- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We sought to develop and characterize outer retinal degeneration induced by intravitreal injection of sodium iodate (SI) after vitrectomy in rabbits. To determine the effective dose
of SI, the right eyes of 19 male New Zealand white rabbits received an intravitreal injection of SI or sham. Based on the dose-dependence results, 0.4 mg of SI in 0.05 mL of total volume
was injected into the right eyes of 10 rabbits at two weeks after vitrectomy. In the dose-dependence study, localized retinal atrophy was observed with 0.3- and 0.4-mg SI injections without
vitrectomy. Severe and diffuse retinal atrophy was identified by spectral-domain optical coherence tomography (SD-OCT) at one month after a 0.5-mg SI injection following vitrectomy. In the
second experiment, 0.4 mg of SI in 0.05 mL was injected, and the severity of outer retinal degeneration was graded as one of two types according to electroretinography (ERG) response change.
There was no response on ERG in complete retinal degeneration, 30% of all 10 rabbits. Intravitreal injection of 0.4 mg of SI into vitrectomized rabbit eyes induces diffuse outer retinal
degeneration, and the degree of retinal degeneration can be evaluated through _in vivo_ ophthalmic examination. SIMILAR CONTENT BEING VIEWED BY OTHERS AN EXPERIMENTAL PIG MODEL WITH OUTER
RETINAL DEGENERATION INDUCED BY TEMPORARY INTRAVITREAL LOADING OF _N_-METHYL-_N_-NITROSOUREA DURING VITRECTOMY Article Open access 08 January 2021 ACUTE SYMPTOMATIC VITREOUS FLOATERS
ASSESSED WITH ULTRA-WIDE FIELD SCANNING LASER OPHTHALMOSCOPY AND SPECTRAL DOMAIN OPTICAL COHERENCE TOMOGRAPHY Article Open access 26 April 2021 ANISEIKONIA AFTER REDUCED-FLUENCE PHOTODYNAMIC
THERAPY IN PATIENTS WITH CENTRAL SEROUS CHORIORETINOPATHY Article Open access 10 October 2023 INTRODUCTION Retinal degeneration, which includes conditions such as retinitis pigmentosa (RP),
choroideremia, and geographic atrophy (GA) of age-related macular degeneration (ARMD), is the main cause of irreversible vision loss and greatly affects quality of life. RP is the most
common inherited retinal dystrophy and leads to irreversible vision loss. Initial degeneration due to RP occurs in the photoreceptors, and inner retinal thickness is gradually decreased in
advanced-stage RP1,2,3. Visual prosthetics such as retinal implants have been developed for treatment of retinal degeneration due to advances in electronic device technology and
biomaterials4,5. Recently, implantation of visual prosthetics has been performed in humans. Therefore, to further develop and refine such medical devices, larger experimental animal models
(e.g., dogs, pigs, cats, rabbits) with specific loss of photoreceptors are inevitably needed. The retinotoxin sodium iodate (SI) is an oxidizing compound toxic to retinal pigment epithelial
(RPE) cells, with secondary effects on photoreceptors and the choriocapillaris6. Specifically, SI primarily induces necrosis in RPE cells7,8, which is followed by choriocapillaris atrophy9
and panretinal degeneration8,10. In addition to these effects on RPE cells and photoreceptors, SI also provokes necrosis of the inner retina8,11. SI induces the production of reactive oxygen
species, which contribute to damage in the RPE cells12. SI retinal toxicity has been demonstrated in many different mammalian species, including sheep7, rabbits13,14, rats10,15, and
mice6,11,16, with varying doses and routes of administration. Most studies have used relatively high doses of SI (50–100 mg/kg) and have reported rapid RPE damage characterized by
defragmentation and loss of RPE cell nuclei. Systemic application of SI leads not only to bilateral retinal degeneration, but also to reduced general health of the experimental animals.
Systemic intoxication of SI after systemic administration includes gastrointestinal problems such as diarrhea, general weakness, and convulsion17,18. A high dose of SI was found to be lethal
in experimental animals17,18. Therefore, local administration of SI is required to avoid its systemic effects. In the present research, we attempted to induce unilateral diffuse homogeneous
outer retinal degeneration of the whole retina by intravitreal administration of SI in rabbits. We hypothesized that this approach would avoid the known systemic side effects. The primary
objective of this study was to elucidate the necessary effects of vitrectomy and the proper intravitreal SI dose following vitrectomy to induce diffuse homogeneous outer retinal degeneration
in rabbits. Secondarily, we evaluated the ability of the determined dose of intravitreally injected SI to induce diffuse outer retinal degeneration. RESULTS RETINAL IMAGING IN THE
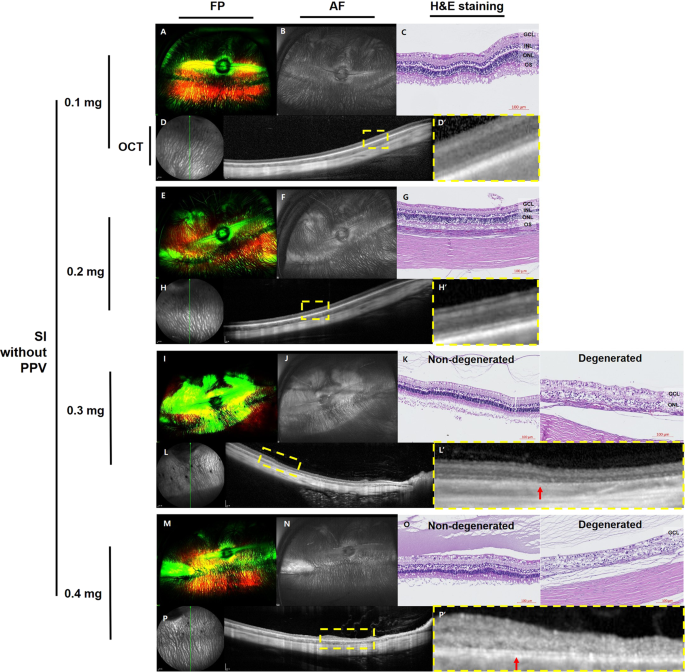
DOSE-DEPENDENCE STUDY OF SODIUM IODATE WITHOUT PARS PLANA VITRECTOMY At one month after SI injection, no significant changes were observed in fundus photography (FP), fundus autofluorescence
(AF), histology with hematoxylin and eosin (H&E) staining, or spectral-domain optical coherence tomography (SD-OCT) images of rabbit eyes injected with 0.1 mg of SI (Fig. 1A–D).
Localized hyper-autofluorescent areas were observed in eyes injected with 0.2 mg, 0.3 mg, or 0.4 mg of SI without vitrectomy (Fig. 1F,J,N, respectively). Both non-degenerated retina and
degenerated retina were observed by histology and SD-OCT in the rabbit eyes injected with 0.3 mg (Fig. 1K,L) or 0.4 mg (Fig. 1O,P) of SI. Disruption of the outer retina and decrease in
retinal thickness were observed in degenerated retina. RETINAL IMAGING IN THE DOSE-DEPENDENCE STUDY OF SODIUM IODATE AFTER PARS PLANA VITRECTOMY At one month after SI injection, no localized
hyper- or hypo autofluorescence was observed by ultra-wide-field color FP or AF (Fig. 2). Additionally, no significant retinal changes were observed with SD-OCT in eyes injected with sham
at one month, and 0.1 mg of SI at one week and one month (Fig. 2D,H,I). However, in the retina of rabbit eyes injected with 0.5 mg of SI, degenerative changes of the outer retina were
observed at one week (Fig. 2R) and degenerative changes became worse for one month (Fig. 2S). The interlayer boundary of the inner and outer retina was unclear, and layers of the outer
retina were replaced with multiple hyper-reflective materials at one month (Fig. 2S). In one of the three rabbit eyes injected with 0.3 mg of SI, degenerative changes of the outer retina
were observed at both one week and one month, while the inner retina remained normal (Fig. 2M,N). An example of histological examination by H&E staining at one month is presented in Fig.
2. In the eye injected with 0.5 mg of SI after vitrectomy, which resulted in severe degenerative changes of the retina observed by SD-OCT, the layers of the retina were disrupted and not
distinguishable (Fig. 2Q). The nuclei of the inner nuclear layer (INL) and outer nuclear layer (ONL) were mixed and scattered, and there was a loss of the photoreceptor layer. In the eyes
injected with 0.3 mg of SI after vitrectomy with selective outer retinal degeneration on SD-OCT, the outer retinal layer was distinguishable from the INL in the area that remained relatively
intact on SD-OCT (Fig. 2L). Following sham and 0.1-mg SI injections after vitrectomy, no significant changes were observed in H&E staining (Fig. 2C,G). RETINAL DEGENERATION INDUCED BY
0.4-MG SODIUM IODATE INJECTION WITH VITRECTOMY Based on the dose-dependence study, 0.4 mg of SI in 0.05 mL of total volume was selected for further evaluation. Six weeks after the 0.4-mg SI
injection, the severity of outer retinal degeneration was graded into two types according to change in OCT images and electroretinography (ERG) response (Fig. 3). For animals classified as
having incomplete or complete retinal degeneration, the FP images showed degenerative changes (Fig. 3A,F), the AF images showed hyper-autofluorescence (Fig. 3B,G), and the ERG response was
abnormal (Fig. 3E,J). FP and AF images did not show any localized degenerative change. Seven of the 10 rabbits showed incomplete retinal degeneration, characterized by a loss of the junction
between inner and outer segments of the photoreceptor layer (IS/OS line), a relatively preserved ONL on SD-OCT, and partially remnant response on ERG (Fig. 3C–E). Three of the 10 rabbits
had complete retinal degeneration including diffuse loss of the IS/OS line and ONL on SD-OCT, which implies diffuse outer retinal degeneration, and complete loss of responses on ERG (Fig.
3H–J). Histology and immunohistochemistry findings demonstrated differences between the control eyes (left eye within the same animal) and SI-injected eyes with incomplete and complete
changes in retinal degeneration (Fig. 4). In the control eyes, all layers of the retina were easily distinguished by histology (Fig. 4A). Additionally, RPE, cone and rod photoreceptors,
bipolar cells, and ganglion cells were all easily identified with RPE65, PNA, rhodopsin, PKCα, and Brn3 staining, respectively (Fig. 4D,G,J,M,V). In eyes with incomplete changes in retinal
degeneration, H&E staining revealed a minor reduction in the photoreceptor layer, and there were fewer cone photoreceptor cells based on PNA staining (Fig. 4B,E). However, the levels of
RPE cells, rod photoreceptor cells, bipolar cells, and ganglion cells remained normal (Fig. 4H,K,N,W). In eyes with complete changes in retinal degeneration, H&E staining demonstrated
severe disruption of the photoreceptor layer, retinal thinning, and almost complete depletion of the cone and rod photoreceptor cells based on PNA and rhodopsin staining (Fig. 4C,F,I).
Additionally, bipolar cell staining and ganglion cell staining were also decreased (Fig. 4L,O). However, the level of RPE cells remained normal (Fig. 4X). Eyes determined to have any retinal
degeneration (either incomplete or complete) demonstrated increased GFAP staining, suggesting a proliferation of glial cells, which was not observed in the control eyes (Fig. 4P–R). Some
apoptotic cells, as determined by the TUNEL assay, were present in eyes with incomplete or complete retinal degeneration (Fig. 4T,U). However, there were no TUNEL-positive cells in the
control eyes (Fig. 4S). Cataracts caused by contact with the instrumental lens during vitrectomy were observed in four out of 10 eyes (40.0%) during examination at six weeks after injection.
We observed no signs of systemic toxicity such as weight loss or death in any rabbit. QUANTITATIVE COMPARISON OF 0.4-MG SODIUM IODATE INJECTION WITHOUT OR WITH VITRECTOMY We compared the
effects of a 0.4-mg SI injection depending on vitrectomy. In non-vitrectomized rabbit eyes, total retinal thickness was significantly decreased after 0.4 mg SI injection (154.06 ± 1.80 μm at
baseline vs. 71.80 ± 6.48 μm at one month after injection; p < 0.01; Fig. 5A). In vitrectomized rabbit eyes, total retinal thickness was also significantly decreased after injection
(154.80 ± 2.08 μm at baseline vs. 135.89 ± 2.99 μm at six weeks after injection; p = 0.009; Fig. 5A). Total retinal thickness of non-vitrectomized eyes showed more thinning than did that of
vitrectomized eyes (p < 0.01; Fig. 5A). In non-vitrectomized rabbit eyes, inner retinal thickness was significantly decreased after 0.4 mg SI injection (70.83 ± 1.43 μm at baseline vs.
47.50 ± 4.00 μm at one month after injection; p < 0.01; Fig. 5A). In vitrectomized rabbit eyes, inner retinal thickness was not significantly decreased after injection (69.55 ± 2.28 μm at
baseline vs. 70.95 ± 1.94 μm at six weeks after injection; p < 0.34; Fig. 5A). Inner retinal thickness of non-vitrectomized eyes presented more significant thinning than that of
vitrectomized eyes (p < 0.01; Fig. 5A). We compared total retinal thickness among the types of retinal degeneration at six weeks after 0.4-mg SI injection in vitrectomized rabbit eyes
(Fig. 5B). According to severity of outer retinal degeneration, retinal thickness change was different among the groups of retinal degeneration severity. The group of complete retinal
degeneration showed more significant retinal thinning than did the groups of incomplete retinal degeneration (145.25 ± 2.96 μm in total retinal layer for incomplete vs. 107.8 ± 3.35 μm in
total retinal layer for complete, 75.83 ± 2.19 μm in inner retinal layer for incomplete vs. 56.3 ± 1.79 μm in inner retinal layer for complete; p < 0.01, respectively; Fig. 5B).
DISCUSSION In this study, we determined that SI injection after vitrectomy was effective in inducing unilateral diffuse homogeneous outer retinal degeneration. A dose of 0.4 mg of SI in 0.05
mL of total volume was determined to be most effective and resulted in complete retinal degeneration, with loss of cone and rod photoreceptors following intravitreal injection in 30% of
subjects. We previously reported the effectiveness of vitrectomy when retinal degeneration was induced by intravitreal injection with a drug, such as N-methyl-N-nitrosourea (MNU)19. In the
previous study, it was hard to induce diffuse outer retinal degeneration by intravitreal injection of MNU without vitrectomy. Based on our previous study, we also tried to compare the
results of intravitreal SI injection without or with vitrectomy. Without vitrectomy, only localized retinal degeneration occurred in SI-injected eyes, whereas diffuse retinal degeneration
was induced in SI-injected eyes after vitrectomy. When different doses of SI were tested after vitrectomy, we found that 0.5 mg of SI induced severe retinal atrophy. However, in eyes
injected with 0.3 mg of SI after vitrectomy, selective outer retinal degeneration developed in only one of three eyes. Therefore, we recognized that the range for a safe and effective dose
of intravitreal SI was quite narrow. Additionally, we observed that 0.4 mg of SI induced two types of retinal degeneration, and all rabbit eyes showed retinal degeneration. These results
support use of the animal rabbit model induced by 0.4 mg SI injection after vitrectomy for further experiment regarding the severity of retinal degeneration. In this study, we used
ultra-wide-field fundus photography and autofluorescence imaging to evaluate whether retinal degeneration was global and diffuse. Additionally, 55-degree SD-OCT images were produced to
visualize retinal changes after the injections because of their wide fields of view. Importantly, the degree of outer retinal degeneration, as graded based on ERG response, was associated
with SD-OCT findings and additionally validated by histological examination with immunohistochemistry. When ERG response was partially decreased, greater damage to the IS/OS with an intact
ONL was observed on SD-OCT. Finally, when all responses were lost on ERG, ONL and the photoreceptor layer were indistinguishable in SD-OCT images. Additionally, eyes with incomplete changes
in retinal degeneration had fewer cone cells based on PNA staining. Eyes with complete changes in retinal degeneration, including those with flat ERG responses, also had less staining with
PNA and rhodopsin. Immunohistochemistry demonstrated fewer bipolar and ganglion cells in eyes with complete changes in retinal degeneration. SI not only affects RPE cells and photoreceptors,
but can also lead to toxicity and necrosis of the inner retina when intravenously injected8,11,20. However, in this experiment, RPE staining remained normal despite retinal degeneration
compared with the control. There was no previous report about RPE staining after intravitreal injection of SI. A previous study reported morphologic changes of RPE with immunohistochemistry
staining after subretinal injection of SI in other species21. In this report, the RPE nuclei became bigger and more rounded after subretinal injection of SI, but RPE remained relatively
stable and appeared to be more resistant than the photoreceptors. The previous and present studies suggest that systemic administration of SI could induce toxicity of RPE, but localized
administration of SI such as subretinal and especially intravitreal injection induce less damage to the RPE. We did find that intravitreal injection of SI after vitrectomy induced direct
damage to the photoreceptor, not the level of RPE. Previous studies reported that, after SI was administered, Müller glial cells proliferated on the third day, and the ratio of GFAP-positive
cells increased markedly for 28 days8,22. In this study, GFAP staining was also increased in all the eyes that received an intravitreal injection of SI, and it is likely that the SI
injection itself caused fibrosis and proliferation of glial cells, regardless of degeneration. The TUNEL assay was positive in eyes with outer retinal degeneration, even though the degree of
staining was not extensive. This could be due to a lack of apoptotic cells. SI induced the death of photoreceptors and may have triggered apoptosis. TUNEL-positive cells were restricted to
the ONL, where photoreceptor nuclei are located6,12. Kondo _et al_.23 reported a transgenic (Tg) rabbit model of progressive retinal degeneration. That rabbit model showed decreased rod ERG
response in 5% of animals at 48 weeks of age. In that study, 15% of newborn rabbits were transgene-positive, and some died. In general, it is difficult to develop a genetically modified
animal model, and it takes a relatively long time for disease to fully manifest. Because of these limits, intravitreal injection of compounds to induce retinal degeneration has been tried
with various drugs in several different kinds of animals24,25,26,27,28,29,30,31. The main advantages of localized application of SI through intravitreal injection are that it spares the
second eye as a control and avoids unwanted systemic side effects. With a known effective drug dose, many adequate animal models for retinal degeneration could be developed quickly. Animal
models with monocular drug–induced retinal degeneration could be useful because they do not show binocular blindness. Cho _et al_.30 reported that intravitreal injection of SI induced
monocular retinal degeneration in New Zealand white rabbit eyes. They further reported that retinal damage was reversible at low doses (0.1 and 0.2 mg of SI) but irreversible at higher doses
(0.4 and 0.8 mg of SI). In the 0.4-mg SI group in that study, the outer retina was significantly destroyed, whereas the inner retina was relatively preserved. Conversely, in the 0.8-mg SI
group, the entire retinal layers were irreversibly destroyed. Those authors reported that monocular intravitreal injection with SI provided an animal model for monocular retinal
degeneration. However, Cho _et al_.30 only tested the performance of intravitreal SI injection and did not perform vitrectomy. Our study showed that intravitreal SI injection without
vitrectomy did not induce diffuse retinal degeneration and produced only localized, widely varying degrees of retinal degeneration. In addition, intravitreal SI injection without vitrectomy
could induce severe retinal thinning to affect the inner retinal layer. With intravitreal injection after vitrectomy, retinal degeneration was relatively uniform, and the RPE and inner
retinal layer remained relatively intact per the results of immunohistochemistry and quantitative outcomes of retinal thickness. Additionally, because only conventional FP without
autofluorescence imaging was used instead of ultra-wide-field FP with AF imaging, those authors were limited in their ability to determine the distribution of effects in the peripheral
retina. In the present study, we demonstrated that intravitreal injection of SI after vitrectomy was an effective method to produce a globally diffuse outer retinal degeneration animal model
with no systemic complications and low cost. Furthermore, if future researchers screen the degree of outer retinal degeneration before experimentation, the two types of outer retinal
degeneration models induced here by SI injection can be effectively used for other study purposes. Notably, there were some limitations in this study. First, the intravitreally injected SI
dose was different between the post-vitrectomy group and the without-vitrectomy group. Because we learned that diffuse homogenous retinal degeneration in the whole retina could not be
induced with intravitreal SI injection without vitrectomy in the dose-dependence study, we did not try to inject a higher SI dose than 0.4 mg. In the dose-dependent study of SI with
vitrectomy, we confirmed that intravitreal injection of SI after vitrectomy affected the whole retina homogeneously based on wide AF and SD-OCT images and focused on detecting the effective
dose that could induce diffuse retinal degeneration. We initially could not predict the effect of intravitreal SI injection after vitrectomy and could not rule out the possibility that even
lower doses of SI might induce retinal degeneration in the vitrectomized eye. Therefore, sham and a higher dose of SI (0.5 mg) were also injected in vitrectomized eyes. Second, the follow-up
periods of the dose-dependent study and the second study were different. In the dose-dependent study, the purpose was to find the effective dose to induce diffuse retinal degeneration, so
rabbits were observed for four weeks after intravitreal injection. In the second study, the purpose was to evaluate the retinal change after a 0.4-mg SI intravitreal injection, so rabbits
were sacrificed at six weeks after intravitreal injection. Third, the numbers of animals included were relatively small. However, with the rabbits used, we could compare the effects of
vitrectomy on the induction of diffuse retinal degeneration and determine the effective dose. Furthermore, in the second study, complete retinal degeneration was induced at 30% among 10
rabbits. So, while minimizing the number of sacrificed animals, the aim of this study was achieved. This SI-induced method provides an animal model that includes incomplete and complete
outer retinal degeneration and will be useful for evaluating therapeutic strategies for diseases involving retinal degeneration. After vitrectomy, an animal model induced by intravitreal SI
injection showed diffuse and uniform outer retinal degeneration. In conclusion, a vitrectomized rabbit model with an intravitreal a 0.4-mg/0.05-mL SI injection induced diffuse outer retinal
degeneration with disruption of photoreceptors. MATERIALS AND METHODS ANIMALS In a dose-dependent study of SI, the right eyes of male New Zealand white rabbits (n = 19), aged five months and
weighing between 2.5 and 3.5 kg, received either an intravitreal injection of SI without vitrectomy or an intravitreal injection of SI or sham injection at two weeks after vitrectomy. For
all injections, each dose of SI was diluted in 0.05 mL of phosphate-buffered saline (PBS). Two rabbits per concentration received the following intravitreal injections of SI without
vitrectomy: 0.4 mg, 0.3 mg, 0.2 mg, and 0.1 mg. Intravitreal injection of SI after vitrectomy was performed as follows: sham (0.05 mL of PBS; n = 2 rabbits) or 0.5 mg, 0.3 mg, or 0.1 mg of
SI (n = 3 rabbits per concentration). To identify morphological changes to the retina, ultra-wide-field color FP, fundus AF imaging, and SD-OCT were performed at one month after intravitreal
SI injection. Histological examinations after H&E staining were also completed on select rabbit eyes at one month after injection. Additionally, to analyze the change of retinal
degeneration over time, SD-OCT was also performed at one week after intravitreal SI injection in the vitrectomized eyes. Based on the results of the dose-dependence study, 0.4 mg of SI in
0.05 mL of PBS was chosen for injection into the right eyes of rabbits (n = 10) at two weeks after vitrectomy. The rabbits in the efficacy experiments had a mean body weight of 3.32 kg and
mean axial length according to A-scan ultrasonography of 15.96 mm. In addition, FP, AF imaging, SD-OCT, and histology were performed at six weeks after injection. To identify physiological
changes to the retina, ERG was also performed at six weeks after injection. All procedures adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of
Animals in Ophthalmic and Vision Research (ARVO Animal Policy). Approval for this study was obtained from the Institutional Animal Care and Use Committee of Korea University College of
Medicine in Seoul, Korea. VITRECTOMY The rabbits were anesthetized by injection of alfaxalone (5 mg/kg, Alfaxan®; Jurox Pty Ltd., Rutherford, Australia) into the marginal auricular vein and
intramuscular injection of xylazine (4 mg/kg, Rompun®; Bayer AG, Leverkusen, Germany). After anesthesia, 0.5% tropicamide and 0.5% phenylephrine (Tropherine®; Hanmi Pharm Co., Ltd., Seoul,
Korea) were administered for pupil dilatation, and then the eye was irrigated with 5% povidone iodide and draped for surgery. Two-port, 23-gauge core vitrectomy (Associate; DORC, Zuidland,
the Netherlands) was performed with a direct biconcave lens. The two ports were prepared by inserting a trocar cannula into the sclera at 4 mm from the limbus on the superoventral and
superodosal sides. A surgical microscope was used for lighting. The vitreous was removed using a vitreous cutter while continually supplying balanced salt solution (BSS; Alcon, Fort Worth,
TX, USA). INTRAVITREAL INJECTION OF SODIUM IODATE Animals were anesthetized as described above. Immediately before the injections, SI (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
PBS. The right eye of each rabbit was prepared, and the corresponding dose of SI (in a total volume of 0.05 mL) was injected intravitreally at 4 mm posterior to the limbus using a 30-gauge
needle. No injections were performed in the left eyes. ULTRA-WIDE-FIELD IMAGING AND SPECTRAL-DOMAIN OPTICAL COHERENCE TOMOGRAPHY FP and AF images were captured using an ultra-wide-field
scanning laser ophthalmoscope (OPTOS 200 TX; Optos PLC, Dunfermline, UK). SD-OCT was performed using the Spectralis® OCT system (Heidelberg Engineering GmbH, Heidelberg, Germany). The area
of the visual streak below the optic disc was evaluated. Vertical line scans, horizontal line scans, and raster scans (33 B-scans over a 16.5-mm × 16.5-mm area in a 55-degree image) were
performed in high-resolution mode (1,536 A-scans per B-scan, lateral resolution = 10 µm/pixel in a 55-degree image). Up to 100 single images were averaged in automatic real-time mode to
obtain a high-quality mean image. ELECTRORETINOGRAPHY The ERG protocol was based on the international standard for ERG from the International Society for Clinical Electrophysiology of Vision
(ISCEV)32,33,34. The rabbits were anesthetized as described above, dark-adapted for 30 minutes, and their pupils were dilated. One eye per animal was studied to avoid accidental
contralateral light adaptation. Light stimulation and ERG signal recording were performed with a commercial system (RETIcom; Roland Consult, Brandenburg an der Havel, Germany) using a
contact lens electrode with a built-in light resource (Kooijman/Damhof ERG lens; Medical Workshop BV, Groningen, the Netherlands). The reference and ground electrodes were platinum subdermal
needle electrodes. The reference electrodes were placed in the skin near the lateral canthus of the eyes, while the ground electrode was placed on the forehead between the two eyes.
HISTOLOGICAL EXAMINATION Immediately after euthanasia, both eyes were enucleated, immersion-fixed in Davidson’s solution for 24 hours, dehydrated, and embedded in paraffin. Sections
measuring 4 µm were cut and stained with H&E. The slides were examined for pathological retinal changes using a light microscope (BX-53; Olympus Corp., Tokyo, Japan) and photographed
with a digital camera (INFINITY3-1UR; Lumenera Corp., Ottawa, ON, Canada). IMMUNOHISTOCHEMISTRY Tissue sections were deparaffinized, rehydrated, and microwave-heated in antigen retrieval
buffer [1 mM of ethylenediaminetetraacetic acid (EDTA), 0.05% Tween 20; pH: 8.0]. Sections were then blocked with 4% horse serum in PBS, followed by primary antibody incubation at 4 °C
overnight. Anti-RPE65 (Invitrogen, Carlsbad, CA, USA) staining was performed following the manufacturer’s protocol. For anti-PKCα (Invitrogen, Carlsbad, CA, USA) and rhodamine-labeled
anti-peanut agglutinin (PNA) (Vector Laboratories, Burlingame, CA, USA) coimmunostaining, fluorescent detection was performed with an Alexa Fluor 488–conjugated goat anti-mouse secondary
antibody (Invitrogen, Carlsbad, CA, USA). For anti-PKCα and anti-rhodopsin (Rockland Immunochemicals, Pottstown, PA, USA) coimmunostaining, fluorescence detection was performed with Alexa
Fluor 488–conjugated goat anti-mouse and Alexa Fluor 594–conjugated goat anti-mouse secondary antibodies (Invitrogen, Carlsbad, CA, USA). For anti-Brn3 (Santa Cruz Biotechnology, Dallas, TX,
USA) immunostaining, fluorescence detection was performed with an Alexa Fluor 594–conjugated goat anti-mouse secondary antibody. Lastly, for anti-GFAP (Novus Biological, Littleton, CO, USA)
staining, sections were incubated for two hours at room temperature and then for one hour with Alexa Fluor 594–conjugated goat anti-mouse secondary antibody. A deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay (Merck Millipore, Burlington, MA, USA) was performed following the manufacturer’s protocol. Nuclei were counterstained with
4,6-diamidino-2-phenylindole (DAPI) (AnaSpec Inc., Fremont, CA, USA). Cells stained by TUNEL were evaluated using fluorescence microscopy (T2000-U; Nikon, Tokyo, Japan). RETINAL THICKNESS
MEASUREMENT AND STATISTICAL ANALYSIS FOR RETINAL THINNING INDUCED BY SODIUM IODATE INJECTION In each rabbit, we measured total retinal thickness and inner retinal thickness at 10 different
inferior retinal sites using linear horizontal SD-OCT imaging performed at baseline and at one month after a 0.4-mg SI/0.05-mL injection in the non-vitrectomized and vitrectomized eyes,
respectively. Total retinal layer was defined as from ganglion cell layer (GCL) to RPE layer and inner retinal layer was defined as from GCL to INL. To compare the effects of SI injection on
total retinal thickness and inner retinal thickness between baseline and post-injection, statistical analysis was conducted using a Wilcoxon signed-rank test. To compare the effects of
vitrectomy on total retinal thickness and inner retinal thickness, statistical analysis was conducted using the Mann–Whitney U test. To compare the type of retinal degeneration after SI
injection, statistical analysis was conducted using the Mann–Whitney U test or Kruskal Wallis test. Data are presented as mean ± standard error (SE). Differences were considered
statistically significant at p < 0.05. REFERENCES * Asakawa, K. _et al_. Histopathological Changes of Inner Retina, Optic Disc, and Optic Nerve in Rabbit with Advanced Retinitis
Pigmentosa. _Neuroophthalmology_ 40, 286–291, https://doi.org/10.1080/01658107.2016.1229339 (2016). Article PubMed PubMed Central Google Scholar * Vamos, R. _et al_. The structure and
function of the macula in patients with advanced retinitis pigmentosa. _Invest Ophthalmol Vis Sci_ 52, 8425–8432, https://doi.org/10.1167/iovs.11-7780 (2011). Article PubMed PubMed Central
Google Scholar * Strettoi, E., Porciatti, V., Falsini, B., Pignatelli, V. & Rossi, C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. _J Neurosci_
22, 5492–5504, 20026533 (2002). * da Cruz, L. _et al_. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss.
_Br J Ophthalmol_ 97, 632–636, https://doi.org/10.1136/bjophthalmol-2012-301525 (2013). Article PubMed PubMed Central Google Scholar * Humayun, M. S. _et al_. Visual perception in a
blind subject with a chronic microelectronic retinal prosthesis. _Vision Res_ 43, 2573–2581 (2003). Article Google Scholar * Balmer, J., Zulliger, R., Roberti, S. & Enzmann, V. Retinal
Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways. _Int J Mol Sci_ 16, 15086–15103, https://doi.org/10.3390/ijms160715086
(2015). Article CAS PubMed PubMed Central Google Scholar * Nilsson, S. E., Knave, B. & Persson, H. E. Changes in ultrastructure and function of the sheep pigment epithelium and
retina induced by sodium iodate. II. Early effects. _Acta Ophthalmol (Copenh)_ 55, 1007–1026 (1977). Article CAS Google Scholar * Kiuchi, K., Yoshizawa, K., Shikata, N., Moriguchi, K.
& Tsubura, A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. _Curr Eye Res_ 25, 373–379 (2002). Article Google Scholar * Korte, G. E., Reppucci,
V. & Henkind, P. RPE destruction causes choriocapillary atrophy. _Investigative ophthalmology & visual science_ 25, 1135–1145 (1984). CAS Google Scholar * Hariri, S., Moayed, A.
A., Choh, V. & Bizheva, K. _In vivo_ assessment of thickness and reflectivity in a rat outer retinal degeneration model with ultrahigh resolution optical coherence tomography. _Invest
Ophthalmol Vis Sci_ 53, 1982–1989, https://doi.org/10.1167/iovs.11-8395 (2012). Article PubMed Google Scholar * Chowers, G. _et al_. Course of Sodium Iodate-Induced Retinal Degeneration
in Albino and Pigmented Mice. _Invest Ophthalmol Vis Sci_ 58, 2239–2249, https://doi.org/10.1167/iovs.16-21255 (2017). Article CAS PubMed Google Scholar * Hanus, J., Anderson, C.,
Sarraf, D., Ma, J. & Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. _Cell Death Discov_ 2, 16054, https://doi.org/10.1038/cddiscovery.2016.54 (2016).
Article CAS PubMed PubMed Central Google Scholar * Clifton, L. & Makous, W. Iodate poisoning: early effect on regeneration of rhodopsin and the ERG. _Vision Res_ 13, 919–924 (1973).
Article CAS Google Scholar * Obata, R. _et al_. Retinal degeneration is delayed by tissue factor pathway inhibitor-2 in RCS rats and a sodium-iodate-induced model in rabbits. _Eye
(Lond)_ 19, 464–468, https://doi.org/10.1038/sj.eye.6701531 (2005). Article CAS Google Scholar * Hariri, S. _et al_. Noninvasive imaging of the early effect of sodium iodate toxicity in a
rat model of outer retina degeneration with spectral domain optical coherence tomography. _J Biomed Opt_ 18, 26017, https://doi.org/10.1117/1.JBO.18.2.026017 (2013). Article CAS PubMed
Google Scholar * Enzmann, V. _et al_. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. _Exp Eye Res_ 82, 441–448,
https://doi.org/10.1016/j.exer.2005.08.002 (2006). Article CAS PubMed Google Scholar * Andersen, F. A. Final Report on the Safety Assessment of Sodium Iodate. _J Am Coll Toxicol_ 14,
231–239 (1995). Article CAS Google Scholar * Webster, S. H., Rice, M. E., Highman, B. & Vonoettingen, W. F. The Toxicology of Potassium and Sodium Iodates - Acute Toxicity in Mice.
_Journal of Pharmacology and Experimental Therapeutics_ 120, 171–178 (1957). CAS PubMed Google Scholar * Ahn, S. M. _et al_. Development of a Post-vitrectomy Injection of
N-methyl-N-nitrosourea as a Localized Retinal Degeneration Rabbit Model. _Exp Neurobiol_ 28, 62–73, https://doi.org/10.5607/en.2019.28.1.62 (2019). Article PubMed PubMed Central Google
Scholar * Wang, J., Iacovelli, J., Spencer, C. & Saint-Geniez, M. Direct effect of sodium iodate on neurosensory retina. _Invest Ophthalmol Vis Sci_ 55, 1941–1953,
https://doi.org/10.1167/iovs.13-13075 (2014). Article CAS PubMed PubMed Central Google Scholar * Mones, J. _et al_. A Swine Model of Selective Geographic Atrophy of Outer Retinal Layers
Mimicking Atrophic AMD: A Phase I Escalating Dose of Subretinal Sodium Iodate. _Invest Ophthalmol Vis Sci_ 57, 3974–3983, https://doi.org/10.1167/iovs.16-19355 (2016). Article CAS PubMed
Google Scholar * Chen, X., Li, Q., Xu, H. & Yin, Z. Q. Sodium iodate influences the apoptosis, proliferation and differentiation potential of radial glial cells _in vitro_. _Cellular
physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology_ 34, 1109–1124, https://doi.org/10.1159/000366325 (2014). Article CAS
Google Scholar * Kondo, M. _et al_. Generation of a transgenic rabbit model of retinal degeneration. _Invest Ophthalmol Vis Sci_ 50, 1371–1377, https://doi.org/10.1167/iovs.08-2863
(2009). Article PubMed Google Scholar * Rosch, S. _et al_. Selective photoreceptor degeneration by intravitreal injection of N-methyl-N-nitrosourea. _Investigative ophthalmology &
visual science_ 55, 1711–1723, https://doi.org/10.1167/iovs.13-13242 (2014). Article CAS ADS Google Scholar * Aplin, F. P. _et al_. Stimulation of a Suprachoroidal Retinal Prosthesis
Drives Cortical Responses in a Feline Model of Retinal Degeneration. _Investigative ophthalmology & visual science_ 57, 5216–5229, https://doi.org/10.1167/iovs.16-19926 (2016). Article
CAS Google Scholar * Aplin, F. P. _et al_. ATP-induced photoreceptor death in a feline model of retinal degeneration. _Investigative ophthalmology & visual science_ 55, 8319–8329,
https://doi.org/10.1167/iovs.14-15732 (2014). Article CAS Google Scholar * Aplin, F. P. _et al_. Retinal Changes in an ATP-Induced Model of Retinal Degeneration. _Frontiers in
neuroanatomy_ 10, 46, https://doi.org/10.3389/fnana.2016.00046 (2016). Article CAS PubMed PubMed Central Google Scholar * Halupka, K. J. _et al_. Neural Responses to Multielectrode
Stimulation of Healthy and Degenerate Retina. _Investigative ophthalmology & visual science_ 58, 3770–3784, https://doi.org/10.1167/iovs.16-21290 (2017). Article CAS Google Scholar *
Vessey, K. A. _et al_. Adenosine triphosphate-induced photoreceptor death and retinal remodeling in rats. _The Journal of comparative neurology_ 522, 2928–2950,
https://doi.org/10.1002/cne.23558 (2014). Article CAS PubMed PubMed Central Google Scholar * Cho, B. J., Seo, J. M., Yu, H. G. & Chung, H. Monocular retinal degeneration induced by
intravitreal injection of sodium iodate in rabbit eyes. _Japanese journal of ophthalmology_ 60, 226–237, https://doi.org/10.1007/s10384-016-0429-1 (2016). Article CAS PubMed Google
Scholar * Rosch, S., Werner, C., Muller, F. & Walter, P. Photoreceptor degeneration by intravitreal injection of N-methyl-N-nitrosourea (MNU) in rabbits: a pilot study. _Graefe’s
archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie_ 255, 317–331, https://doi.org/10.1007/s00417-016-3531-7
(2017). Article CAS PubMed Google Scholar * Amaral, A. V. C. _et al_. Electroretinography and immunohistochemistry of retina in rabbits treated with sildenafil citrate. _Arq Bras Med Vet
Zoo_ 67, 1589–1598, https://doi.org/10.1590/1678-4162-7464 (2015). Article Google Scholar * Gjorloff, K., Andreasson, S. & Ehinger, B. Standardized full-field electroretinography in
rabbits. _Doc Ophthalmol_ 109, 163–168 (2004). Article Google Scholar * Luebke, J., Anton, A. & Bach, M. Test-retest reliability of scotopic full-field electroretinograms in rabbits.
_Doc Ophthalmol_ 134, 157–165, https://doi.org/10.1007/s10633-017-9582-1 (2017). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This research was supported in part by
the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1A02937018); by the Bio & Medical Technology
Development Program of the NRF funded in part by the Korean government, Ministry of Science and ICT (MSIP) (NRF-2017M3A9E2056458 and 2017M3A9E2056460); and in part by the 2016 Korea
University Ansan Hospital R&D support project through the support of the Vice President for Medical Affairs of Korea University Special Research Funds (K1613771); by a Korea University
grant. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ophthalmology, Korea University College of Medicine, Seoul, Korea So Min Ahn, Cheolmin Yun & Seong-Woo Kim * Department
of Physiology, Chungbuk National University School of Medicine, Cheongju, Korea Jungryul Ahn, Seongkwang Cha & Yong Sook Goo * Department of Ophthalmology, Bucheon Hospital,
Soonchunhyang University College of Medicine, Bucheon, Korea Tae Kwann Park * Medical Device Development Center, Osong Medical Innovation Foundation, Cheongju, Korea Young-Jin Kim Authors *
So Min Ahn View author publications You can also search for this author inPubMed Google Scholar * Jungryul Ahn View author publications You can also search for this author inPubMed Google
Scholar * Seongkwang Cha View author publications You can also search for this author inPubMed Google Scholar * Cheolmin Yun View author publications You can also search for this author
inPubMed Google Scholar * Tae Kwann Park View author publications You can also search for this author inPubMed Google Scholar * Young-Jin Kim View author publications You can also search for
this author inPubMed Google Scholar * Yong Sook Goo View author publications You can also search for this author inPubMed Google Scholar * Seong-Woo Kim View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS Study design (S.K.); study conduct (S.A., S.K.); data collection (S.A., S.K.); data analysis and interpretation (S.A., J.A.,
S.C., Y.G., S.K.); and preparation, review, and approval of the manuscript (S.A., J.A., S.C., C.Y., T.P., Y.K., Y.G., S.K.). CORRESPONDING AUTHORS Correspondence to Yong Sook Goo or
Seong-Woo Kim. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ahn, S.M., Ahn, J., Cha, S. _et al._ The effects of intravitreal sodium iodate
injection on retinal degeneration following vitrectomy in rabbits. _Sci Rep_ 9, 15696 (2019). https://doi.org/10.1038/s41598-019-52172-y Download citation * Received: 01 August 2019 *
Accepted: 12 October 2019 * Published: 30 October 2019 * DOI: https://doi.org/10.1038/s41598-019-52172-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative