- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The correlation between molecular orientation and optoelectrical properties is most critical to the future design of molecular materials. We made highly-anisotropic microcrystalline
array structures with an organic semiconductor, a methoxy-substituted thiophene/phenylene co-oligomer (TPCO), by depositing it on friction-transferred poly(tetrafluoroethylene) (PTFE)
layers fabricated on substrates with several heat treatments. Polarising microscope observation, polarised emission and absorption spectra measurements indicated that the TPCO molecules
aligned along the drawing direction of PTFE. Using these films, we fabricated two types of field-effect transistors (FETs) and compared them with those using non-heated TPCO films which
provide aligned pleats structures. Ones had the channel length direction parallel to the drawing direction of PTFE and the others had the channel length direction perpendicular to that
drawing direction. As for the microcrystalline array films, the mobility ratio of the former FET to that of the latter device was about 27 in the saturation region, while the emission
polarisation ratio was 4.5. The heat treatment promoted the crystal growth to enhance the mobility while retaining the high anisotropy. The results demonstrate that the heat treatments of
the TPCO films on the friction-transferred layers were useful for controlling crystallinity and orientation of the molecules. SIMILAR CONTENT BEING VIEWED BY OTHERS LARGE AREA POLYMER
SEMICONDUCTOR SUB-MICROWIRE ARRAYS BY COAXIAL FOCUSED ELECTROHYDRODYNAMIC JET PRINTING FOR HIGH-PERFORMANCE OFETS Article Open access 20 October 2022 WAFER SCALE SYNTHESIS OF ORGANIC
SEMICONDUCTOR NANOSHEETS FOR VAN DER WAALS HETEROJUNCTION DEVICES Article Open access 03 December 2021 CRYSTALLINE STRUCTURE, MOLECULAR MOTION AND PHOTOCARRIER FORMATION IN THIN FILMS OF
MONODISPERSE POLY(3-HEXYLTHIOPHENE) WITH VARIOUS MOLECULAR WEIGHTS Article 07 October 2022 INTRODUCTION Organic materials are expected to be applied to unique devices including electronic
papers and flexible displays by virtue of useful characteristics e.g. light-weight, flexible and printable. In applying the organic materials to devices, electronic and optical properties
are affected by not only the characteristics of these materials but also structural anisotropy and spatial overlap of the molecules such as crystallinity and orientation1. These are also
influenced by the quality and morphology of the organic films and the device structures2. For molecular orientation, we can use the friction-transfer technique3. This readily produces
oriented polymer films by drawing a polymer block on a substrate without using solvents and vacuum1,4,5,6,7. Since Wittmann and Smith reported friction-transferred poly(tetrafluoroethylene)
(PTFE) films in 19913, this technique was developed and widely used as the effective molecular orientation method8,9,10,11. In the cases of organic light-emitting diodes, the device
performance was improved by controlling molecular orientation for carriers to transport effectively perpendicular to the substrate7. As for organic field-effect transistors (OFETs), the
molecular orientation for the effective lateral carrier transport was preferred1. Previously we made highly oriented films of thiophene/phenylene co-oligomers (TPCOs) by depositing them on
friction-transferred PTFE layers fabricated on substrates12. Even though these TPCO films indicated the anisotropic optical and electronic properties12, it is desired to enhance the
crystallinity as well. In the present studies, we have fabricated highly-anisotropic microcrystalline array structures by applying heat treatment to orientation films of a
methoxy-substituted TPCO deposited on friction-transferred PTFE layers to enhance the crystallinity and carrier mobility while retaining the high anisotropy. We examined optical properties
of the resulting films, carried out their microscopies and measured current-voltage (_I_–_V_) characteristics of OFETs made of these films. For comparison, we also prepared the OFETs with
orientation films on the PTFE layers with or without heat treatment during TPCO deposition. RESULTS AND DISCUSSION SAMPLE PREPARATION We chose
5,5″-bis(4′-methoxybiphenyl-4-yl)-2,2′:5′,2″-terthiophene (BP3T-OMe), that is 5,5″-bis(4-biphenylyl)-2,2′:5′,2″-terthiophene (BP3T)13 substituted with methoxy groups at both molecular
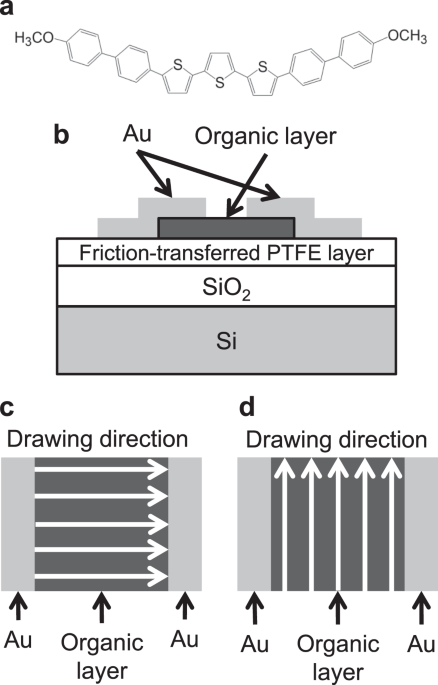
terminals. Figure 1(a) shows the structural formula of BP3T-OMe. The synthetic method is described in the Supplementary Information. We prepared friction-transferred PTFE layers on Si
substrates covered with a SiO2 layer and quartz substrates. We deposited 100-nm-thick BP3T-OMe on the untreated SiO2/Si substrate, the friction-transferred SiO2/Si substrates and the
friction-transferred quartz substrates. After film deposition, we heated untreated and friction-transferred PTFE substrates at 100, 150 or 200 °C for ~1 h. Hereafter, we call this treatment
“postheat” treatment. As another heat treatment, we deposited in a vacuum a 100-nm-thick BP3T-OMe film on the friction-transferred PTFE substrates that were preliminarily heated and kept at
70 or 100 °C in a vacuum (substrate heating). Table 1 shows a summary of the film fabrication conditions. The table includes sample numbers. We fabricated OFETs using the deposited films on
the friction-transferred SiO2/Si substrates [Fig. 1(b)]. We fabricated two types of devices where the drawing directions of PTFE were parallel and perpendicular to the channel length
direction [see Fig. 1(c,d)]. Here, the channel length direction means the direction perpendicular to the parallel edges of the Au layers (used as source and drain contacts). We also
fabricated an OFET with the BP3T-OMe film on the substrate without PTFE layer. POLARISING MICROSCOPE OBSERVATION The polarising micrographs of the BP3T-OMe film deposited on the substrates
without a PTFE layer (Sample 1) were dark at any position under the crossed Nicols [Fig. 2(a)], indicating the isotropic film. Figure 2(b,c) show polarising micrographs of the BP3T-OMe film
deposited on the friction-transferred PTFE layer without heat treatment (Sample 5). The bright lines of the film image [Fig. 2(b)] disappeared when the drawing direction accorded with the
polarising direction of the polariser or the analyser [Fig. 2(c)], indicating the molecular orientation of the film. The same molecular orientations were observed in the postheat-treated
films at 100, 150 and 200 °C and in the films deposited on the heated substrates at 70 and 100 °C (Samples 6–10) [Fig. 2(d–h)]. The postheat-treated film images [Samples 7 and 8, Fig.
2(e,f)] were dark as well as the non-polarising micrographs. X-RAY DIFFRACTION X-ray diffraction (XRD) patterns of the BP3T-OMe films deposited under the various conditions are shown in Fig.
S1 (Supplementary Information). We summarised major peak positions and plane separations in Table S1 (Supplementary Information). For Sample 1 (without a friction-transferred layer or
heat-treatment), we observed first-, third-, fourth-, fifth-, eighth- and ninth-order peaks related to a long plane separation of ~3.2 nm [marked with asterisks in Fig. S1(a), Supplementary
Information] and first-order peaks associated with short separations of ~0.46 (a triangle) and ~0.38 nm (a diamond). If the crystal system of BP3T-OMe is assumed to be monoclinic or
orthorhombic and the molecules stand against the crystal plane as shown in unsubstituted TPCOs or methoxy-substituted TPCOs14,15,16,17, ~3.2 nm is a half of the distance between the planes
composed of the crystallographic short and intermediate axes. This distance agrees with the calculated molecular length of BP3T-OMe (3.6 nm). The short separations ~0.38 and ~0.46 nm
correspond to the length of the crystallographic intermediate axis and the distance between the hypotenuse and the point of the right angle made by the crystallographic short and
intermediate axes, respectively. The results indicated that the BP3T-OMe molecules both stood and lay down on the substrate in Sample 1. As for Sample 5 (with a friction-transferred layer
without heat-treatment), the weak first-order peak associated with the separation ~3.2 nm was observed at 2_θ_ = 2.77°. The peak associated with ~0.38 nm (2_θ_ = 23.60°) increased [see Fig.
S1(b), Supplementary Information] compared with that in Sample 1. This showed that the proportion of the molecules lying down (standing) on the substrate increased (decreased) and that the
lying-down molecules were dominant in Sample 5. Although the same was observed in the postheat-treated films and the films deposited on the heated substrates [see Fig. S1(c,e–g),
Supplementary Information], the peak associated with ~0.38 nm disappeared and the peak at 2_θ_ = 28.05° became noticeable in Sample 7 [postheat-treated at 150 °C, Fig. S1(d), Supplementary
Information]. This suggests that the molecular reorientation occurred at around 150 °C in the postheat-treated films. In Sample 8, the diffraction peak associated with ~0.38 nm was dominant
at 2_θ_ = 23.54° [Fig. S1(e), Supplementary Information]. This indicates that the molecules were aligned so that the crystallographic intermediate axis stood against the substrate surface.
This crystal alignment was clarified by scanning electron microscope (SEM) observation (vide infra). POLARISED EMISSION SPECTRA The polarised emission spectra are shown in Fig. S2
(Supplementary Information). For all films, the parallel emissions relative to the drawing direction were larger than the perpendicular ones. This suggests that the direction of transition
dipole moment (the molecular long axis) of BP3T-OMe was in agreement with the drawing direction. We calculated their intensity ratios of the parallel component to the perpendicular one
around the major peak positions and summarise them in Table S2 (Supplementary Information). Sample 5 showed peaks at 573 and 615 nm [see Fig. S2(a), Supplementary Information] and the ratios
were 2.7 and 2.5, respectively. For the postheat-treated films at 100 (Sample 6) and 150 °C (Sample 7) [Fig. S2(b,c), Supplementary Information) these ratios decreased to 1.7 and 2.4 at 590
and 592 nm, respectively. Sample 8 [postheat-treated at 200 °C, see Fig. S2(d), Supplementary Information] showed the ratio 4.5 at 591 nm. This value was ~1.7 times larger than that of
Sample 5. As for the films deposited on the heated substrates, Sample 9 [at 70 °C, see Fig. S2(e), Supplementary Information] showed the ratio of 5.0 at 572 nm and Sample 10 [at 100 °C, see
Fig. S2(f), Supplementary Information] indicated 4.9 at 571 nm and a maximum of 5.2 at 614 nm. The value 5.2 was almost twice as large as that of Sample 5. This indicates that the BP3T-OMe
molecular long axis was highly oriented in the drawing direction in Sample 10. Among the samples, Samples 8–10 show the higher polarisation ratios ~5 (see Table S2, Supplementary
Information). This indicates that the molecules were well-aligned in these films. POLARISING ABSORPTION SPECTRA Figure 3 shows the polarised absorption spectra. The spectrum of Sample 1
(isotropic film) showed a maximum peak at ~360 nm [Fig. 3(a)]. In Samples 5 and 6 [Fig. 3(b,c)] the parallel components _A_// of absorption spectrum relative to the drawing direction showed
a maximum peak at ~360 nm and these maxima decreased in the perpendicular component _A_⊥. For the postheat-treated films at 150 (Sample 7) and 200 °C (Sample 8) [Fig. 3(d,e)], the absorption
bands at longer wavelength increased and the peak observed in Samples 5 and 6 around 360 nm became flat. These were related to the dark polarising micrograph images of the postheat-treated
films [Fig. 2(e,f)]. From the polarised absorption spectra, we estimated the dichroic ratios _D_ and the degrees of orientation _F_ and summarise the results in Table 2. Of all the samples,
Sample 10 [Fig. 3(g)] indicated the maximum _D_ = 5.4 and _F_ = 0.59 at 355 nm, indicating highly molecular orientation along the drawing direction. SEM OBSERVATION Figure 4 shows the SEM
images of the friction-transferred PTFE film and the BP3T-OMe films deposited on the untreated substrate (Sample 1), the friction-transferred substrates without heat treatment (Sample 5),
postheat-treated at 200 °C (Sample 8) and under substrate heating at 100 °C (Sample 10). Friction-transferred PTFE showed many straight lines on the substrate [Fig. 4(a)], as shown in the
micrograph in our previous study12. The height of PTFE was ~10 nm [see Fig. 4(b)]. In Samples 1 and 5, BP3T-OMe films were composed of pleats [Fig. 4(c,d)]. Sample 5 indicated
longitudinally-aligned pleats that were laterally stretched [Fig. 4(d)]. The friction-transferred PTFE lines were suggested to be under these aligned pleats because the alignment of the
pleats was parallel to the drawing direction. In Sample 8, the substrate surface was composed of longitudinally aligned lines made of horizontally long blocks [Fig. 4(e)]. Cross-section
observation of Sample 8 indicated that these blocks were hexagonal cylinders standing on the substrate [Fig. 4(f)]. These hexagonal shapes mean the crystal growth through the postheat
treatment18,19 and suggest that the crystallographic intermediate axis stood against the substrate surface, as indicated in the XRD measurements. From the point of view of molecular
orientation, the molecules were most highly oriented in the film of Sample 8. Figure 4(g) indicates that these hexagonal cylinders were closely aligned along the drawing direction. This may
affect the carrier mobility along this direction (vide infra). Although larger pleats were observed in Sample 10 [Fig. 4(h)] than in Samples 1 and 5 [Fig. 4(c,d)], no aligned pleats were
observed. The similar film morphologies composed of pleats in Samples 5 and 10 [Fig. 4(d,h)] may result in the similar XRD patterns [see Fig. S1(b,g), Supplementary Information] and the
similar absorbance spectra [see Fig. 3(b,g)]. Figure 4(i,j) compare the cross-sections of Sample 10 cut along the perpendicular and parallel directions relative to the drawing direction,
respectively. Although both the cross-section images show similar film morphology, the pleats seemed to spread along the drawing direction compared to the direction perpendicular to the
drawing direction. This may also affect the carrier mobility as in the case with Sample 8. Another possible reason why Sample 10 showed the higher anisotropic optoelectrical properties
(Tables 2, 3 and S2) despite no apparent aligned pleats is as follows: In the case of substrate heating, molecular alignment may improve at the location closer to the substrate rather than
to the top surface along the film thickness direction. This is because the deposited molecules closer to the substrate were heated for a longer time. MOLECULAR ORIENTATION From the results
of polarising microscope observation, XRD and optical measurements and SEM observation, Fig. 5(a–c) schematically summarise molecular orientation of BP3T-OMe on the substrates. BP3T-OMe
deposition on an untreated substrate (without friction transfer or heat treatment) produced clusters in which the molecules either stood or lay down against the substrate [Fig. 5(a)]. The
lying molecules are predominant in clusters of the BP3T-OMe layer deposited on a friction-transferred substrate [Fig. 5(b)]. In the layer, the longer direction of the BP3T-OMe molecules is
parallel to the drawing direction of PTFE. The aggregates of the clusters were supposed to form the pleats [see Fig. 4(d,h)]. Meanwhile, postheat treatment of the deposited BP3T-OMe layer on
a friction-transferred substrate gave aligned hexagonal crystals along the transferred PTFE [Fig. 5(c)]. The longer direction of the molecules in the crystal is parallel to the drawing
direction. In TPCO crystals, the longer direction of molecules usually rises on the hexagonal surface, and the transition dipole moments are large along this direction20. The crystals
absorbed most of light polarised parallel to the longer direction of molecules. The dark polarising micrographs [Fig. 2(e,f)] and the flat absorption spectra [Fig. 3(d,e)] of Samples 7 and 8
are attributed to the strong light absorption caused by the high degree of molecular orientation21,22. Figure 5(d) shows incident-angle-dependent polarised absorption spectra of Sample 8.
The rotation axis was perpendicular to the three directions of light traveling, light polarisation and PTFE drawing. At 0°, the drawing direction was parallel to the light polarisation
direction. With deviating the polarisation direction of light from 0° to 50°, the absorption spectra over 500 nm decreased, coming close to _A_⊥ [see Fig. 3(e)]. OFET CHARACTERISTICS The
output characteristics are shown in Fig. S3 (Supplementary Information) for OFETs made from Samples 1–10. All OFETs indicated well-defined p-type conduction. We estimated carrier mobilities
in the linear and saturation regions and summarise them in Table 3 and Fig. S5(a,b) (Supplementary Information). In the table, devices were indicated by sample number (integer) and the
drawing direction relative to the channel length direction (parallel “//” or perpendicular “⊥”). For some devices with the PTFE layer friction-transferred along ⊥ direction, it is difficult
to estimate the accurate mobility from the linear regions due to the disordered current increases around the origin. Hence, we used the mobility in the saturation regions for a fair
comparison. The OFETs with postheat-treated films showed the following tendency. For the OFETs without friction-transferred PTFE layers (Devices 2–4), the mobility increased up to 2.6 × 10−2
cm2 V−1 s−1 in the saturation region (Device 4) with the postheat treatment temperature. However, the postheat treated sample at 100 °C (Device 2) showed the lower mobility than the
unheated one (Device 1). The same was observed in the OFETs with the PTFE layer friction-transferred along // direction (Devices 5-//, 6-//, 7-// and 8-//). In contrast, the mobility was
almost independent with the postheat treatment temperature for the OFETs with the PTFE friction-transferred along ⊥ direction (Devices 5-⊥, 6-⊥, 7-⊥ and 8-⊥). For substrate heating, the
mobilities of both Devices 9-// (1.0 × 10−2 cm2 V−1 s−1) and 10-// (2.2 × 10−2 cm2 V−1 s−1) showed similar values to that of Device 8-// (1.4 × 10−2 cm2 V−1 s−1). The mobility of Device 5-//
(7.9 × 10−3 cm2 V−1 s−1 in the saturation region) was larger than that of Device 5-⊥ (3.9 × 10−4 cm2 V−1 s−1), as shown in Devices 6–10, but smaller than that of Device 1 (1.4 × 10−2 cm2
V−1 s−1). The mobility ratio of Device 5-// to Device 5-⊥ is ~20, indicating the high anisotropy. The increases in the drain current from the origin were observed in the devices having the
channel length direction parallel to the drawing direction [see Fig. S3(f,h,j,l,n,p), Supplementary Information]. This indicates the good electrical contact between the Au electrodes and the
BP3T-OMe film. Among the devices, Devices 8-// and 10-// indicated the larger mobilities, and the latter device indicated the largest mobility of 2.2 × 10−2 cm2 V−1 s−1 in the saturation
region. This value was almost 1.5 times as large as that of Device 1 (1.4 × 10−2 cm2 V−1 s−1) and ~67 times larger than that of Device 10-⊥ (3.3 × 10−4 cm2 V−1 s−1). In the case of Sample 8,
the crystals were closely aligned along the drawing direction [Fig. 4(g)]. This makes the mobility along the drawing direction larger. This may cause ~27 times larger mobility of Device
8-// (1.4 × 10−2 cm2 V−1 s−1 in the saturation region) than that of Device 8-⊥ (5.1 × 10−4 cm2 V−1 s−1). Different from Samples 5 and 8, longitudinally aligned lines composed of
laterally-stretched pleats or horizontally long blocks were not observed in Sample 10 [see Fig. 4(d,e,h)]. However, the largest polarisation ratio of the emissions [Fig. S2(f) and Table S2,
Supplementary Information] and degree of orientation (Table 2) were also observed along with the largest mobility in Sample 10. This suggests that Sample 10 had the highly molecular
orientation which was not directly observed by microscopic observation. As seen in Fig. 4(j), the continuously spread pleats along the drawing direction caused these results. Meanwhile, one
possible reason that the mobilities were smaller along the direction perpendicular to the drawing direction than parallel to that direction is that the channel was interrupted by the
discontinuous separation between adjacent alignments of pleats or hexagonal crystals. Device 8-// showed the slightly smaller mobility (1.4 × 10−2 cm2 V−1 s−1) than Device 10-// (2.2 × 10−2
cm2 V−1 s−1) in the saturation region, even though the molecules seemed to be highly aligned in Sample 8 than in Sample 10 (Tables 2 and S2). The slightly smaller mobility may be caused by
chasms between the crystal blocks in Sample 8 [Fig. 4(e)]. These chasms might obstruct carrier transfer in the direction parallel to the channel. In contrast, the pleats seem to be in close
contact with each other in Sample 10 [Fig. 4(h)]. We also evaluated the transfer characteristics of the devices in Fig. S4 (Supplementary Information) to examine the effect of the
friction-transferred PTFE layer to interface characteristics of the channel layer. The OFETs with the friction-transferred PTFE layer showed anisotropy in on/off ratio and subthreshold slope
[Table 3 and Fig. S5(c,d)]. Among Devices 1–8, the on/off ratio and subthreshold slope were improved in all postheat-treated OFETs (Devices 2–4 and 6–8) up to 2010 and 9.1 V dec−1 (Device
3), respectively. In samples with substrate heating (Devices 9 and 10), the higher temperature (Device 10-//) was more effective to improve both on/off ratio (1926) and subthreshold slope
(10.7 V dec−1), which are comparable with the best results (Device 3). These results indicate that, although insertion of a friction-transferred layer lowers the mobility, the heat
treatments not only recover the mobility but also improve the on/off ratio and subthreshold slope. On the whole, the postheat-treated OFET at 200 °C (Device 8-//) and the OFET with substrate
heating at 100 °C (Device 10-//) showed the better performance than that without a friction-transferred film or heat treatment (Device 1). CONCLUSION In the present studies, we made
highly-anisotropic microcrystalline array structures through thermal crystal growth of BP3T-OMe orientation films on friction-transferred PTFE layers. We heated substrates after BP3T-OMe
deposition or deposited it on the heated substrates. The orientation film without heat treatment was also prepared. We examined optical properties and morphology of the resulting films. The
BP3T-OMe molecules lying-down on the friction-transferred substrate were dominant and their molecular long axis was in agreement with the drawing direction. The non-heated films showed
longitudinally-aligned pleats structure. On the other hand, the microcrystalline array structures which were highly-aligned along the drawing direction were observed when the substrate was
heated at 200 °C after BP3T-OMe deposition (“postheat” treatment). The BP3T-OMe film deposited on the heated substrate at 100 °C (substrate heating) provided no aligned pleats structures but
intriguingly indicated the highest degree of orientation. Using these films, we fabricated OFETs. The OFETs having the channel length direction parallel to the drawing direction of PTFE
showed a linear increase in the drain current from the origin, indicating the good electrical contact between the BP3T-OMe film and electrodes. The OFET with microcrystalline array films
(non-heated films) indicated the mobility 1.4 × 10−2 cm2 V−1 s−1 (7.9 × 10−3 cm2 V−1 s−1) in the saturation region. This value was almost 27 times (20 times) larger than the corresponding
value 5.1 × 10−4 cm2 V−1 s−1 (3.9 × 10−4 cm2 V−1 s−1) of the OFET having the channel length direction perpendicular to the drawing direction. Even though the films treated with substrate
heating had no aligned pleats structures, its OFET indicated the highest mobility and anisotropy. The results demonstrate that the heat treatments enhanced the crystallinity and carrier
mobility of the BP3T-OMe orientation films on the friction-transferred PTFE substrates while retaining the high anisotropy, showing usefulness of the heat treatments for controlling
crystallinity and orientation of TPCO molecules. We expect the microcrystalline array structures to be utilised in organic solar cells due to the high crystallinity, the strong light
absorption property and the convex-concave surface patterns associated with an interpenetration structure. METHODS FABRICATION OF FRICTION-TRANSFERRED PTFE LAYER We used Si substrates (10 mm
× 10 mm) covered with an SiO2 layer (300 nm in thickness) and quartz substrates (10 mm × 10 mm). We cleaned them sequentially in acetone, 2-propanol, ethanol and distilled water with an
ultrasonic cleaner for 10 min each and dried them for ~30 min in an oven. Its temperature was set at 120 °C. We prepared a PTFE sheet (10 mm in width, 40 mm in length and 0.8 mm in
thickness), folded it in half and made its crease flat by polishing with sandpaper. We pressed the half fold PTFE sheet with a compression pressure of 1.5 kgf cm−2 onto the substrate that
was preliminarily heated at 150 °C, and mechanically rubbed the sheet once at a rate of 60 mm min−1 12,23. The resulting friction-transferred area was ~8 mm × 8 mm. The micrograph and XRD
pattern of the transferred PTFE layer were described in the literature12. The aligned PTFE polymer chains lay horizontally on the substrate12. ORIENTED TPCO FILMS FABRICATION We used an
Ulvac vacuum coater VPC-260F and deposited 100-nm-thick BP3T-OMe films on the substrates with a deposition rate of 0.02 nm s−1 in the vacuum of ~10−3 Pa through a handmade mask (hole size:
~8 mm × 10 mm). We determined the thickness by using an Ulvac deposition controller CRTM-6000 with a quartz oscillator. For postheat treatment, we used a hot plate and controlled the
temperature of its top panel. The postheat treatment was carried out in a nitrogen atmosphere to prevent material thermal oxidation. For substrate heating, we used a heating unit of
VPC-260F. We observed macroscopic morphology and orientation of the BP3T-OMe films with a Nikon Eclipse LV100 POL polarising microscope and a Rigaku RINT 2500 X-ray diffractometer,
respectively. We measured diffractions (_θ_/2_θ_-scans) in the angle range 2_θ_ = 2–60° using a Cu-Kα X-ray source (wavelength: 0.15418 nm). We observed microscopic film morphology using a
JEOL JSM-7001F scanning electron microscope. For the emission measurements, we used a Hamamatsu Photonics PMA-11 photonic multichannel analyser using the same setup as before12,20. We
irradiated ultraviolet excitation light (330–380 nm) through an objective lens (magnification was 50 or 100) of Eclipse LV100 POL perpendicular to the BP3T-OMe film surface and measured
emission perpendicular to that surface. We measured parallel and perpendicular components of the emissions to the drawing direction by placing a polariser between the BP3T-OMe film and
PMA-11. We measured the polarised light absorption of the BP3T-OMe films using a Shimadzu UV-3600 UV-VIS-NIR spectrophotometer. For this, we used the BP3T-OMe films deposited on the
friction-transferred quartz substrates with various heat treatments (see Table 1). Two polarisers were located between the light source and the film and between the film and the detector and
their polarising directions were set parallel to each other. We irradiated monochromatic light (wavelength range: 300–600 nm or 300–900 nm) perpendicular to the substrate and measured
polarised absorption along the direction both parallel and perpendicular to the drawing direction. We estimated a dichroic ratio _D_ = _A_///_A_⊥ and a degree of orientation _F_ = (_D_ −
1)/(_D_ + 2) from absorbance components _A_// and _A_⊥ polarised along the directions parallel and perpendicular to the drawing direction, respectively. As an option, we measured
incident-angle-dependent polarised absorption spectra for Sample 8. We manually rotated the sample on a handmade holder to vary an incident angle of the light from the normal (defined as 0°)
to 50° in 10° step. OFET FABRICATION AND _I_–_V_ CHARACTERISTICS We fabricated OFETs with the BP3T-OMe films deposited on the friction transferred SiO2/Si substrates with various heat
treatments (see Table 1). We formed source and drain contacts (area: ~1 mm × 4 mm) by depositing 200-nm-thick Au layers with a deposition rate of 0.15 nm s−1 over a tungsten wire (50 μm in
diameter) to make a channel of the OFET. The channel lengths and widths were ranged ~19–50 μm and ~0.8–1.1 mm, respectively. We carried out _I_–_V_ characteristics measurements using a
Keithley 4200-SCS semiconductor characterisation system in a vacuum (~10−3 Pa) in the dark. We measured drain currents _I_D with one of Au electrodes grounded as the source contact24 and
applied direct current (DC) voltages _V_DS ranging from 10 to −60 V (or 10 to −100 V) to the other Au electrode (used as the drain contact) with various DC gate voltages _V_G from 10 to −60
V (or 10 to −100 V) as a parameter. We obtained the output characteristics from the _I_–_V_ measurements, and then, transfer curves were constructed based on the output characteristics data.
We estimated carrier mobilities _µ_ in the linear and saturation regions by the following equations25: $${I}_{{\rm{D}}}=\frac{W}{L}\mu
{C}_{{\rm{i}}}({V}_{{\rm{G}}}-{V}_{{\rm{th}}}){V}_{{\rm{DS}}}$$ (1) $${I}_{{\rm{D}}}=\frac{W}{2L}\mu {C}_{{\rm{i}}}{({V}_{{\rm{G}}}-{V}_{{\rm{th}}})}^{2}$$ (2) where _W_ is the channel
width, _L_ is the channel length, _C_i is the insulator capacitance (per unit area), and the _V_th is the threshold voltage. For the calculations, we neglected the effects of the PTFE layer
because this layer was much thinner (~10 nm, vide supra) than SiO2 (300 nm). We also calculated the on/off ratio and subthreshold slope of the devices from the transfer curves at _V_DS = −60
V. The on/off ratio was calculated by dividing maximum _I_D by minimum _I_D. The subthreshold slope was determined as an absolute value of the reciprocal value of a transfer curve slope in
a subthreshold region, where the vertical axis is log(_I_D) and the horizontal axis is _V_G. QUANTUM CHEMICAL CALCULATION We estimated the molecular length of BP3T-OMe from the atomic
geometry optimised by a molecular orbital (MO) calculation using the Gaussian 09 program26 with the B3LYP method and the 6–31 G(d) basis set. The molecular length was defined by the distance
between the terminal hydrogens of the molecule [see Fig. 1(a)]. The length includes the van der Waals radius of a hydrogen atom (1.2 Å)27. REFERENCES * Hosokawa, Y. _et al_. Molecular
orientation and anisotropic carrier mobility in poorly soluble polythiophene thin films. _Appl. Phys. Lett._ 100, 203305 (2012). Article ADS Google Scholar * Dimitrakopoulos, C. D. &
Malenfant, P. R. L. Organic Thin Film Transistors for Large Area Electronics. _Adv. Mater._ 14, 99–117 (2002). Article CAS Google Scholar * Wittmann, J. C. & Smith, P. Highly oriented
thin films of poly(tetrafluoroethylene) as a substrate for oriented growth of materials. _Nature_ 352, 414–417 (1991). Article ADS CAS Google Scholar * Nagamatsu, S. _et al_. Polymer
field-effect transistors by a drawing method. _Appl. Phys. Lett._ 84, 4608–4610 (2004). Article ADS CAS Google Scholar * Misaki, M. _et al_. Highly polarized polymer light-emitting
diodes utilizing friction-transferred poly(9,9-dioctylfluorene) thin films. _Appl. Phys. Lett._ 87, 243503 (2005). Article ADS Google Scholar * Nagamatsu, S. _et al_. Crystal Structure of
Friction-Transferred Poly(2,5-dioctyloxy-1,4-phenylenevinylene). _J. Phys. Chem. B_ 111, 4349–4354 (2007). Article CAS Google Scholar * Misaki, M. _et al_. Highly efficient polarized
polymer light-emitting diodes utilizing oriented films of _β_-phase poly(9,9-dioctylfluorene). _Appl. Phys. Lett._ 93, 023304 (2008). Article ADS Google Scholar * Gill, R. E.,
Hadziioannou, G., Lang, P., Garnier, F. & Wittmann, J. C. Highly oriented thin films of a substituted oligo(_para_-phenylenevinylene) on friction-transferred PTFE substrates. _Adv.
Mater._ 9, 331–334 (1997). Article CAS Google Scholar * Chen, X. L., Bao, Z., Sapjeta, B. J., Lovinger, A. J. & Crone, B. Polarized Electroluminescence from Aligned Chromophores by
the Friction Transfer Method. _Adv. Mater._ 12, 344–347 (2000). Article CAS Google Scholar * Yoshida, Y., Tanigaki, N., Yase, K. & Hotta, S. Color-Tunable Highly Polarized Emissions
from Uniaxially Aligned Thin Films of Thiophene/Phenylene Co-oligomers. _Adv. Mater._ 12, 1587–1591 (2000). Article CAS Google Scholar * Nagamatsu, S. _et al_. Multi-Layered Oriented
Polyfluorene Films. _J. Phys. Chem. B_ 113, 5746–5751 (2009). Article CAS Google Scholar * Kouda, M., Hirase, R., Yamao, T., Hotta, S. & Yoshida, Y. Orientation-controlled films of
thiophene/phenylene co-oligomers. _IEICE Trans. Electron._ E98C, 73–79 (2015). Article ADS Google Scholar * Hotta, S., Kimura, H., Lee, S. A. & Tamaki, T. Synthesis of
Thiophene/Phenylene Co-oligomers. II. Block and Alternating Co-oligomers. _J. Heterocycl. Chem._ 37, 281–286 (2000). Article CAS Google Scholar * Hotta, S. & Goto, M. Crystal
Structure Analysis of 2,5-Bis(4-biphenylyl)thiophene. _Adv. Mater._ 14, 498–501 (2002). Article CAS Google Scholar * Hotta, S. _et al_. Crystal Structures of Thiophene/Phenylene
Co-Oligomers with Different Molecular Shapes. _Chem. Mater._ 16, 237–241 (2004). Article CAS Google Scholar * Hotta, S., Goto, M. & Azumi, R. Peculiar Crystal Structure of a
Thiophene/Phenylene Co-oligomer of 2,5-Bis(4′-methoxybiphenyl-4-yl)thiophene. _Chem. Lett._ 36, 270–271 (2007). Article CAS Google Scholar * Mizuno, H. _et al_. Single Crystals of
5,5′-Bis(4′-methoxybiphenyl-4-yl)-2,2′-bithiophene for Organic Laser Media. _Adv. Mater._ 24, 5744–5749 (2012). Article CAS Google Scholar * Yamao, T. _et al_. Direct Formation of Thin
Single Crystals of Organic Semiconductors onto a Substrate. _Chem. Mater._ 19, 3748–3753 (2007). Article CAS Google Scholar * Yamao, T., Juri, K., Kamoi, A. & Hotta, S. Field-effect
transistors based on organic single crystals grown by an improved vapor phase method. _Org. Electron._ 10, 1241–1247 (2009). Article CAS Google Scholar * Yamao, T. _et al_. Polarized
emissions from single crystals of thiophene/phenylene co-oligomers measured by microspectroscopy. _Jpn. J. Appl. Phys._ 47, 4719–4723 (2008). Article ADS CAS Google Scholar * Lee, S. A.,
Yoshida, Y., Fukuyama, M. & Hotta, S. Phenyl-capped oligothiophenes: Novel light-emitting materials with different molecular alignments in thin films. _Synth. Met._ 106, 39–43 (1999).
Article CAS Google Scholar * Hotta, S., Ichino, Y., Yoshida, Y. & Yoshida, M. Spectroscopic features of thin films of thiophene/phenylene co-oligomers with vertical molecular
alignment. _J. Phys. Chem. B_ 104, 10316–10320 (2000). Article CAS Google Scholar * Yamao, T., Akagami, H., Nishimoto, Y., Hotta, S. & Yoshida, Y. Improved device performance of
organic crystal field-effect transistors fabricated on friction-transferred substrates. _J. Nanosci. Nanotechnol._ 9, 6271–6276 (2009). Article CAS Google Scholar * Sze, S. M. _Physics of
Semiconductor Devices_. (John Wiley & Sons, Inc., 1981). * Horowitz, G. Organic Field-Effect Transistors. _Adv. Mater._ 10, 365–377 (1998). Article CAS Google Scholar * Gaussian 09
Revision B.01 (Gaussian, Inc., Wallingford, CT, 2009). * Bondi, A. van der Waals Volumes and Radii. _J. Phys. Chem._ 68, 441–451 (1964). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by Grant-in-Aid for Scientific Research A (Grant No. 25248045) from the Japan Society for the Promotion of Science (JSPS) and The Iwatani Naoji
Foundation’s Research Grant. AUTHOR INFORMATION Author notes * Yuhi Inada and Takeshi Yamao contributed equally. AUTHORS AND AFFILIATIONS * Faculty of Materials Science and Engineering,
Kyoto Institute of Technology, Kyoto, 606-8585, Japan Yuhi Inada, Masashi Koda, Yuji Urabe, Takeshi Yamao & Shu Hotta * Sumitomo Seika Chemicals Co., Ltd., Harima, Hyogo, 675-0145, Japan
Toshifumi Katagiri * National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, 305-8565, Japan Yuji Yoshida Authors * Yuhi Inada View author publications You can
also search for this author inPubMed Google Scholar * Masashi Koda View author publications You can also search for this author inPubMed Google Scholar * Yuji Urabe View author publications
You can also search for this author inPubMed Google Scholar * Toshifumi Katagiri View author publications You can also search for this author inPubMed Google Scholar * Takeshi Yamao View
author publications You can also search for this author inPubMed Google Scholar * Yuji Yoshida View author publications You can also search for this author inPubMed Google Scholar * Shu
Hotta View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.I., T.Y. and S.H. designed the study, Y.I. and T.Y. wrote the paper, Y.I., M.K.,
Y.U., T.K. and T.Y. conducted the experiments and analysed the results, Y.Y. supported the experiment for friction-transfer technique. CORRESPONDING AUTHORS Correspondence to Yuhi Inada or
Takeshi Yamao. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Inada, Y., Koda, M., Urabe, Y. _et al._ Microcrystalline array
structures induced by heat treatment of friction-transferred organic semiconductor films. _Sci Rep_ 9, 9739 (2019). https://doi.org/10.1038/s41598-019-46212-w Download citation * Received:
04 January 2019 * Accepted: 18 June 2019 * Published: 05 July 2019 * DOI: https://doi.org/10.1038/s41598-019-46212-w SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative




