters are listed in Table 4. In Table 4, the subscript “1”
represents water, while the “2” represents the ionic liquid [EMIM][MeSO3]. \(\Delta {g}_{12-11}\) is the interaction energy change as a result of breaking an H2O-H2O interaction and forming
a [EMIM][MeSO3]-H2O interaction. As shown in Table 4, the interaction energy parameters \(\Delta g\) follow the order [EMIM][MeSO3]-[EMIM][MeSO3] > [EMIM][MeSO3]-H2O ≫ H2O-H2O. The large
negative value of \(\Delta {g}_{12-11}\) indicates that the intermolecular attractive force between [EMIM][MeSO3] and H2O is much stronger than that between H2O and H2O. The absolute value
of interaction energy \(\Delta {g}_{12-11}\) and \(\Delta {g}_{12-22}\) decreases with increasing temperature, which could be attributed to the increasing thermal motion of the
molecules18.
Table 4 Interaction Energy and Non-Randomness parameters for the [EMIM][MeSO3]/water binary system at different temperatures.Full size tableFor comparison, the exchange in interaction energy \(\Delta {g}_{12-11}\) and \(\Delta {g}_{12-22}\) in some other ionic liquids/water binary solutions were summarized in Table 5
19,20,24,25. It should be noted that the \(\Delta {g}_{12-11}\) in the [EMIM][MeSO3]/water binary solution was found to be larger than that in 1-ethyl-3-methylimidazolium ethylsulfate
([EMIM][EtSO4])/water solution25, which is another promising ionic liquid for moisture removal and shares similar chemical structure26,27. The difference in interaction energy may result
from the shorter alkyl group in [EMIM][MeSO3] anion, which is favorable for the bonding with water molecule28,29.
Table 5 Interaction Energy parameters for other ionic liquid/water binarysystems.Full size table
The interaction energy between water molecules \({g}_{11}\) is the molar vaporization energy of water (i.e., cohesive energy) but has a negative sign on it30, which is available in
liteature31. The interaction energy between the ionic liquid and water molecules \({g}_{12}\) can be calculated using the formula: \({g}_{12}=\Delta {g}_{12-11}+{g}_{11}\). Similarly, the
interaction energy between the ionic liquid molecules \({g}_{22}\) can be determined by \({g}_{22}=\Delta {g}_{12-22}+{g}_{12}\). The obtained interaction energies \({g}_{11},\,{g}_{12}\)
and \({g}_{22}\) are summarized in Table 4. These molecular interaction energies have negative signs due to the intermolecular attractive forces. It is found in Table 4 that the molecular
interaction energies become less negative when the temperature increases. The magnitude of the interaction energy between the [EMIM][MeSO3] and water molecules was found to be in the range
of 45~49 kJ/mol, which was 20% larger than that between the water molecules in the [EMIM][MeSO3]/water system. The large interaction energy between the ionic liquid [EMIM][MeSO3] and water
molecules can explain many reported macroscopic thermodynamic properties, such as small water activity coefficient and strong hygroscopicity.
The parameters \({\alpha }_{12}\) is related to the non-randomness in the liquid mixture; when \({\alpha }_{12}\) is zero, the local distribution around the center molecule is completely
random. The non-randomness parameters \({\alpha }_{12}\) in the [EMIM][MeSO3]/water binary solutions were found to be around 0.5, as shown in Table 4. The values of α12 are generally
consistent with those reported for other water/hygroscopic ionic liquid binary solutions17,20,24. The non-zero α12 in the [EMIM][MeSO3]/water binary solutions are mainly due to the
difference in interaction energy and size between water and [EMIM][MeSO3].
The extent of the correlation between the experimental data and the NRTL model was evaluated by calculating the absolute relative deviation (ARD)24:
$${\rm{ARD}}=\frac{\sum|\frac{{{\rm{\gamma }}}_{\exp }-{{\rm{\gamma }}}_{{\rm{NRTL}}}}{{{\rm{\gamma }}}_{\exp }}|}{{\rm{n}}}$$ (13)
where n is the number of data points, \({\gamma }_{\exp }\) is the γ value calculated from experimental data, and \({\gamma }_{{\rm{NRTL}}}\) is the γ value calculated from the NRTL model.
The values of ARD are also listed in Table 4, which implies a satisfactory correlation in the test temperature range.
Heat of desorptionOne application of these molecular interaction energies is that they can be used to determine the heat of desorption of the aqueous ionic liquid solutions. The internal energy in the aqueous
ionic liquid solution Ul is the sum of the excess internal energy of the solution UE and the molar internal energy of the pure component
Ui:
$${U}_{l}={U}^{E}+{n}_{1}{U}_{1}+{n}_{2}{U}_{2}$$ (14)where n1 and n2 are the mole number of component 1 (i.e., water) and component 2 (i.e., ionic liquid), respectively. In the evaporation process, the intermolecular interaction energy is
dominant, and therefore the intramolecular interaction energy can be neglected in the internal energy. The excess internal energy of the aqueous ionic liquid solution UE can be evaluated
as18,32:
$${U}^{E}={(g}^{(1)}-{g}_{11})\,{n}_{1}+{(g}^{(2)}-{g}_{22})\,{n}_{2}$$ (15)where \({g}_{11}\) and \({g}_{22}\) are the molar interaction energy between water molecules and between the ionic liquid and water molecules, respectively, and \({g}^{(1)}\) and
\({g}^{(2)}\) represent the molar residual Gibbs energy for molecule cells having component 1 and component 2 at the center respectively33. \({g}^{(1)}\) and \({g}^{(2)}\) can be calculated
as:
$${g}^{(1)}={x}_{11}{g}_{11}+{x}_{21}{g}_{12}={g}_{11}+{x}_{21}\Delta {g}_{12-11}$$ (16) $${g}^{(2)}={x}_{12}{g}_{12}+{x}_{22}{g}_{22}={g}_{22}+{x}_{12}\Delta {g}_{12-22}$$ (17)where x11, x22, x21 and x12 represent the local mole fractions. For example, x21 is the local mole fraction of component 2 around the center component 1. x21 and x12 depend on the global
concentration according to the NRTL model,
$${x}_{21}=\frac{{x}_{2}\ast exp(\,-\,{\alpha }_{12}\Delta {g}_{12-11}/RT)}{{x}_{1}+{x}_{2}\ast exp(\,-\,{\alpha }_{12}\Delta{g}_{12-11}/RT)}=\frac{{n}_{2}\ast exp(\,-\,{\alpha }_{12}\Delta {g}_{12-11}/RT)}{{n}_{1}+{n}_{2}\ast exp(\,-\,{\alpha }_{12}\Delta {g}_{12-11}/RT)}$$ (18) $${x}_{12}=\frac{{x}_{1}\ast \exp
(\,-\,{\alpha }_{12}\Delta {g}_{12-22}/RT)}{{x}_{2}+{x}_{1}\ast \exp (\,-\,{\alpha }_{12}\Delta {g}_{12-22}/RT)}=\frac{{n}_{1}\ast \exp (\,-\,{\alpha }_{12}\Delta
{g}_{12-22}/RT)}{{n}_{2}+{n}_{1}\ast \exp (\,-\,{\alpha }_{12}\Delta {g}_{12-22}/RT)}$$ (19)
Differentiating Eq. (14) leads to the change of internal energy in the liquid solution for the evaporation of one mole of water:
$$\Delta {U}_{l}=-{g}_{11}-\frac{\Delta {g}_{12-11}\ast{{x}_{2}}^{2}\ast exp(-2{\alpha }_{12}\Delta {g}_{12-11}/RT)}{{({x}_{1}+{x}_{2}\ast exp(-{\alpha }_{12}\Delta {g}_{12-11}/RT))}^{2}}-\frac{\Delta {g}_{12-22}\ast {{x}_{2}}^{2}\ast \exp
(-{\alpha }_{12}\Delta {g}_{12-22}/RT)}{{({x}_{2}+{x}_{1}\ast \exp (-{\alpha }_{12}\Delta {g}_{12-22}/RT))}^{2}}$$ (20)
Considering the volume work in the evaporation process, which is equal to RT based on the ideal gas assumption, the heat of desorption can be calculated using the interaction
energies:
$$\begin{array}{ccc}\Delta {H}_{v} & = & -{g}_{11}+RT-\frac{\Delta {g}_{12-11}\ast {{x}_{2}}^{2}\ast exp(-\,2{\alpha }_{12}\Delta {g}_{12-11}/RT)}{{({x}_{1}+{x}_{2}\astexp(-{\alpha }_{12}\Delta {g}_{12-11}/RT))}^{2}}\\ & & -\,\frac{\Delta {g}_{12-22}\ast {{x}_{2}}^{2}\ast \exp (-\,{\alpha }_{12}\Delta {g}_{12-22}/RT)}{{({x}_{2}+{x}_{1}\ast \exp (-{\alpha
}_{12}\Delta {g}_{12-22}/RT))}^{2}}\end{array}$$ (21)
The formula can be used to predict the heat of desorption in the aqueous binary solutions when the interaction energies are given.
Figure 4 shows the heat of desorption calculated from the interaction energies in the [EMIM][MeSO3]/water binary solutions with water fraction from 0% to 100% at temperatures 303 K, 323 K,
343 K, and 363 K. The desorption heat calculated by the Clausius-Clapeyron Equation is also shown for comparison. As shown in Fig. 4, they are in good agreement. The desorption heat \(\Delta
{H}_{v}\) decreases with increasing temperature for a given water concentration. This trend is consistent with the temperature dependence of the interaction energy parameters \(\Delta
{g}_{12-11}\) and \(\Delta {g}_{12-22}\) listed in Table 4, which could be attributed to the increasing thermal motion of the molecules at elevated temperatures. Due to the strong bonding
forces between water and [EMIM][MeSO3], the desorption heat or enthalpy of vaporization of water in the [EMIM][MeSO3]/water solutions is always higher than the enthalpy of vaporization of
pure water at the same temperature, but the difference becomes smaller when the water concentration approaches 100%.
Figure 4Desorption heat calculated from the interaction energy in the temperature range of 303 K to 363 K and water fraction range of 0% to 100%. (The desorption heat calculated by the
Clausius-Clapeyron Equation is also shown for comparison).
Full size imageConclusionIn this work, molecular thermodynamic properties such as interaction energies and non-randomness parameter of the [EMIM][MeSO3]/water binary system were determined from the water activity
coefficient data using the Non-Random Two-Liquid (NRTL) model. The water activity coefficient of this binary system was measured with molar concentrations of water from 18% to 92% at
temperatures 303 K, 323 K, 343 K, and 363 K. The interaction energy between the ionic liquid [EMIM][MeSO3] and water molecules (\({g}_{12}\)) was found to be ~20% larger than that between
the water molecules (\({g}_{11}\)). The exchange in interaction energy \(\Delta g\) followed the order [EMIM][MeSO3]-[EMIM][MeSO3] > [EMIM][MeSO3]-H2O» H2O-H2O. The large negative value of
\(\Delta {g}_{12-11}\) (−7427.51 J/mol to −7283.79 J/mol) indicated that the intermolecular attractive force between [EMIM][MeSO3] and H2O was much stronger than that between H2O and H2O.
This can explain the observed strong hygroscopicity in the ionic liquid [EMIM][MeSO3]. With the molecular interaction energies, the heat of desorption was predicted in the
[EMIM][MeSO3]/water binary system. The obtained heat of desorption was in good agreement with that calculated from the conventional Clausius-Clapeyron Equation.
Data availabilityAll data generated or analyzed during this study are included in this published article.
References Saihara, K., Yoshimura, Y., Ohta, S. & Shimizu, A. Properties of water confined in ionic liquids. Scientific reports 5, 10619 (2015).
Article ADS Google Scholar
Kwon, H.-N., Jang, S.-J., Kang, Y. C. & Roh, K. C. The effect of ILs as co-salts in electrolytes for high voltage supercapacitors. Scientific reports 9, 1180 (2019).
Article ADS Google Scholar
Sun, J., Fu, L. & Zhang, S. A review of working fluids of absorption cycles. Renewable and Sustainable Energy Reviews 16, 1899–1906 (2012).
Article CAS Google Scholar
Wasserscheid, P. & Seiler, M. Leveraging gigawatt potentials by smart heat‐pump technologies using ionic liquids. ChemSusChem 4, 459–463 (2011).
Article CAS Google Scholar
Collier, R., Barlow, R. & Arnold, F. An overview of open-cycle desiccant-cooling systems and materials. Journal of Solar Energy Engineering 104, 28–34 (1982).
Article CAS Google Scholar
Herold, K. E., Radermacher, R. & Klein, S. A. Absorption chillers and heat pumps. (CRC press, 2016).
Krannich, M., Heym, F. & Jess, A. Characterization of six hygroscopic ionic liquids with regard to their suitability for gas dehydration: density, viscosity, thermal and oxidative stability,
vapor pressure, diffusion coefficient, and activity coefficient of water. Journal of Chemical & Engineering Data 61, 1162–1176 (2016).
Article CAS Google Scholar
Krannich, M., Heym, F. & Jess, A. Continuous gas dehydration using the hygroscopic ionic liquid [EMIM][MeSO3] as a promising alternative absorbent. Chemical Engineering & Technology 39,
343–353 (2016).
Article CAS Google Scholar
Stark, A., Zidell, A. W. & Hoffmann, M. M. Is the ionic liquid 1-ethyl-3-methylimidazolium methanesulfonate [emim][MeSO3] capable of rigidly binding water? Journal of Molecular Liquids 160,
166–179 (2011).
Article CAS Google Scholar
Ficke, L. E., Novak, R. R. & Brennecke, J. F. Thermodynamic and thermophysical properties of ionic liquid+ water systems. Journal of Chemical & Engineering Data 55, 4946–4950 (2010).
Article CAS Google Scholar
Hasse, B. et al. Viscosity, interfacial tension, density, and refractive index of ionic liquids [EMIM][MeSO3],[EMIM][MeOHPO2],[EMIM][OcSO4], and [BBIM][NTf2] in dependence on temperature at
atmospheric pressure. Journal of Chemical & Engineering Data 54, 2576–2583 (2009).
Article CAS Google Scholar
Ficke, L. E. & Brennecke, J. F. Interactions of ionic liquids and water. The Journal of Physical Chemistry B 114, 10496–10501 (2010).
Article CAS Google Scholar
Safarov, J., Huseynova, G., Bashirov, M., Hassel, E. & Abdulagatov, I. Viscosity of 1-ethyl-3-methylimidazolium methanesulfonate over a wide range of temperature and Vogel–Tamman–Fulcher
model. Physics and Chemistry of Liquids 56, 703–717 (2018).
Article CAS Google Scholar
Russo, J. W. & Hoffmann, M. M. Measurements of surface tension and chemical shift on several binary mixtures of water and ionic liquids and their comparison for assessing aggregation.
Journal of Chemical & Engineering Data 56, 3703–3710 (2011).
Article CAS Google Scholar
Amado-Gonzalez, E., Esteso, M. A. & Gomez-Jaramillo, W. Mean activity coefficients for NaCl in the mixtures containing ionic liquids [Emim][MeSO3]+ H2O and [Emim][EtSO4]+ H2O at 298.15 K.
Journal of Chemical & Engineering Data 62, 752–761 (2017).
Article CAS Google Scholar
Domańska, U. & Królikowski, M. Measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-ethyl-3-methylimidazolium methanesulfonate. The
Journal of Chemical Thermodynamics 54, 20–27 (2012).
Article Google Scholar
Calvar, N., González, B., Gómez, E. & Domínguez, Á. Vapor–liquid equilibria for the ternary system ethanol+ water+ 1-ethyl-3-methylimidazolium ethylsulfate and the corresponding binary
systems containing the ionic liquid at 101.3 kPa. Journal of Chemical & Engineering Data 53, 820–825 (2008).
Article CAS Google Scholar
Schmidt, K., Maham, Y. & Mather, A. Use of the NRTL equation for simultaneous correlation of vapour-liquid equilibria and excess enthalpy: applications to aqueous alkanolamine systems.
Journal of thermal analysis and calorimetry 89, 61–72 (2007).
Article CAS Google Scholar
Döker, M. & Gmehling, J. Measurement and prediction of vapor–liquid equilibria of ternary systems containing ionic liquids. Fluid phase equilibria 227, 255–266 (2005).
Article Google Scholar
Wang, J.-F., Li, C.-X., Wang, Z.-H., Li, Z.-J. & Jiang, Y.-B. Vapor pressure measurement for water, methanol, ethanol, and their binary mixtures in the presence of an ionic liquid
1-ethyl-3-methylimidazolium dimethylphosphate. Fluid Phase Equilibria 255, 186–192 (2007).
Article CAS Google Scholar
Koutsoyiannis, D. Clausius–Clapeyron equation and saturation vapour pressure: simple theory reconciled with practice. European Journal of physics 33, 295 (2012).
Article ADS Google Scholar
Henderson-Sellers, B. A new formula for latent heat of vaporization of water as a function of temperature. Quarterly Journal of the Royal Meteorological Society 110, 1186–1190 (1984).
Article ADS Google Scholar
Moffat, R. J. Describing the uncertainties in experimental results. Experimental thermal and fluid science 1, 3–17 (1988).
Article ADS Google Scholar
Zhao, J., Jiang, X.-C., Li, C.-X. & Wang, Z.-H. Vapor pressure measurement for binary and ternary systems containing a phosphoric ionic liquid. Fluid phase equilibria 247, 190–198 (2006).
Article CAS Google Scholar
Zuo, G., Zhao, Z., Yan, S. & Zhang, X. Thermodynamic properties of a new working pair: 1-Ethyl-3-methylimidazolium ethylsulfate and water. Chemical engineering journal 156, 613–617 (2010).
Article CAS Google Scholar
Irfan, M., Rolker, J. & Schneider, R. Process for dehumidifying moist gas mixtures. U.S. Patent Application 15/619,561 (2017).
Zehnacker, O. & Willy, B. Process and absorbent for dehumidifying moist gas mixtures. U.S. Patent 10,105,644 (2018).
Kohno, Y. & Ohno, H. Temperature-responsive ionic liquid/water interfaces: relation between hydrophilicity of ions and dynamic phase change. Physical Chemistry Chemical Physics 14, 5063–5070
(2012).
Article CAS Google Scholar
Kurnia, K. A., Pinho, S. P. & Coutinho, J. A. Designing ionic liquids for absorptive cooling. Green Chemistry 16, 3741–3745 (2014).
Article CAS Google Scholar
Tassios, D. A single-parameter equation for isothermal vapor‐liquid equilibrium correlations. AIChE Journal 17, 1367–1371 (1971).
Article CAS Google Scholar
Cabane, B. & Vuilleumier, R. The physics of liquid water. Comptes Rendus Geoscience 337, 159–171 (2005).
Article CAS ADS Google Scholar
Sandler, S. I. An introduction to applied statistical thermodynamics. (John Wiley & Sons, 2010).
Renon, H. & Prausnitz, J. M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE journal 14, 135–144 (1968).
Article CAS Google Scholar
Download references
AcknowledgementsThis research is financially supported by DOE under Grant DEEE0008672.
Author informationAuthors and Affiliations University of Maryland, Department of Mechanical Engineering, College Park, MD, 20742, USA
Chaolun Zheng, Jian Zhou, Yong Pei & Bao Yang
AuthorsChaolun ZhengView author publications You can also search for this author inPubMed Google Scholar
Jian ZhouView author publications You can also search for this author inPubMed Google Scholar
Yong PeiView author publications You can also search for this author inPubMed Google Scholar
Bao YangView author publications You can also search for this author inPubMed Google Scholar
ContributionsB.Y. and C.Z. conceived the experiment and developed the theoretical model. C.Z. set up the experimental system. C.Z. and J.Z. performed the experiments. C.Z. and Y.P. conducted the data
analysis. C.Z. and B.Y. wrote the main manuscript. All authors reviewed the manuscript.
Corresponding author Correspondence to Bao Yang.
Ethics declarations Competing interestsThe authors declare no competing interests.
Additional informationPublisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissionsOpen Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this articleCite this article Zheng, C., Zhou, J., Pei, Y. et al. Equilibrium Thermodynamic Properties of Aqueous Solutions of Ionic Liquid 1-Ethyl-3-Methylimidazolium Methanesulfonate
[EMIM][MeSO3]. Sci Rep 10, 3174 (2020). https://doi.org/10.1038/s41598-020-59702-z
Download citation
Received: 21 June 2019
Accepted: 02 December 2019
Published: 21 February 2020
DOI: https://doi.org/10.1038/s41598-020-59702-z
Share this article Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article.
Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative


:max_bytes(150000):strip_icc():focal(999x0:1001x2)/cycling-2000-1588038a08114b83ae2e541e7692eccd.jpg)


:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)
:max_bytes(150000):strip_icc():focal(647x0:649x2)/gettyimages-1166899212-2000-88270930f0a04da3aa29f041db856c3d.jpg)