- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT _Phomopsis_ sp. XP-8, an endophytic fungus from the bark of Tu-Chung (_Eucommia ulmoides_ Oliv) showed capability to biosynthesize pinoresinol (Pin) and pinoresinol diglucoside
(PDG) from glucose (glu) and phenylalanine (Phe). To verify the mass flow in the biosynthesis pathway, [13C6]-labeled glu and [13C6]-labeled Phe were separately fed to the strain as sole
substrates and [13C6]-labeled products were detected by ultra-high-performance liquid chromatography-quadrupole time of flight mass spectrometry. As results, [13C6]-labeled Phe was
incorporated into [13C6]-cinnamylic acid (Ca) and _p_-coumaric acid (_p_-Co), and [13C12]-labeled Pin, which revealed that the Pin benzene ring came from Phe via the phenylpropane pathway.
[13C6]-Labeled Ca and _p_-Co, [13C12]-labeled Pin, [13C18]-labeled pinoresinol monoglucoside (PMG), and [13C18]-labeled PDG products were found when [13C6]-labeled glu was used,
demonstrating that the benzene ring and glucoside of PDG originated from glu. It was also determined that PMG was not the direct precursor of PDG in the biosynthetic pathway. The study
identified the occurrence of phenylalanine- lignan biosynthesis pathway in fungi at the level of mass flow. SIMILAR CONTENT BEING VIEWED BY OTHERS DEREPLICATION OF SECONDARY METABOLITES FROM
_SOPHORA FLAVESCENS_ USING AN LC–MS/MS-BASED MOLECULAR NETWORKING STRATEGY Article Open access 24 March 2025 DISCOVERY OF NOVEL NEUTRAL GLYCOSPHINGOLIPIDS IN CEREAL CROPS: RAPID PROFILING
USING REVERSED-PHASED HPLC–ESI–QQTOF WITH PARALLEL REACTION MONITORING Article Open access 19 December 2023 STABLE ISOTOPE AND CHEMICAL INHIBITION ANALYSES SUGGESTED THE EXISTENCE OF A
NON-MEVALONATE-LIKE PATHWAY IN THE YEAST _YARROWIA LIPOLYTICA_ Article Open access 10 March 2021 INTRODUCTION Pinoresinol diglucoside (PDG), (+)-1-pinoresinol 4, 4′-di-O-β-D-glucopyranoside,
is a major antihypertensive compound found in Tu-Chung, a traditional herb medicine with excellent efficacy for lowering blood pressure1. PDG also possesses the potential to prevent
osteoporosis2. Additionally, in the human intestine, PDG can be converted to enterolignans by intestinal microflora3, and enterolignans have potential to reduce the risk of breast cancer4
and other estrogen-dependent cancers5. PDG is found primarily in plants as lignans1,6 but yields are very low. _Phomopsis_ sp. XP-8 is an endophytic fungus isolated from the bark of Tu-Chung
that was previously found to produce PDG _in vitro_7, thus, providing an alternative resource to obtain PDG. This is the first report on the capability of a microorganism to synthesize
lignan. However, the PDG production by _Phomopsis_ sp. XP-8 was very low, which might be enhanced by regulatory controls based on the biosynthetic pathways. Therefore, it is essential to
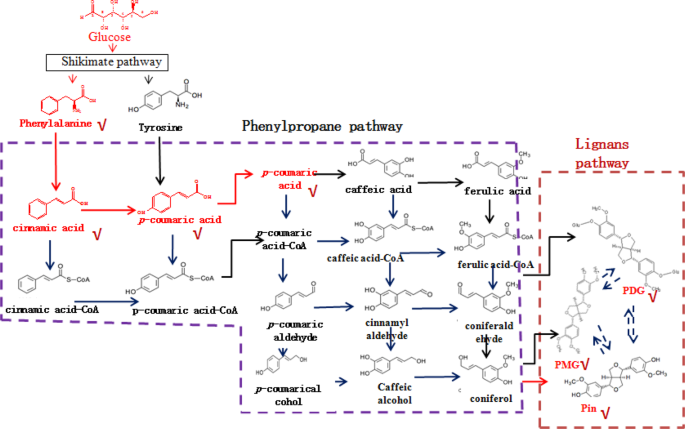
identify the PDG biosynthetic pathway in this strain. The lignan biosynthetic pathway has only been reported in plants until now8,9. Synthesis of Pin in plants occurs via oxidative coupling
of monolignols, which are synthesized through the phenylpropanoid pathway with Phe, Ca, _p_-Co, _p_-coumaroyl-CoA, caffeate, ferulate, feruloy-CoA, coniferylaldehyde, and coniferyl alcohol
as intermediates or precursors10,11 (Fig. 1). PMG and PDG are converted from Pin by UDP-glucose-dependent glucosyltransferase8. However, the biosynthesis of PDG from Pin has not been
detected in plants and the Pin, PMG, and PDG biosynthetic pathways have not been elucidated in microorganisms. We previously reported that _Phomopsis_ sp. XP-8 converts mung bean starch and
polysaccharides to Pin, PMG, and PDG. Phe, cinnamic acid, and _p_-coumaric acid have been detected as products of the bioconversion12,13. Precursor feeding and enzymatic activity
measurements indicate that this strain synthesizes PDG via many steps, such as during mass flow of the phenylpropanoid pathway14. Genomic annotation indicates that the phenylpropane pathway
exists in this strain15 and some other microorganisms16. However, the functions of the denoted genes have not been verified until now. Therefore, it is necessary to verify the entire PDG
biosynthetic pathway in _Phomopsis_ sp. XP-8. Using stable or radioactive isotope-labeled compounds is an efficient and reliable strategy to verify the mass flow of unknown biosynthetic
pathways by tracing the isotope-labeled compounds from substrates to products17. 13C-labeled substrates have been used to shed light on the biodegradation pathways of organic pollutants18.
Isotope labeling combined with high-resolution mass spectrometry have also been used to track the abiotic transformation of pollutants in aqueous mixtures19. In recent years, liquid
chromatography-mass spectrometry (LC–MS) and ultra-high-performance liquid chromatography (UPLC) systems have been developed to facilitate the analysis of many substances at the same time
with high sensitivity and selectivity20. Stable isotope-labeled compounds have also been employed in several areas of biomedical research21. The combination of stable isotope-labeling
techniques with MS has allowed rapid acquisition and interpretation of data and has been used in many fields, including distribution, metabolism, food, and excretion studies22,23,24. The
biochemical pathway of the aromatic compounds in tea has been also been revealed using the stable isotope labeling method25. In this study, we applied stable isotopes labeling and MS to
trace the PDG biosynthetic pathway. Stable isotope-labeled 13C6 glu and 13C6 Phe were used as the substrates and ultra-high-performance liquid chromatography-quadrupole time of flight mass
spectrometry (UPLC-Q-TOF-MS/MS) was used to identify the products. MATERIALS AND METHODS MICROORGANISM AND CHEMICALS _Phomopsis_ sp. XP-8 previously isolated from the bark of Tu-Chung and
stored at the China Center for Type Culture Collection (Wuhan, China) (code: _Phomopsis_ sp. CCTCC M 209291) was used in this study. Phe (purity ≥98%, Sigma, St. Louis, MO, USA), Ca and
_p_-Co (purity ≥98%; Aladdin, Shanghai, China), PDG, PMG, and Pin (purity ≥99%; National Institutes for Food and Drug Control, Beijing, China) were used as the standards (dissolved in
methanol) for the structural analysis and product identification. [13C6]-Labeled Phe and glu were purchased from the Qingdao TrachinoidCo (≥99%; Qingdao, China). The purity of the
[13C6]-labeled Phe and glu was 99%. Methanol (HPLC grade) was purchased from Fisher Scientific (Fairlawn, NJ, USA). The water used in the experiment was purified using a Milli-Q water
purification system (18.5 M) (Millipore Corp., Bedford, MA, USA). Other reagents and chemicals were of analytical grade. PREPARATION OF _PHOMOPSIS_ SP. XP-8 CELLS _Phomopsis_ sp. XP-8 was
grown at 28 °C on potato dextrose agar plates for 5 days. Then, three pieces of mycelia (5 mm in diameter) were inoculated into 100 mL liquid potato dextrose broth in a 250-mL flask and
cultivated at 28 °C on a rotary shaker (180 rpm). After 4 days, the cells were collected by centrifugation at 4 °C (1,136 × g for10 min) using a centrifuge (HC-3018R, Anhui USTC Zonkia
Scientific Instruments Co., Ltd., Anhui, China). The cells were washed twice with sterile water and used for bioconversion according to the experimental design. BIOCONVERSION SYSTEMS The
bioconversion with unlabeled glu as the sole substrate was carried out in a 250-mL flask containing 100 mL of ultrapure water (pH 7), 5 g/L glu, and the prepared _Phomopsis_ sp. XP-8 cell
set a ratio of 10 g cells (wet weight) per 100 mL medium. To track the mass flow from glu to PDG, glu was changed to 5 g/L [13C6]-labeled glu in the above medium and the same conditions were
used for bioconversion. Bioconversion with Phe as the sole substrate was carried out in medium without glu, 7 mM [13C6]-labeled phenylalanine, and the prepared _Phomopsis_ sp. XP-8 cells at
a ratio of 10 g wet cells per 100 mL medium. All bioconversions were carried out for 48 h at 28 °C and 180 rpm. At the end of bioconversion, the broth was collected and filtered through an
intermediate speed qualitative filter paper before the products were detected. IDENTIFICATION OF THE ACCUMULATED PRODUCTS DURING BIOCONVERSION The products were extracted from the
vacuum-evaporated (0.09 MPa, 50 °C) bioconversion broth with methanol and adjusted to 4 mL for the UPLC measurements after filtration through a membrane (0.45 µm, 13 mm diameter; Millipore,
Billerica, MA, USA). The UPLC analysis was performed on a Waters Acquity UPLC system (Waters Corp., Milford, MA, USA), equipped with a binary pump, a thermostatically controlled column
compartment, and a UV detector. Gradient elution was performed on an Acquity UPLCTM BEH C18 column (50 mm × 2.1 mm I.D., 1.7 m; Waters) and the column temperature was maintained at 30 °C,
while sample temperature was 10 °C13. The MS analysis of the products was carried out on a Q-TOF PremierTM with an ESI source (Waters Corp.) at the optimized parameters of: capillary
voltage, 2.8 kV; sampling cone voltage, 20 V; extractor voltage, 4 V; source temperature, 100 °C; desolvation temperature, 250 °C, and flow rate of the desolvation gas (N2), 400 L/h. The
collision cell parameters for the Q-TOF-MS/MS analysis were: collision gas (Argon) flow rate, 0.45 L/h; collision energy, 15–35 eV. The mass spectra were recorded using full scan mode over a
mass range of _m/z_ 100–800 in negative ion mode. The MS acquisition rate was set to 1.0 s, with a 0.02 s interscan delay. The Q-TOF-MS/MS experiments were carried out by setting the
quadrupole to allow ions of interest to pass prior to fragmentation in the collision cell. Accurate mass measurements were obtained by means of a lock mass that introduces a low flow rate (3
L/min) of a chrysophanol (253.0499) calibrating solution in the ESI-Q-TOF-MS and ESI-Q-TOF-MS/MS. All operations and acquisition and data analyses were controlled by Masslynx V4.1 software
(Waters Corp.). DATA PROCESSING Peak detection, alignment, and identification of the detected compounds were performed using Masslynx V4.1 software (Waters Corp.). The MS/MS fragmentation
patterns were used for informative non-targeted metabolic profiling of the LC-MS data, and the acquired LC-MS/MS spectrum was identified after comparison with spectra proposed by the Mass
bank database (www.massbank.jp), the KEGG database, and related reports. RESULTS DETECTION OF PRODUCTS CONVERTED FROM UNLABELED GLU Production of PDG, PMG, Pin, Phe, _p_-Co, and Ca were
detected in bioconversion systems using glu as the sole substrate. Data in Figs. 2–5 show the mass spectra of these compounds accumulated in the bioconversion systems and the corresponding
standards. Production of Phe was detected as _m/z_ = 164.08, and _m/z_ = 147.06 (Fig. 2A-4), which was consistent with the data obtained from the corresponding standards (Fig. 2A-2).
Similarly, production of PDG, PMG, Pin, _p_-Co, and Ca was also detected in the bioconversion system, indicating that glu was converted to these products by _Phomopsis_ sp. XP-8, as only glu
was provided in the bioconversion system. IDENTIFICATION OF PRODUCTS CONVERTED FROM [13C6]-LABELED PHE The phenylpropanoid pathway in plants starts with Phe and ends with _p-_Co. The same
mass flow was previously detected during PDG biofrom glu by _Phomopsis_ sp. XP-813. To verify this finding and the role of the Phe pathway in the biosynthesis of PDG, PMG, and Pin,
[13C6]-labeled Phe was used as the sole substrate in the bioconversion system without glu (mainly used as the glucoside donor). As results, 13C labeled Pin, Phe, _p_-Co, and Ca were
successfully detected (Fig. 6). The products were successfully detected at the same RT of their corresponding unlabeled standard substrates. All 13C-labeled product data and their
corresponding standard substrates are summarized in Table S1 (Supporting information). As shown in Fig. 6B, 13C-labeled Ca was detected as _m/z_ = 153.07, indicating that six 13C from
[13C6]-labeled Phe were incorporated into Ca. A daughter ion of 13C-labeled Ca was obtained at _m/z_ = 109.08, indicating six 13C referring to the standard Ca (_m/z_ = 103.06). The structure
of 13C-labeled Ca without -COO− was observed at _m/z_ = 109.08. Therefore, it was deduced that the six 13C were incorporated into the benzene ring of Ca not into –COO−. _P_-Co produced in
the conversion system was detected as _m/z_ = 169.05 and revealed six 13C by consulting the _p_-Co standard (Fig. 6C). A daughter ion of 13C-labeled _p_-Co was obtained at _m/z_ = 125.07,
indicating 6 Da mass shift than _p_-Co standard (_m/z_ = 119.06). The structure of 13C-labeled _p_-Co without –COO− (44 Da lost) was observed at _m/z_ = 125.07. Therefore, it was deduced
that the six 13C might be distributed in the benzene ring. 13C-labeled Pin was detected (Fig. 6D-1) and compared with the mass spectra of the Pin standard (C20H22O6, RT = 9.736 min, detected
as _m/z_ = 357.13 and _m/z_ = 151.04 respectively) (Table S2, Supporting information). 13C-labeled Pin was detected as _m/z_ = 369.05, indicating 12 Da mass shift than Pin standard (_m/z_ =
357.13). A daughter ion of 13C-labeled Pin was observed at _m/z_ = 157.06, which showed a mass increase of 6 Da than Pin standard (_m/z_ = 151.04). The structure of 13C-labeled Pin with
loss of a benzene ring was identified as the major daughter ion of _m/z_ = 157.06 (Fig. 6D-1). This result confirmed that the six13C were distributed in a benzene ring, whereas the other
six13C might be in a symmetrical benzene ring. Therefore, we deduced that the Pin with 12 13C was bio-converted from the [13C6]-labeled Phe, Ca, or/and _p_-Co. This finding also confirmed
that the benzene ring in Pin came from Phe, which is consistent with that of the lignan biosynthetic pathway in plants. IDENTIFICATION OF PRODUCTS CONVERTED FROM [13C6]-LABELED GLUCOSE To
explore where Phe originated from the Pin biosynthetic pathway, [13C6]-labeled glu was supplied as the sole substrate in the bioconversion system with _Phomopsis_ sp. XP-8 cells. As results,
13C labeled PDG, PMG, Pin, Phe, _p_-Co, and Ca were detected (Figs. 7 and 8). The isotopic patterns observed in the MS and MS/MS spectra suggest the 13C from 13C6-labeled glu were
incorporated into the products of Phe (Fig. 7A), Ca (Fig. 7B) _p_-Co (Fig. 7C), PDG (Fig. 7D), PMG (Fig. 8A), Pin (Fig. 8B), respectively. The observed mass shifts, indicating the number of
incorporated 13C, were shown in the spectra. The detailed information on the products and possible positions of 13C in the products are summarized in Table S2 (Supporting information).
Interestingly, the analysis revealed that 13C6-labeled glu were incorporated into the core structure of PDG and PMG, and their glycosides. Additionally, the maximum of 16 13C was detected in
the formed Pin (C20H22O6), indicating the [13C6]-labeled glu partly contributed to the formation of Pin. The possible positions of 13C in the structures are summarized in Table S2
(Supporting information). Taken together, the mass flow from [13C6]- Phe to [13C6]-Ca, [13C6]-_p_-Co, and [13C12]-Pin was verified by the experiments using [13C6]-labeled Phe as the sole
substrate (Fig. 9A). The mass flow from [13C6]- glu to [13C]-Phe, [13C]-Ca, [13C]-_p_-Co, [13C]-Pin, [13C]-PMG, and[13C]-PDG was verified by the data obtained using [13C6]- glu as the sole
substrate (Fig. 9B). POSSIBLE PATHWAYS FOR BIOSYNTHESIS OF PDG AND PMG The evidences for the possible biosynthetic pathways of PDG, PMG, and Pin are summarized in Figs. 9 and 10. The pathway
from Phe to Pin, glu to Phe, Pin, PMG and PDG was verified (Fig. 9A,B, Supporting information Table S1 and Table S1). In addition, the bioconversion between PDG and PMG in _Phomopsis_ sp.
XP-8 was reported for the first time, and the analysis was showed in Fig. 10. As shown in Fig. 10, two structures of PMG were detected: one was [13C12]-PMG with two benzene rings converted
from 13C-labeled glu and an unlabeled glycoside (PMG _m/z_ 531.29), and the other was [13C18]-PMG with both benzene ring structures converted and a glucoside from 13C-labeled glu (PMG _m/z_
537.33). Similarly, two PDG structures were detected: one was [13C18]-PDG with a two benzene ring structure and one glycoside converted from 13C-labeled glu (PDG _m/z_ 699.27); the other one
was [13C24]- PDG with two benzene rings and two glycosides from 13C-labeled glu (PDG _m/z_ 705.26). If PMG was the direct precursor of PDG, PMG _m/z_ 531.29 would be converted to PDG _m/z_
699.27 by bonding one [13C6]-labeled glycoside through glycosylation; PMG _m/z_ 537.33 could also be converted to PDG _m/z_ 699.27 by bonding one unlabeled glycoside through glycosylation
and to PDG _m/z_ 705.26 by bonding one [13C6]-labeled glycoside. If this is true, PDG _m/z_ 699.27 would have two glucoside sources, whereas PDG _m/z_ 705.26 would have only one glucoside
source. Therefore, the concentration of PDG _m/z_ 705.26 should be lower than PDG _m/z_ 699.27. However, the data show that the relative abundance of PDG _m/z_ 705.26 was much higher than
that of PDG _m/z_ 699.27 (Fig. 7D-3). Therefore, PMG was not the precursor of PDG. In contrast, if PDG was the direct precursor of PMG, PDG _m/z_ 699.27 would be converted to PMG _m/z_
531.29 by hydrolyzation of one [13C6]-labeled glycoside and to PMG _m/z_ 537.33 by hydrolyzation of one unlabeled glycoside; PDG _m/z_ 705.26 would be converted to PMG _m/z_ 537.33 by
hydrolyzation of one [13C6]-labeled glycoside. If this is true, PMG _m/z_ 537.33 would have two glycoside sources, whereas PMG _m/z_ 531.29 would have only one source. The concentration of
PMG _m/z_ 531.29 should be lower than PMG _m/z_ 537.33. However, the data show that relative abundance of _m/z_ = 531.29 was higher than that of _m/z_ = 537.33 (Fig. 8A-3). Therefore, PDG
was not the precursor of PMG. DISCUSSION The 13C stable isotope labeling method was successfully used in this study to verify the phenylpropanoid-pinoresinol and biosynthetic pathway of its
glycosides in _Phomopsis_ sp. XP-8 during mass flow. This lignan biosynthetic pathway was only reported in plants until now8,9, so it was very significant to verify the occurrence of this
pathway in microorganisms. Stable Isotope-assisted metabolomics is an efficient way to trace and identify bio-transformed products and the metabolic pathways involved in their formation,
such as understanding the fate of organic pollutants in environmental samples17. It was the first time to use this method to verify the Phenylpropanoid-pinoresinol in a microorganism. In our
previous studies, many methods such as precursor feeding13, detection of enzyme activity14, and genomic annotation15 have been used to analyze the Phenylpropanoid-pinoresinol biosynthetic
pathway in _Phomopsis_ sp. XP-8. Through these studies, the precursors, enzymes activity and genes of PDG biosynthetic pathway have been found. The 13C stable isotope labeling method gave
further verification on the occurrence of lignan biosynthetic pathway in microorganisms by now. In addition to this, it is the first time that differences between the PDG and PMG
biosynthetic pathways have been verified. The results obtained in this study verify the existence of the phenylpropanoid-lignan metabolic pathway in _Phomopsis_ sp. XP-8. Genomic annotation
is an efficient way to discover the pathways that are normally difficult to reveal by metabolic and enzymatic evidence due to low intermediate accumulation, low end-product, and silent gene
expression under normal conditions. This method has been successfully used to identify the existence of a phenylpropanoid metabolic pathway in _Aspergillus oryzae_26, and the molecular
genetics of naringenin biosynthesis, a typical plant secondary metabolite in _Streptomyces clavuligerus_27, and the occurrence of the phenylpropanoid-lignan pathway in _Phomopsis_ sp.
XP-815. This study reports the existence of the phenylpropanoid-lignan pathway in _Phomopsis_ sp. XP-8 during mass flow and identified the metabolites. Additional studies should illustrate
the origin of the genes in the phenylpropanoid-lignan pathway of _Phomopsis_ sp. XP-8. Horizontal gene transfer (HGT) has long been recognized as an important force in the evolution of
organisms28. HGT occurs among different bacteria and plays important roles in the adaptation of microorganisms to different hosts or environmental conditions29. More and more evidence for
gene transfer between distantly related eukaryotic groups has been presented28.Therefore, we cannot exclude the possibility that XP-8 may have acquired the genes related to the lignan
biosynthetic pathway from its host plant by HGT during long-term symbiosis and evolution. However, further evidence is still needed to verify this proposed process. The results obtained in
this study provide useful information on the biosynthesis of lignans and their glycosides via microbial fermentation. Biosynthesis of lignans is of great interest to organic chemists as it
provides a model for biomimetic chemistry and has extensive applications30. Improvement has been made in the techniques to biosynthesize lignan products by regulating the lignan biosynthetic
pathway in trees through genetic modifications31. However, the lignan biosynthetic pathway has rarely been reported. More importantly, the bioconversion sequence from Pin to PDG and the
direct precursor of PDG have remained unclear until now. In previous studies on _Phomopsis_ sp. XP-8, the highest production of PDG and PMG did not occur simultaneously12 and PMG was not the
precursor of PDG because PDG production decreased and/or disappeared when PMG yield increased13. The present study demonstrated that PMG was not the precursor of PDG, and PDG was not the
precursor of PMG, indicating that Pin might be converted to PMG and PDG via two different pathways in _Phomopsis_ sp. XP-8, which has not been revealed in plants. Furthermore, this study
revealed that the bioconversion of Pin, PMG, and PDG from glu occurred simultaneously as that from Phe. We found that the benzene ring structure of Phe did not open throughout the entire Pin
bioconversion process in _Phomopsis_ sp. XP-8 when Phe was used as the sole substrate, indicating that the Pin benzene ring originated from Phe. Glu was converted to Phe and was the sole
glycoside donor for PDG biosynthesis. Therefore, glu not only participated in the formation of glycosides in PDG, but also provided the PDG benzene ring structure. This is different from
that found in plants, indicating there might be some other different pathways to produce these products in _Phomopsis_ sp. XP-8. Not all intermediates in the KEGG-identified plant-lignan
biosynthetic pathway related to Pin, PMG, and PDG formation were found in _Phomopsis_ sp. XP-8, such as caffeic acid, ferulic acid, and coniferyl alcohol (Fig. 1). This may be because the
pathways after _p_-Co are different in XP-8 from those in plants, or the accumulation of these intermediates was too low to be detected. Further studies are needed to verify this hypothesis.
In conclusion, the capability of _Phomopsis_ sp. XP-8 to biosynthesize Pin, PMG and PDG from [13C6]-Phe and [13C6]-glu was verified. The study illustrated the phenylpropanoid-pinoresinol
biosynthetic pathway in microorganism by using stable isotope assisted UPLC-Q-TOF-MS/MS, thus, demonstrating a completely new way to produce Pin, PMG and PDG by bioconversion process. In the
further studies, _Phomopsis_ sp. XP-8 could be used in producing these lignans and their derivatives by microbial fermentation or enzymatic reaction. In addition, the microbial fermentation
production of Pin, PMG and PDG could be enhanced by regulatory controls based on the biosynthetic pathways proved in this study. REFERENCES * Charles, J. S., Ravikumt, P. R. & Huang, F.
C. Isolation and synthesis of Pinoresinol diglucoside, a major antihypertensive principle of Tu-Chung (_Eucommia ulmoides Oliv_.). _J. Am. Chem. Soc._ 98, 5412–5413 (1976). Article Google
Scholar * Saleem, M., Kim, H. J., Ali, M. S. & Lee, Y. S. An update on bioactive plant lignans. _Nat. Prod. Rep._ 22, 696–716 (2005). Article CAS Google Scholar * Xie, L. H., Akao,
T., Hamasaki, K., Deyama, T. & Hattori, M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of _Enterococcus faecalis_
strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. _Chem. Pharm. Bull_ 51, 508–515 (2003). Article CAS Google Scholar * Xie, J. _et al_. Plasma
enterolactone and breast cancer risk in the Nurses’ Health Study II. _Breast Cancer Res. Tr._ 139, 801–809 (2013). Article CAS Google Scholar * Adlercreutz, H. Phyto-oestrogens and
cancer. _Lancet Oncol._ 3, 364–373 (2002). Article Google Scholar * Luo, L. F. _et al_. Antihypertensive effect of _Eucommia ulmoides_ Oliv. extracts in spontaneously hypertensive rats.
_J. Ethnopharmac._ 129, 238–243 (2010). Article Google Scholar * Shi, J. L., Liu, C., Liu, L. P., Yang, B. W. & Zhang, Y. Z. Structure identification and fermentation characteristics
of Pinoresinol diglucoside produced by Phomopsis sp. isolated from _Eucommia ulmoides_ Oliv. _Appl. Microbiol. Biotechnol._ 93, 1475–1483 (2012). Article CAS Google Scholar * Satake, H.,
Ono, E. & Murata, J. Recent advances in the metabolic engineering of lignan biosynthesis pathways for the production of transgenic plant-based foods and supplements. _J. Agric. Food
Chem._ 61, 11721–11729 (2013). Article CAS Google Scholar * Pastor, V., Sanchez-Bel, P., Gamir, J., Pozo, M. J. & Flors, V. Accurate and easy method for systemin quantification and
examining metabolic changes under different endogenous levels. _Plant Methods_ 14, 33 (2018). Article Google Scholar * Eudes, A., Liang, Y., Mitra, P. & Loqué, D. Lignin
bioengineering. _Curr. Opin. Biotechnol._ 28, 189–198 (2014). Article Google Scholar * Zhou, Y. H. _et al_. Transcriptomic and biochemical analysis of highlighted induction of
phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid, Pichia membranaefaciens and oligochitosan. _Postharvest Biol. Tec._ 142, 81–92 (2018). Article CAS Google
Scholar * Zhang, Y. _et al_. Comparison of pinoresinol diglucoside production by _Phomopsis_ sp. XP-8 in different media and the characterization and product profiles of the cultivation in
mung bean. _J. Sci. Food Agr._ 96(12), 4015–4025 (2016). Article CAS Google Scholar * Zhang, Y. _et al_. Production of pinoresinol diglucoside, pinoresinol monoglucoside, and pinoresinol
by _Phomopsis_ sp. XP-8 using mung bean and its major components. _Appl. Microbiol. Biotechnol._ 99, 4629–4643 (2015). Article CAS Google Scholar * Zhang, Y. _et al_. Bioconversion of
Pinoresinol Diglucoside and Pinoresinol from Substrates in the Phenylpropanoid Pathway by Resting Cells of _Phomopsis_ sp. XP-8. _Plos One_ 10, e0137066 (2015). Article Google Scholar *
Gao, Z. H. _et al_. Genomic analysis reveals the biosynthesis pathways of diverse secondary metabolites and pinoresinol and its glycoside derivatives in _Phomopsis_ sp. XP-8. _Acta.
Microbiologica. Sinica._ 58(5), 939–954 (2018). Google Scholar * Zhou, J. _et al_. Identification of membrane proteins associated with phenylpropanoid tolerance and transport in
_Escherichia coli_ BL21. _J. Proteomics_ 113, 15–28 (2015). Article CAS Google Scholar * Tian, Z. Y., Vila, J. Q., Yu, M., Bodnar, W. & Aitken, M. D. Tracing the Biotransformation of
Polycyclic Aromatic Hydrocarbons in Contaminated Soil Using Stable Isotope-Assisted Metabolomics. _Environ. Sci. Techno._ 5(2), 103–109 (2018). CAS Google Scholar * Morasch, B., Hunkeler,
D., Zopfi, J., Temime, B. & Höhener, P. Intrinsic biodegradation potential of aromatic hydrocarbons in an alluvial aquifer – potentials and limits of signature metabolite analysis and
two stable isotope-based techniques. _Water Res._ 45, 4459–4469 (2011). Article CAS Google Scholar * Fischer, A., Manefield, M. & Bombach, P. Application of stable isotope tools for
evaluating natural and stimulated biodegradation of organic pollutants in field studies. _Curr. Opin. Biotechnol._ 41, 99–107 (2016). Article CAS Google Scholar * Angel, S. I. _et al_.
Model selection for within-batch effect correction in UPLC-MS metabolomics using quality control - Support vector regression. _Anal. Chim. Acta._ 1026, 62–68 (2018). Article Google Scholar
* Ren, S. _et al_. 34S, A New Opportunity for the Efficient Synthesis of Stable Isotope Labeled Compounds. _Chemistry_ 24(28), 7133–7136 (2018). Article CAS Google Scholar * Robey, M.
T. _et al_. Identification of the First Diketomorpholine Biosynthetic Pathway Using FAC-MS Technology. _Acs Chemical Biology_ 13(5), 1142–1147 (2018). Article CAS Google Scholar * Li, J.,
Liu, H., Wang, C., Yang, J. & Han, G. Stable isotope labeling-assisted GC/MS/MS method for determination of methyleugenol in food samples. _J. Sci. Food Agr._ 98(9), 3485–3491 (2018).
Article CAS Google Scholar * Mutlib, A. E. Application of stable isotope-labeled compounds in metabolism and in metabolism-mediated toxicity studies. _Chem. Res. Toxicol._ 21(9),
1672–1689 (2008). Article Google Scholar * Zhou, Y. _et al_. Study of the biochemical formation pathway of aroma compound 1-phenylethanol in tea _Camellia sinensis_ L. O. Kuntze. flowers
and other plants. _Food Chem._ 258, 352–358 (2018). Article CAS Google Scholar * Seshime, Y., Juvvadi, P. R., Fujii, I. & Kitamoto, K. Genomic evidences for the existence of a
phenylpropanoid metabolic pathway in _Aspergillus oryzae_. _Biochem. Bioph. Res. Co._ 337(3), 747–751 (2005). Article CAS Google Scholar * Álvarez-Álvarez, R. _et al_. Molecular genetics
of naringenin biosynthesis, a typical plant secondary metabolite produced by Streptomyces clavuligerus. _Microb. Cell Fact._ 141, 1–12 (2015). Google Scholar * Soucy, S. M., Huang, J. L.
& Gogarten, J. P. Horizontal gene transfer, building the web of life. _Nat Rev Genet_ 16(8), 472–482 (2015). Article CAS Google Scholar * Li, M., Zhao, J., Tang, N. W., Sun, H. &
Huang, J. L. Horizontal Gene Transfer From Bacteria and Plants to the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis. _Front Plant Sci._ 9, 701 (2018). Article Google Scholar *
Umezawa, T. Biosynthesis of lignans, lignins, and norlignans. _Kagaku to Seibutsu_ 43, 461–467 (2005). Article CAS Google Scholar * Chiang, V. L. Monolignol biosynthesis and genetic
engineering of lignin in trees, a review. _Environ. Chem. Lett._ 4, 143–146 (2006). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We acknowledge funding by the National
Natural Science Foundation of China (grant no. 31471718), the Modern Agricultural Industry Technology System (CARS-30), the National Key Technology R&D Program (2015BAD16B02), the
National Natural Science Foundation of China (grant no. 31760446), and the Start-up funding of Shihezi University (RCSX201713), and Key research and development plan of Shaanxi Province
(2017ZDXL-NY-0304). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Key Laboratory for Space Bioscience and Biotechnology, School of Life Sciences, Northwestern Polytechnical University, 127
Youyi West Road, Xi’an, Shaanxi Province, 710072, China Junling Shi, Xixi Zhao & Zhenhong Gao * College of Food, Shihezi University, Road Beisi, Shihezi, Xinjiang Province, 832003, China
Yan Zhang, Yongqing Ni & Zhixia Zhao * College of Enology, Northwest A & F University, Yangling, Shaanxi Province, 712100, China Yanlin Liu Authors * Yan Zhang View author
publications You can also search for this author inPubMed Google Scholar * Junling Shi View author publications You can also search for this author inPubMed Google Scholar * Yongqing Ni View
author publications You can also search for this author inPubMed Google Scholar * Yanlin Liu View author publications You can also search for this author inPubMed Google Scholar * Zhixia
Zhao View author publications You can also search for this author inPubMed Google Scholar * Xixi Zhao View author publications You can also search for this author inPubMed Google Scholar *
Zhenhong Gao View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.Z. and J.L.S. designed and performed the cultivations, analysis of
metabolites and co-wrote the manuscript. Y.Q.N. and Y.L.L. performed the data analysis. Z.X.Z., X.X.Z. and Z.H.G. co-wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Junling Shi.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPORTING INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, Y., Shi, J., Ni, Y. _et al._ Tracing the mass flow from glucose and
phenylalanine to pinoresinol and its glycosides in _Phomopsis_ sp. XP-8 using stable isotope assisted TOF-MS. _Sci Rep_ 9, 18495 (2019). https://doi.org/10.1038/s41598-019-54836-1 Download
citation * Received: 20 December 2018 * Accepted: 19 November 2019 * Published: 06 December 2019 * DOI: https://doi.org/10.1038/s41598-019-54836-1 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative





