- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Exposure of the vertebrate embryo to maternal hormones can have long-lasting effects on its phenotype, which has been studied extensively by experimentally manipulating maternal
steroids, mostly androgens, in bird eggs. Yet, there is a severe lack of understanding of how and when these effects are actually mediated, hampering both underlying proximate and ultimate
explanations. Here we report a novel finding that the embryo expresses androgen receptor (AR) and estrogen receptor (ERα) mRNA in its extraembryonic membranes (EMs) as early as before its
own hormone production starts, suggesting a novel substrate for action of maternal hormones on the offspring. We also report the first experimental evidence for steroid receptor regulation
in the avian embryo in response to yolk steroid levels: the level of AR is dependent on yolk androgen levels only in the EMs but not in body tissues, suggesting embryonic adaptation to
maternal hormones. The results also solve the problem of uptake of lipophilic steroids from the yolk, why they affect multiple traits, and how they could mediate maternal effects without
affecting embryonic sexual differentiation. SIMILAR CONTENT BEING VIEWED BY OTHERS PLASTICITY IN METABOLISM OF MATERNAL ANDROGENS IN AVIAN EMBRYOS Article Open access 18 May 2023 UNVEILING
THE CRITICAL ROLE OF ANDROGEN RECEPTOR SIGNALING IN AVIAN SEXUAL DEVELOPMENT Article Open access 17 October 2024 TESTING FOR CONTEXT-DEPENDENT EFFECTS OF PRENATAL THYROID HORMONES ON
OFFSPRING SURVIVAL AND PHYSIOLOGY: AN EXPERIMENTAL TEMPERATURE MANIPULATION Article Open access 03 September 2020 INTRODUCTION In many animal taxa, including vertebrates, the embryo is
exposed to maternal hormones, which can have long-lasting effects on its phenotype (fish1, reptiles2, birds3,4,5, mammals6,7). Several studies have injected steroids, mostly androgens, into
bird eggs, the most widely used model, mimicking variation in maternal yolk deposition and finding a wide array of effects on the offspring phenotype3,4,5,8. The mechanisms underlying such
effects are largely ignored, hampering further progress in this prevalent field of research9. In order to be functional, the androgens must reach the embryonic tissues and those tissues must
have androgen receptors (AR). However, very early in incubation, yolk androgens seem to be rapidly metabolized to inactive forms by the embryo10,11,12. Moreover, in spite of being polar,
steroid hormones are lipophilic and do not easily dissolve in water. Therefore, it remains an enigma how the embryo is able to take up these hormones from the lipid rich yolk into its watery
circulation for their transport to body tissues where they can exert their effects. We tested the hypothesis that the embryo expresses AR and/or estrogen receptors (ERα, as alpha is the
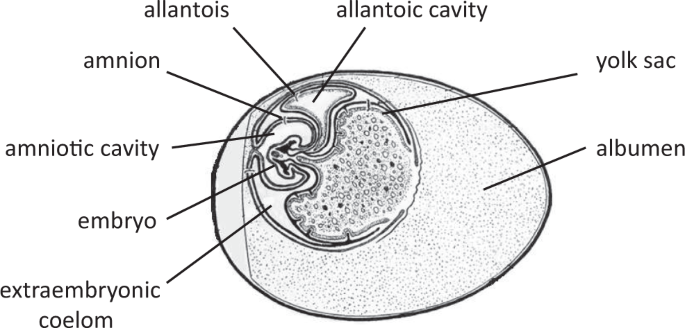
most commonly studied isoform in bird species) in the extraembryonic membranes (EMs) where maternal hormones could act without the need to reach to body tissues. The embryo produces EMs –
yolk sac, amnion, chorion, and allantois, that support embryo’s nutrition, physical protection, respiration, and excretion13, having similar functions as the fetal placenta in mammals. The
EMs are at the immediate interface of the maternal egg yolk containing the maternal hormones and the circulation of developing embryo (Fig. 1), making these a potential candidate for
mediating effects of maternal hormones on the embryo. It has been shown earlier that yolk androgens can affect AR expression in the brain of the young chick14. However, it remains unknown to
what extent such receptors are present and influenced by yolk hormones at the interface of the yolk and embryonic circulation, already early in embryonic development, before these hormones
are metabolized during the first days of incubation. Therefore, we also tested the hypothesis whether the androgen treatment could induce changes in AR and/or ERα expression in embryonic
tissues. If so, this would indicate that the embryo is an active player in the translation of the maternal signal. We assessed the effect of elevated yolk testosterone (T) and, in other
eggs, androstenedione (A4), within the physiological range of the species on AR and ERα expression in the EMs and in embryonic body tissues (the head and the decapitated body) analysed by
quantitative PCR (qPCR), using chicken eggs incubated for five days. This time-period was chosen because the gonadal differentiation15,16 and the surge of the endogenous steroid production17
in the chicken embryo starts only after this period. RESULTS AR mRNA was expressed in all three embryonic fractions: head, decapitated body, and EMs (Fig. 2a–c). It shall be noted that the
receptor expression levels are inversely related to the normalized threshold cycle (Ct) values of the qPCR procedure. There was no significant overall effect of the egg treatment on AR
expression levels (F2,48 = 0.011, p = 0.989), but there was a significant interaction effect between egg treatment and embryonic tissue (F4,48 = 3.266, p = 0.019). Tukey’s post-hoc
comparisons revealed a significant downregulation of AR expression under A4 treatment only in the EMs (p = 0.016, Table 1). ERα mRNA was also expressed in all three embryonic fractions, but
to much lower levels than AR (Fig. 2d–f). There was no significant overall effect of the egg treatment on ERα expression levels (F2,48 = 0.754, p = 0.476), and neither was any significant
interaction effect between egg treatment and embryonic tissue (F4,48 = 1.737, p = 0.157). DISCUSSION It is generally assumed that avian maternal hormones in the egg can be functional only if
they reach embryonic body tissues. Here we report that both ARs and ERs are expressed in avian EMs (Fig. 2) as early as approximately one-fourth of the entire egg incubation period until
hatching, before the embryo’s own hormone production starts15,16,17, opening up a novel, potential pathway for hormone mediated maternal effects. The EMs, particularly the yolk sac, provide
potent sites for embryonic contact with yolk contents due to their relatively larger surface area and denser blood vessel networks, compared to the embryonic body tissues (Fig. 1).
Furthermore, we found that AR expression is dependent on yolk A4 levels only in the EMs, suggesting embryonic adaptation to its exposure to maternal androgens in the egg as the EMs are right
at the interface of maternal yolk environment and embryonic circulation. The importance of the EMs for yolk hormones have also been shown by the fact that the EMs express enzymes that are
important for regulating steroid metabolism, as found in a turtle species18,19. One of the steroid metabolites is etiocholanolone, which is an androgen metabolite formed during egg
incubation10,11, and it has been suggested that etiocholanolone might influence erythropoiesis via yolk sac membrane10, but for which there is as yet no experimental evidence. There was no
effect of T treatment on AR expression in the EMs, which could simply be due to the fact that the amount of injected T was much lower than A4. There was no effect of increased T or A4 yolk
levels on the AR and ERα mRNA expression in the embryonic body tissues (Fig. 2a,b,d,e). This suggests it is unlikely that elevated concentrations of maternal yolk androgens affect offspring
phenotype by their effect on early embryonic responsiveness to its own endogenous steroids later in development (i.e. after five days of incubation). However, it should be studied further
whether the androgen treatment might affect the embryonic AR and ERα receptor expression in the body tissues at later developmental stages. The levels of the ERα mRNA expression were much
lower than the AR in all the embryonic fractions examined (Fig. 2). Though several studies have previously reported steroid receptors in avian embryonic body tissues (AR20,21, ER14,21,22,23,
progesterone receptor (PR)24,25, glucocorticoid receptor (GR)26), the data on receptors in the EMs are scarce. Two of these membranes, chorion and allantois, in combination form a tissue
lining at the inner surface of the eggshell, known as the chorioallantoic membrane. Chorioallantoic membrane tissue was found to express AR27, ER28, and PR25 in 8 to18 days old chicken
embryos, chicken embryos partly cultured in petri dishes29, as well as in reptiles27,30. However, the chorioallantoic membrane starts to develop only after day 4 and at a very slow rate31,
contributing less than 5% to the total EMs dry weight by day 532. This indicates that the high receptor expression that we found is very likely to be localized in the yolk sac membrane
itself and should be further verified. The yolk sac membrane is in a much better position than the chorioallantoic membrane for translating yolk hormones to the embryo as the chorioallantoic
membrane does not have direct access to the yolk and hence maternal hormones. The mammalian fetal placenta, an equivalent of part of the avian EMs, has also been found to express AR33,
ER34, and GR35,36,37, mediating effects of maternal condition, however their presence has always been measured at much later stages of embryonic development. That is typically at the time of
delivery with only one exception of about 55% completion of fetal development34 while we measured the receptors already at 24% of the total embryonic development period. Another
long-standing question in the field is how the gonadal sex-steroids in the egg mediate maternal effects without interfering with embryonic sexual differentiation processes38. One potential
explanation is very early embryonic metabolism of maternal steroids, i.e. prior to the critical time-window for sexual differentiation11,39. Our proposed mechanism, activating ARs in the EM
very early, provides an additional potential solution to this problem as we postulate that maternal steroids need not reach embryonic body tissues to mediate maternal effects. Furthermore,
maternal hormones could induce receptor mediated transcription long before organs that undergo sexual differentiation, such as the hypothalamus, are developed. Additionally, the activation
of the receptors in the EMs so early in the process of building a new organism and its expression not being limited to specific brain or other tissues might also explain the wide array of
maternal hormone effects observed in the literature. The location of these receptors may explain how the lipophilic hormones in the yolk that would be difficult to extract and taken up in
the embryo’s circulation, can affect the embryo. However, it remains to explore further what kind of molecular and physiological effects are elicited via AR activation in the EMs. Finally,
the receptor downregulation caused by increased yolk A4 levels indicates that the embryo can to some extent negate potential effects of elevated hormone exposure, suggesting that the embryo
is not simply a passive receiver of the mother’s signals but may play its own role in mother-offspring conflict40,41,42,43. METHODS ANIMAL ETHICS This study used five days old chicken
embryos, which does not require an ethical license or approval from an animal experimentation committee. EXPERIMENTAL DESIGN Fertilized unincubated chicken eggs of Lohman Brown Classic
strain were randomly collected from a local chicken farm, and randomly allocated to the three weight-matched treatment groups. Each egg was injected with 100 µl of sterilized sesame oil with
either 0.2 µg/ml stable isotope labelled T, or 0.58 µg/ml stable isotope labelled A4, or only oil as a control, with seven eggs per group. Stable isotope labelled androgens were used in
order to track steroid metabolism using mass spectrometry as part of another study. Due to a lack of prior studies on the effect of egg hormone treatment on the embryonic receptor
expression, it was not possible to make a reliable estimate of the effect size for sample size prediction. Therefore, a sample size of seven was chosen which is just above the minimum
required sample size to perform statistical tests. The injected hormone values were within two standard deviations of the mean yolk hormone concentrations found in our earlier study12 (T =
0.74 ± 0.13 pg mg−1; A4 = 23.24 ± 2.20 pg mg−1; means ± s.d.). The eggs were subsequently incubated for five days at 37 °C at a relative humidity of 60% in an incubator (Brinsea Ova-Easy
Advance). At the end of five days of incubation each individual embryo (of either sex) was separated into three fractions: embryonic head, decapitated body, and EMs, which were frozen at −80
°C until AR and ERα mRNA expression analysis took place. AR AND ERΑ MRNA EXPRESSION ANALYSIS The receptor mRNA expression was analysed by qPCR by a technician blind to the treatment groups.
We started with seven different embryos for each of the three treatments (T, A4, oil) per tissue type (embryonic head, decapitated body, and EMs). Tissue was homogenized (Tissue Ruptor,
Qiagen). Total RNA was isolated from deep frozen tissue according to the kit instructions (RNeasy Mini kit, Qiagen). RNA Quality was measured using Bioanalyzer 2100 (Agilent) and quantified
using Nanodrop (Peqlab), of which the descriptive statistics (average, standard deviation, and range per treatment group for each tissue type) is provided in Supplementary Table 1. Out of 63
samples, 6 did not yield sufficient RNA and thus could not be analysed further. For the remaining 57 samples, cDNA was synthesized from total RNA according to the kit instructions
(SuperScriptIII, Invitrogen) and was diluted 1:5 in water as template in qPCR experiment. Power SYBr green qPCR mastermix was used from Thermo Fisher Scientific. PCR protocol included the
following steps: denaturation at 95 °C for 30 seconds; annealing at 60 °C for 60 seconds; elongation at 72 °C for 30 seconds; cycle repeat for 35 times. The primers used are listed in Table
2. The primer efficiency was tested by a dilution series and their amplicons were sequenced (MWG Operon Eurofins Genomics). The Ct values were normalized using GenEx6 software for the
efficiency of primers, sample amount (RNA input into cDNA synthesis), qPCR repeats (duplicates), and for two reference genes – hydroxymethylbilane synthase (HMBS) and tyrosine
3-monooxygenase/tryptophan 5-monooxygenase (YWHAZ). There was no significant effect of egg treatment (F2,15.5 = 0.686, p = 0.518 for HMBS; F2,16.8 = 1.890, p = 0.182 for YWHAZ) or
interaction effect between egg treatment and embryonic tissue (F4, 30.7 = 1.319, p = 0.285 for HMBS; F4,31.5 = 0.291, p = 0.881 for YWHAZ) for either of the reference genes (data is provided
in Supplementary Table 2). All samples were run on one plate. The intra-assay coefficient of variation was 7.3% for AR and 5.5% for ERα. STATISTICS The data were analysed by linear mixed
model using IBM-SPSS (version 23) and R44 (version 3.5.3) software. The normalized Ct values were analysed for each receptor gene (AR or ERα) and reference gene (HMBS or YWHAZ) by taking the
Ct value as a dependent variable, egg treatment (three levels: oil, T, and A4) and embryonic tissue (three levels: head, decapitated body, and EMs) as well as their interaction as fixed
factors, and the embryo identity as a random factor, followed by Tukey’s post-hoc tests for multiple comparisons. DATA AVAILABILITY The datasets supporting this article are provided in the
supplementary material. REFERENCES * Brown, C. L. _et al_. Maternal triiodothyronine injections cause increases in swimbladder inflation and survival rates in larval striped bass, Morone
saxatilis. _J. Exp. Zool._ 248, 168–176 (1988). Article CAS Google Scholar * Radder, R. S. Maternally derived egg yolk steroid hormones and sex determination: review of a paradox in
reptiles. _J. Biosci._ 32, 1213–1220 (2007). Article CAS Google Scholar * von Engelhardt, N. & Groothuis, T. G. G. Maternal hormones in avian eggs. in _Hormones and Reproduction of
Vertebrates, Volume 4: Birds_ (ed. David O. Norris and Kristin H. Lopez) 91–127, https://doi.org/10.1196/annals.1343.015 (Academic Press, 2011). Article ADS CAS Google Scholar *
Groothuis, T. G. G., Müller, W., von Engelhardt, N., Carere, C. & Eising, C. Maternal hormones as a tool to adjust offspring phenotype in avian species. _Neurosci. Biobehav. Rev._ 29,
329–352 (2005). Article CAS Google Scholar * Gil, D. Hormones in avian eggs: Physiology, ecology and behavior. _Adv. Study Behav._ 38, 337–398 (2008). Article Google Scholar * Del
Giudice, M. Fetal programming by maternal stress: Insights from a conflict perspective. _Psychoneuroendocrinology_ 37, 1614–1629 (2012). Article Google Scholar * Braun, T., Challis, J. R.,
Newnham, J. P. & Sloboda, D. M. Early-life glucocorticoid exposure: The hypothalamic-pituitary-adrenal axis, placental function, and longterm disease risk. _Endocr. Rev._ 34, 885–916
(2013). Article CAS Google Scholar * Schwabl, H. Yolk is a source of maternal testosterone for developing birds. _Proc. Natl. Acad. Sci. USA_ 90, 11446–11450 (1993). Article ADS CAS
Google Scholar * Groothuis, T. G. G. & Schwabl, H. Hormone mediated maternal effects in birds: mechanisms matter but what do we know of them? _Philos. Trans. R. Soc. B-Biological Sci._
363, 1647–1661 (2008). Article CAS Google Scholar * Paitz, R. T., Bowden, R. M. & Casto, J. M. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris).
_Proc. R. Soc. B-Biological Sci._ 278, 99–106 (2011). Article CAS Google Scholar * Kumar, N., van Faassen, M., Kema, I., Gahr, M. & Groothuis, T. G. G. Early embryonic modification of
maternal hormones differs systematically among embryos of different laying order: A study in birds. _Gen. Comp. Endocrinol._ 269C, 53–59 (2018). Article Google Scholar * Kumar, N. _et
al_. Avian yolk androgens are metabolized instead of taken up by the embryo during the first days of incubation. _J. Exp. Biol. jeb_.193961, https://doi.org/10.1242/jeb.193961 (2019).
Article Google Scholar * Ferner, K. & Mess, A. Evolution and development of fetal membranes and placentation in amniote vertebrates. _Respir. Physiol. Neurobiol._ 178, 39–50 (2011).
Article Google Scholar * Pfannkuche, K. A. _et al_. Examining a pathway for hormone mediated maternal effects – Yolk testosterone affects androgen receptor expression and endogenous
testosterone production in young chicks (Gallus gallus domesticus). _Gen. Comp. Endocrinol._ 172, 487–493 (2011). Article CAS Google Scholar * Smith, C. A., Andrews, J. E. & Sinclair,
A. H. Gonadal sex differentiation in chicken embryos: Expression of estrogen receptor and aromatase genes. _J. Steroid Biochem. Mol. Biol._ 60, 295–302 (1997). Article CAS Google Scholar
* Yoshida, K., Shimada, K. & Saito, N. Expression of P450(17 alpha) hydroxylase and P450 aromatase genes in the chicken gonad before and after sexual differentiation. _Gen Comp
Endocrinol_ 102, 233–240 (1996). Article CAS Google Scholar * Woods, J. E., Simpson, R. M. & Moore, P. L. Plasma testosterone levels in the chick embryo. _Gen. Comp. Endocrinol._ 27,
543–547 (1975). Article CAS Google Scholar * Paitz, R. T. & Bowden, R. M. A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. _Integr. Comp.
Biol._ 48, 419–427 (2008). Article Google Scholar * Paitz, R. T., Duffield, K. R. & Bowden, R. M. Characterizing the distribution of steroid sulfatase during embryonic development:
when and where might metabolites of maternal steroids be reactivated? _J. Exp. Biol._ 220, 4567–4570 (2017). Article Google Scholar * Gasc, J. M., Stumpf, W. E. & Sar, M. Androgen
target cells in the pituitary of the chick embryo. _J. Steroid Biochem._ 11, 1201–1203 (1979). Article CAS Google Scholar * Endo, D., Murakami, S., Akazome, Y. & Park, M. K. Sex
difference in Ad4BP/SF-1 mRNA expression in the chick-embryo brain before gonadal sexual differentiation. _Zoolog. Sci._ 24, 877–882 (2007). Article CAS Google Scholar * Gasc, J. M.
Estrogen target cells in gonads of the chicken embryo during sexual differentiation. _J. Embryol. Exp. Morphol._ 55, 331–342 (1980). CAS PubMed Google Scholar * Andrews, J. E., Smith, C.
A. & Sinclair, A. H. Sites of estrogen receptor and aromatase expression in the chicken embryo. _Gen. Comp. Endocrinol._ 108, 182–190 (1997). Article CAS Google Scholar * Guennoun,
R., Reyssbrion, M. & Gasc, J. M. Progesterone Receptors in Hypothalamus and Pituitary during the Embryonic-Development of the Chick - Regulation by Sex Steroid-Hormones. _Dev. Brain
Res._ 37, 1–9 (1987). Article CAS Google Scholar * Albergotti, L. C., Hamlin, H. J., McCoy, M. W. & Guillette, L. J. Jr. Endocrine Activity of Extraembryonic Membranes Extends beyond
Placental Amniotes. _PLoS One_ 4, e5452–e5452 (2009). Article ADS Google Scholar * Pavlik, A., Novotna, B. & Jelinek, R. Glucocorticoid Receptor-Mediated Teratogenesis and
Cell-Proliferation in the Limbs and Face of the Chick-Embryo. _Teratog. Carcinog. Mutagen._ 6, 441–450 (1986). Article CAS Google Scholar * Griffith, O. W., Brandley, M. C., Whittington,
C. M., Belov, K. & Thompson, M. B. Comparative genomics of hormonal signaling in the chorioallantoic membrane of oviparous and viviparous amniotes. _Gen. Comp. Endocrinol._ 244, 19–29
(2017). Article CAS Google Scholar * Grzegorzewska, A. K., Lis, M. W. & Sechman, A. Immunolocalization of Leptin Receptor and mRNA Expression of Leptin and Estrogen Receptors as well
as Caspases in the Chorioallantoic Membrane (CAM) of the Chicken Embryo. _Folia Biol._ 64, 79–87 (2016). Article CAS Google Scholar * McNatt, L. G., Weimer, L., Yanni, J. & Clark, A.
F. Angiostatic Activity of Steroids in the Chick Embryo CAM and Rabbit Cornea Models of Neovascularization. _J. Ocul. Pharmacol. Ther._ 15, 413–423 (1999). Article CAS Google Scholar *
Cruze, L., Hamlin, H. J., Kohno, S., McCoy, M. W. & Guillette, L. J. Evidence of steroid hormone activity in the chorioallantoic membrane of a Turtle (Pseudemys nelsoni). _Gen. Comp.
Endocrinol._ 186, 50–57 (2013). Article CAS Google Scholar * Nowak-Sliwinska, P., Segura, T. & Iruela-Arispe, M. L. The chicken chorioallantoic membrane model in biology, medicine and
bioengineering. _Angiogenesis_ 17, 779–804 (2014). Article CAS Google Scholar * Byerly, T. Growth of the chick embryo in relation to its food supply. _J. Exp. Biol._ 9, 15–44 (1932).
Google Scholar * Hsu, T. Y. _et al_. Expression of Androgen Receptor in Human Placentas from Normal and Preeclamptic Pregnancies. _Taiwan. J. Obstet. Gynecol._ 48, 262–267 (2009). Article
Google Scholar * Kim, S. C., Park, M.-N., Lee, Y. J., Joo, J. K. & An, B.-S. Interaction of steroid receptor coactivators and estrogen receptors in the human placenta. _J. Mol.
Endocrinol._ 56, 239–247 (2016). Article CAS Google Scholar * Filiberto, A. C. _et al_. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human
placenta. _Epigenetics_ 6, 566–572 (2011). Article CAS Google Scholar * Mparmpakas, D. _et al_. Differential expression of placental glucocorticoid receptors and growth arrest-specific
transcript 5 in term and preterm pregnancies: evidence for involvement of maternal stress. _Obstet. Gynecol. Int._ 2014, 1–9 (2014). Article Google Scholar * Saif, Z. _et al_.
Identification of eight different isoforms of the glucocorticoid receptor in Guinea pig placenta: Relationship to preterm delivery, sex and betamethasone exposure. _PLoS One_ 11, e0148226
(2016). Article Google Scholar * Carere, C. & Balthazart, J. Sexual versus individual differentiation: the controversial role of avian maternal hormones. _Trends Endocrinol. Metab._
18, 73–80 (2007). Article CAS Google Scholar * Paitz, R. T. & Bowden, R. M. Progesterone metabolites, ‘xenobiotic-sensing’ nuclear receptors, and the metabolism of maternal steroids.
_Gen. Comp. Endocrinol._ 166, 217–221 (2010). Article CAS Google Scholar * Winkler, D. W. Testosterone in Egg-Yolks - an Ornithologists Perspective. _Proc. Natl. Acad. Sci. USA_ 90,
11439–11441 (1993). Article ADS CAS Google Scholar * Mock, D. W. & Forbes, L. S. Life-History Consequences of Avian Brood Reduction. _Auk_ 111, 115–123 (1994). Article Google
Scholar * Wilson, A. J. _et al_. Selection on mothers and offspring: Whose phenotype is it and does it matter? _Evolution (N. Y)._ 59, 451–463 (2005). Google Scholar * Müller, W.,
Lessells, C. M., Korsten, P. & von Engelhardt, N. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. _Am. Nat._ 169, E84–E96 (2007). Article
Google Scholar * R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/,
https://doi.org/10.1348/000712608X366867 (2013). Article Google Scholar * Patten, B. M. _The early embryology of the chick_., https://doi.org/10.5962/bhl.title.17672 (Philadelphia: P.
Blakiston, 1920). Download references ACKNOWLEDGEMENTS We thank Ido Pen and Shane Wright for discussion on statistics. This research was supported by Ubbo Emmius research grant by the
University of Groningen to TG in collaboration with the Max Planck Institute for Ornithology. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Behavioural Biology, Groningen Institute for
Evolutionary Life Sciences, University of Groningen, Groningen, The Netherlands Neeraj Kumar & Ton G. G. Groothuis * Behavioural Neurobiology, Max Planck Institute for Ornithology,
Seewiesen, Germany Neeraj Kumar, Anja Lohrentz & Manfred Gahr Authors * Neeraj Kumar View author publications You can also search for this author inPubMed Google Scholar * Anja Lohrentz
View author publications You can also search for this author inPubMed Google Scholar * Manfred Gahr View author publications You can also search for this author inPubMed Google Scholar * Ton
G. G. Groothuis View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS The work was based on a grant from the University of Groningen to T.G.
N.K., MG., and T.G. designed the details of the experiments, discussed, and interpreted the results. N.K. and A.L. performed the experiments. N.K. analysed the data and prepared the
manuscript, on which M.G. and T.G. provided feedback. All authors gave final approval for publication. CORRESPONDING AUTHOR Correspondence to Neeraj Kumar. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DATASET 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and
the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kumar, N., Lohrentz, A., Gahr, M. _et al._ Steroid receptors and their regulation
in avian extraembryonic membranes provide a novel substrate for hormone mediated maternal effects. _Sci Rep_ 9, 11501 (2019). https://doi.org/10.1038/s41598-019-48001-x Download citation *
Received: 19 September 2018 * Accepted: 03 July 2019 * Published: 08 August 2019 * DOI: https://doi.org/10.1038/s41598-019-48001-x SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative



:max_bytes(150000):strip_icc():focal(699x303:701x305)/Richard-Simmons-Teresa-Reveles-photo1.072624jpg-266074941a594c9c9320051619b4c35c.jpg)