- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In patients with chronic kidney disease (CKD), reverse left ventricular (LV) remodelling, including reduction in LV mass, can be observed following long-term haemodialysis (HD) and
has been attributed to regression of LV hypertrophy. However, LV mass can vary in response to changes in myocyte volume, edema, or fibrosis. The aims of this study were to investigate the
acute changes in structural (myocardial mass and biventricular volumes) and tissue characterization parameters (native T1 and T2) following HD using cardiovascular magnetic resonance (CMR).
Twenty-five stable HD patients underwent non-contrast CMR including volumetric assessment and native T1 and T2 mapping immediately pre- and post-HD. The mean time between the first and
second scan was 9.1 ± 1.1 hours and mean time from completion of dialysis to the second scan was 3.5 ± 1.3 hours. Post-HD, there was reduction in LV mass (pre-dialysis 98.9 ± 36.9 g/m2 vs
post-dialysis 93.3 ± 35.8 g/m2, p = 0.003), which correlated with change in body weight (r = 0.717, p < 0.001). Both native T1 and T2 reduced significantly following HD (Native T1:
pre-dialysis 1085 ± 43 ms, post-dialysis 1072 ± 43 ms; T2: pre-dialysis 53.3 ± 3.0 ms, post-dialysis 51.8 ± 3.1 ms, both p < 0.05). These changes presumably reflect acute reduction in
myocardial water content rather than regression of LV hypertrophy. CMR with multiparametric mapping is a promising tool to assess the cardiac changes associated with HD. SIMILAR CONTENT
BEING VIEWED BY OTHERS QUANTIFICATION OF EXTRACELLULAR VOLUME FRACTION BY CARDIAC COMPUTED TOMOGRAPHY FOR NONINVASIVE ASSESSMENT OF MYOCARDIAL FIBROSIS IN HEMODIALYSIS PATIENTS Article Open
access 21 September 2020 CHARACTERIZING MYOCARDIAL EDEMA AND FIBROSIS IN HYPERTENSIVE CRISIS WITH CARDIOVASCULAR MAGNETIC RESONANCE IMAGING Article Open access 09 October 2024 IMPAIRED
AORTIC STRAIN AND DISTENSIBILITY BY CARDIAC MRI IN CHILDREN WITH CHRONIC KIDNEY DISEASE Article Open access 30 June 2022 INTRODUCTION Patients with end-stage renal failure (ESRF) have
increased risk of cardiovascular morbidity and mortality, and around 50% of all deaths in patients on haemodialysis (HD) are due to cardiovascular disease1. Left ventricular hypertrophy
(LVH) and left ventricular cavity dilatation are common findings in patients with ESRF and are associated with increased mortality and cardiac arrhythmias2,3,4. It has been demonstrated that
daily HD when compared to traditional thrice weekly dialysis results in significant reduction in LV mass5,6. However, interventions to reduce left ventricular (LV) mass do not appear to
translate to a reduction in mortality7. It has been suggested that increased afterload and preload in patients with chronic kidney disease (CKD) results in myocardial fibrosis and myocyte
cell hypertrophy, and that regression in LVH following chronic HD is due to reduction in both fibrosis and myocyte cell volume8. Post-mortem and endomyocardial biopsy studies have both
demonstrated the presence of myocardial fibrosis in patients on HD9,10. Greater myocardial extracellular water content may also cause increase in LV mass but the contribution and effects of
this in patients with CKD have not previously been investigated. Cardiovascular magnetic resonance (CMR) is now established as the gold standard for quantification of ventricular volumes,
mass and function11 but it can also provide unique information on tissue composition. CMR with late gadolinium enhancement is uniquely informative for the detection and quantification of
myocardial fibrosis in a variety of conditions but the use of gadolinium contrast in patients with ESRF is constrained by concerns about the risk of nephrogenic systemic fibrosis12. However,
contemporary T1 and T2 mapping methods can surmount some of the shortcomings of non-contrast CMR. Native T1, measured in milliseconds (ms), is increased when the interstitial space is
expanded, for example by fibrosis or amyloid deposition, or edema13,14,15,16,17. T2 measurements provide an estimate of free tissue water content. For example, T2 is elevated during acute
myocarditis and following acute myocardial infarction, and it is proposed that T2 is elevated in the presence of myocardial edema irrespective of myocardial fibrosis14,17. The aims of this
study were (1) To assess the changes in myocardial volumes and mass pre- and post-HD and (2) To assess the changes in native T1 and myocardial T2 pre- and post-HD. METHODS Twenty-five
patients established on thrice weekly HD were recruited at the Royal Free Hospital, London, United Kingdom between October 2017 and June 2018. All participants provided written informed
consent. Ethical approval was obtained from the Joint University College London/University College London Hospitals Research Ethics Committee (REC reference: 07/H0715/101). All
research-related procedures were performed in accordance with local guidelines and regulations. EXCLUSION CRITERIA Patients with standard contraindications to non-contrast CMR (e.g.
implanted pacemaker, intra-cranial coils, severe claustrophobia, inability to lie flat) were excluded. CMR PROTOCOL All CMR scans were performed at 1.5 T (Magnetom Aera, Siemens Healthcare,
Erlangen, Germany). Patients underwent non-contrast CMR before and then again immediately after a haemodialysis session. Patients were weighed immediately before each CMR scan. A standard
protocol was used that included localizers, cine imaging, native T1 mapping and T2 mapping. Cine imaging (long-axis and short axis stack covering the entire ventricles) was performed using
electrocardiographic (ECG)-gated breath-hold steady state free precession (SSFP) or Realtime where the patient was unable to breath-hold or in the presence of arrhythmia. For native T1 and
T2 mapping, basal, mid and apical short axis, and a 4-chamber long-axis were acquired after regional shimming. T1 mapping used a Siemens research works-in-progress sequence (WIP1041B) that
acquired images using a modified look-locker inversion recovery (MOLLI) protocol using a 5 s (3 s) 3 s sampling scheme: 2 inversions, with images acquired each heartbeat for 5 sec following
the 1st inversion, 3 sec recovery, and images acquired for 3 sec following a 2nd inversion18. Typical protocol parameters for T1 mapping were: matrix 256 × 144, field-of-view 360 × 270 mm2,
spatial resolution 1.4 × 1.9 mm2, slice thickness 8 mm, flip angle 35 degrees, bandwidth 1085 Hz/pixel, echo spacing 2.7 ms. T2 mapping used the Siemens MyoMap sequence which acquired 3 T2
weighted measurements and performed an exponential fit for each pixel after respiratory motion correction. The imaging used a T2-prepared single shot b-SSFP readout with T2 preparation times
(TE) = 0, 25, and 55 ms with a recovery period of 3 heartbeats between measurements. Typical protocol parameters for T2 mapping were: matrix 192 × 108, field-of-view 360 × 270 mm2, spatial
resolution 1.9 × 2.5 mm2, slice thickness 8 mm, flip angle 70 degrees, bandwidth 1184 Hz/pixel, echo spacing 2.6 ms. To assess intra-scan reproducibility, all native T1 and T2 maps were
repeated after at least a 5-minute interval in 7 patient scans. CMR IMAGE ANALYSIS All images were analysed offline using Osirix MD 9.0 (Bernex, Switzerland). The endo- and epicardial
borders were manually delineated for each basal-, mid-ventricular and apical short axis map. Obvious image artefacts and coronary arteries were excluded from the regions of interest. Native
T1 and T2 were averaged over all three short-axis slices to calculate global averages. Initial analysis of the maps was performed by TK. All mapping images were then independently reanalysed
by AMN who was blinded to the initial analysis to assess inter-observer variability. Ventricular cavity volumes and mass were calculated by tracing epicardial and endocardial borders on
each end-diastolic short axis cine and endocardial borders on each end-systolic cine. Papillary muscles and trabeculations were included as part of the ventricular mass. STATISTICAL ANALYSIS
Normally distributed metrics are summarized as mean ± standard deviation and non-normally distributed data are expressed as median (interquartile range, IQR). The paired Student’s T-test
was used when comparing parameters between pre- and post-dialysis scans. Correlations between continuous variables were evaluated using the Pearson’s correlation co-efficient. Inter-observer
and intra-study reproducibility were assessed using intraclass correlation co-efficient (ICC) with 95% confidence intervals (CI), coefficient of variance (CoV) and Bland-Altman analysis
(expressed as bias ± 2 SD for limits of agreement). Reproducibility analysis was performed using MedCalc 13.2.1.0 (Ostend, Belgium). All other statistical analysis was performed using IBM
SPSS statistics version 24 (IBM, Somers, New York). RESULTS Twenty-five HD patients (mean age 64 ± 16 years, 17(68%) male) underwent pre- and post-dialysis scans. Baseline characteristics
are summarised in Table 1 and full CMR data for each subject is included in the Supplementary File. The mean time between the first and second scan was 9.1 ± 1.1 hours and mean time from
completion of dialysis to the second scan was 3.5 ± 1.3 hours. Mean duration of the dialysis session between scans was 3.1 ± 0.8 hours. The median volume of fluid removed in the dialysis
session was 2.0 litres (IQR 0.4–2.2 litres), with 4 patients having no net removal of fluid. The mean reduction in weight was 1.5 ± 1.1 kg (Pre-dialysis 76.6 ± 21.8 kg vs Post-dialysis 75.1
± 21.5 kg, p < 0.001) and mean reduction in body surface area (BSA) 0.02 ± 0.01 m2 (Pre-dialysis 1.88 ± 0.282 vs Post-dialysis 1.86 ± 0.28 m2, p < 0.001). VENTRICULAR VOLUMES AND MASS
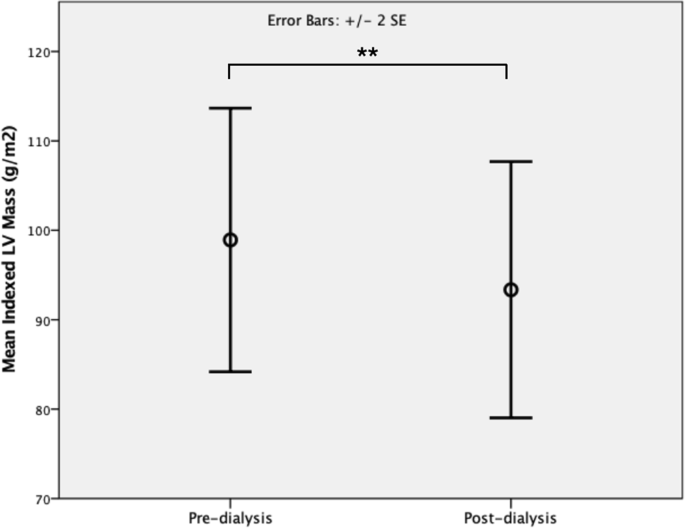
Following HD, there was significant reduction in indexed LV mass (pre-dialysis 98.9 ± 36.9 g/m2 vs post-dialysis 93.3 ± 35.8 g/m2, p = 0.003, Fig. 1) but no significant change in
biventricular volumes (Table 2). There was good correlation between change in indexed LV mass and change in body weight (r = 0.717, p < 0.001) (Fig. 2). In the cohort overall, there was
no significant change in LVEF following HD (pre-HD 54.5 ± 16.5% vs post-HD 56.0 ± 14.4%, p = 0.149). However, in patients with impaired pre-HD LV function (LVEF <45%, n = 7) there was
significant improvement in systolic function post-HD (change in LVEF: impaired LV function group + 5.4 ± 5.6% vs preserved LV function group −0.1 ± 3.8%, p = 0.01) (Fig. 3). There was no
significant change post-HD in RV systolic function or ventricular volumes in patients with impaired LV function compared to those without impaired LV function. NATIVE T1 AND MYOCARDIAL T2
Both Native T1 and myocardial T2 were significantly lower post-HD compared to pre-HD (Native T1: pre-HD 1085 ± 43 ms, post-HD 1072 ± 43 ms, p = 0.043 and T2: pre-HD 53.3 ± 3.0 ms, post-HD
51.8 ± 3.1 ms, p = 0.006) (Figs 4 and 5). The inter-observer reproducibility for both T1 (ICC 0.984 (0.972–0.991), CoV 0.55% (0.44–0.66%), bias 2.4 ms) and T2 mapping (ICC 0.982
(0.968–0.990), CoV 1.32% (1.05–1.59%), bias 0.75 ms) was excellent (Fig. 6). Intra-study reproducibility was also excellent for both T1 (ICC 0.987 (0.927–0.998), CoV 0.36% (0.13–0.60), bias
1.9 ms) and T2 (ICC 0.980 (0.888–0.997), CoV 1.02% (0.35–1.69), bias −0.12 ms). DISCUSSION We demonstrate using contemporary non-contrast CMR techniques that a typical, approximately 3-hour
session of HD is associated with significant acute changes in cardiac structure and tissue characterisation parameters. These include reduction in LV mass, T1 and T2, with change in LV mass
showing a strong correlation with change in body weight. The changes evident on CMR are likely to reflect acute changes in the patients’ overall fluid status, resulting in reduction of
pre-load and myocardial edema. With the ongoing development of non-contrast CMR mapping, some of the limitations of fibrosis imaging in patients on HD seem to have been overcome. Native T1
has emerged as a potentially useful tool to assess myocardial fibrosis and has been shown to have good correlation with fibrosis as assessed on histology19,20. Native T1 is elevated in HD
patients compared to controls and the elevation has been attributed to myocardial fibrosis21. However other mechanisms can contribute to the increase in native T1, such as the presence of
myocardial edema. Patients on HD are exposed to frequent and rapid alterations in fluid status which may be associated with some variable degree of myocardial edema and therefore affect the
native T1 signal. T1 elevation in these patients is likely to be due to fibrosis, myocardial edema or a combination of both. However, the acute reduction in native T1 and T2 following HD is
in keeping with the hypothesis that some of the elevation in T1 is related to myocardial edema and that HD results in some reduction of the tissue free water content. In terms of structural
changes following HD, there was a significant reduction in LV mass. This reduction correlated well with reduction in body weight following the dialysis session, a surrogate marker for the
amount of fluid removed. This observation reinforces the hypothesis that as fluid accumulates between HD sessions, this results in myocardial edema, and that the removal of intravascular
fluid during dialysis results in redistribution of this fluid from the myocardium back onto the intravascular compartment. Several studies have reported reduction in LV mass several months
after commencing HD and this has been attributed to regression of myocyte volume or fibrosis4,5,6. The elevation and subsequent reduction of LV mass coupled with the reduction in native T1
and T2 acutely following HD suggests that myocardial edema is the main cause for this reduction and that the elevation in native T1 is a composite of myocardial fibrosis, a well-recognised
phenomenon in chronic renal failure9 and myocardial edema. Persistent myocardial edema has a detrimental effect on the myocardium eventually resulting in interstitial fibrosis22. It has been
demonstrated that over two-thirds of HD patients with LVEF <40% have improvement in LV function to >50% following renal transplant23. We propose that the fluid shifts associated with
HD provide a recurrent insult to the myocardium resulting in impairment in function. These fluids shifts are removed following renal transplantation. Future CMR mapping studies pre- and
post-transplant could provide further insight regarding the mechanisms of this insult. Furthermore, myocardial edema could be one of the mechanisms contributing to the poor prognosis in HD
patients, that is not currently explained by the traditional risk factors for cardiovascular disease. The T2 mapping sequence used in this study was the single shot based SSFP method which
is part of the SIEMENS MyoMap product, and the T1 mapping sequence was a works-in-progress MOLLI sequence (WIP 1041B) that allowed for a more flexible sampling strategy with acquisition and
recovery periods defined in seconds to further reduce heart rate variability. The technical characteristics of these sequences and comparisons to other methods and protocols have been
described previously24,25. The T1 mapping approach used in the WIP is based on the original published MOLLI design18 with modification of the sampling strategy for reduced heart rate
dependence and inversion pulse design for improved inversion efficiency. The inversion recovery based MOLLI approach was chosen over methods employing saturation recovery since it is the
most widely available sequence and has improved image quality and precision26. The T1 mapping WIP output included both T1 parametric maps as well as estimate standard deviation (SD) maps of
precision27. The mean SD for the native T1 protocol is approximately 30 ms (per pixel). The product T2 mapping sequence did not provide a SD estimate or goodness of fit, however estimates of
the SD for the T2 mapping protocols used in this study are 2–3 ms (per pixel). The precision of the region of interest measurements is improved several-fold over the pixel wise SD depending
on the number of pixels averaged. The inter-observer and intra-study repeatability of both T1 and T2 were excellent and comparable to previously published data28,29,30. LIMITATIONS This was
a small single centre study but is the first to use CMR to assess cardiac changes acutely following dialysis. Dialysis patients included had different aetiologies for their renal failure.
Due to the sample size, the effects of this and other potential confounders such as age, gender, hypertension and diabetes were not investigated. In summary, our findings provide useful
information about the acute effects of HD on cardiac function and tissue changes. It is the first to study patients using CMR pre- and post-dialysis sessions demonstrating reduction in LV
mass following HD which we attribute to reduction in myocardial edema. Our data suggest that T1 and T2 both fall acutely following a dialysis session and this has implications when dialysis
patients undergo repeated follow up scans. When tracking longer term changes (for example over months or years) we would suggest that scans be performed at the same timepoint in their weekly
dialysis schedule (for example on the non-dialysis day following the mid-week dialysis session if serial non-contrast studies are performed) and both T1 and T2 should be measured, together
with standard structural parameters, to better assess changes in myocardial tissue composition. Furthermore, the acute changes in T1, T2 and LV mass could potentially be used to tailor the
HD session to the individual patient, but this would require further large-scale studies. DATA AVAILABILITY The CMR dataset generated and analysed during the current study is provided in the
Supplementary material. Other study-related data is available from the corresponding authors upon reasonable request. REFERENCES * de Jager, D. J. _et al_. Cardiovascular and
noncardiovascular mortality among patients starting dialysis. _JAMA_ 302, 1782–1789, https://doi.org/10.1001/jama.2009.1488 (2009). Article PubMed Google Scholar * Parfrey, P. S. _et al_.
Outcome and risk factors for left ventricular disorders in chronic uraemia. _Nephrol Dial Transplant_ 11, 1277–1285 (1996). Article CAS Google Scholar * Stack, A. G. & Saran, R.
Clinical correlates and mortality impact of left ventricular hypertrophy among new ESRD patients in the United States. _Am J Kidney Dis_ 40, 1202–1210,
https://doi.org/10.1053/ajkd.2002.36881 (2002). Article PubMed Google Scholar * London, G. M. Cardiovascular disease in chronic renal failure: pathophysiologic aspects. _Semin Dial_ 16,
85–94 (2003). Article Google Scholar * Chertow, G. M. _et al_. In-center hemodialysis six times per week versus three times per week. _N Engl J Med_ 363, 2287–2300,
https://doi.org/10.1056/NEJMoa1001593 (2010). Article CAS PubMed Google Scholar * Chan, C. T., Floras, J. S., Miller, J. A., Richardson, R. M. & Pierratos, A. Regression of left
ventricular hypertrophy after conversion to nocturnal hemodialysis. _Kidney Int_ 61, 2235–2239, https://doi.org/10.1046/j.1523-1755.2002.00362.x (2002). Article PubMed Google Scholar *
Badve, S. V. _et al_. The Validity of Left Ventricular Mass as a Surrogate End Point for All-Cause and Cardiovascular Mortality Outcomes in People With CKD: A Systematic Review and
Meta-analysis. _Am J Kidney Dis_ 68, 554–563, https://doi.org/10.1053/j.ajkd.2016.03.418 (2016). Article PubMed Google Scholar * Glassock, R. J., Pecoits-Filho, R. & Barberato, S. H.
Left ventricular mass in chronic kidney disease and ESRD. _Clin J Am Soc Nephrol_ 4(Suppl 1), S79–91, https://doi.org/10.2215/CJN.04860709 (2009). Article PubMed Google Scholar * Mall,
G., Huther, W., Schneider, J., Lundin, P. & Ritz, E. Diffuse intermyocardiocytic fibrosis in uraemic patients. _Nephrol Dial Transplant_ 5, 39–44 (1990). Article CAS Google Scholar *
Aoki, J. _et al_. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. _Kidney Int_ 67, 333–340, https://doi.org/10.1111/j.1523-1755.2005.00086.x
(2005). Article PubMed Google Scholar * Kramer, C. M. _et al_. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board
of trustees task force on standardized protocols. _J Cardiovasc Magn Reson_ 10, 35, https://doi.org/10.1186/1532-429X-10-35 (2008). Article PubMed PubMed Central Google Scholar *
Kribben, A. _et al_. Nephrogenic systemic fibrosis: pathogenesis, diagnosis, and therapy. _J Am Coll Cardiol_ 53, 1621–1628, https://doi.org/10.1016/j.jacc.2008.12.061 (2009). Article CAS
PubMed Google Scholar * Banypersad, S. M. _et al_. T1 mapping and survival in systemic light-chain amyloidosis. _Eur Heart J_ 36, 244–251, https://doi.org/10.1093/eurheartj/ehu444 (2015).
Article PubMed Google Scholar * Bulluck, H. _et al_. T1 mapping and T2 mapping at 3T for quantifying the area-at-risk in reperfused STEMI patients. _J Cardiovasc Magn Reson_ 17, 73,
https://doi.org/10.1186/s12968-015-0173-6 (2015). Article PubMed PubMed Central Google Scholar * Fontana, M. _et al_. Native T1 mapping in transthyretin amyloidosis. _JACC Cardiovasc
Imaging_ 7, 157–165, https://doi.org/10.1016/j.jcmg.2013.10.008 (2014). Article PubMed Google Scholar * Maestrini, V., Treibel, T. A., White, S. K., Fontana, M. & Moon, J. C. T1
Mapping for Characterization of Intracellular and Extracellular Myocardial Diseases in Heart Failure. _Curr Cardiovasc Imaging Rep_ 7, 9287, https://doi.org/10.1007/s12410-014-9287-8 (2014).
Article PubMed PubMed Central Google Scholar * Lurz, P. _et al_. Comprehensive Cardiac Magnetic Resonance Imaging in Patients With Suspected Myocarditis: The MyoRacer-Trial. _J Am Coll
Cardiol_ 67, 1800–1811, https://doi.org/10.1016/j.jacc.2016.02.013 (2016). Article PubMed Google Scholar * Messroghli, D. R. _et al_. Modified Look-Locker inversion recovery (MOLLI) for
high-resolution T1 mapping of the heart. _Magn Reson Med_ 52, 141–146, https://doi.org/10.1002/mrm.20110 (2004). Article PubMed Google Scholar * Bull, S. _et al_. Human non-contrast T1
values and correlation with histology in diffuse fibrosis. _Heart_ 99, 932–937, https://doi.org/10.1136/heartjnl-2012-303052 (2013). Article PubMed PubMed Central Google Scholar *
Sibley, C. T. _et al_. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. _Radiology_ 265, 724–732, https://doi.org/10.1148/radiol.12112721 (2012). Article
PubMed PubMed Central Google Scholar * Rutherford, E. _et al_. Defining myocardial tissue abnormalities in end-stage renal failure with cardiac magnetic resonance imaging using native T1
mapping. _Kidney Int_ 90, 845–852, https://doi.org/10.1016/j.kint.2016.06.014 (2016). Article PubMed PubMed Central Google Scholar * Davis, K. L. _et al_. Effects of myocardial edema on
the development of myocardial interstitial fibrosis. _Microcirculation_ 7, 269–280 (2000). Article CAS Google Scholar * Wali, R. K. _et al_. Effect of kidney transplantation on left
ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. _J Am Coll Cardiol_ 45, 1051–1060, https://doi.org/10.1016/j.jacc.2004.11.061 (2005).
Article PubMed Google Scholar * Kellman, P. & Hansen, M. S. T1-mapping in the heart: accuracy and precision. _J Cardiovasc Magn Reson_ 16, 2, https://doi.org/10.1186/1532-429X-16-2
(2014). Article PubMed PubMed Central Google Scholar * Kellman, P. & Hansen, M. In _Cardiovascular MRI, 3rd Edition_ (eds WJ Manning & D Pennell) 15–29 (Elsevier, 2018). *
Weingärtner, S. _et al_. Myocardial T. _J Cardiovasc Magn Reson_ 18, 84, https://doi.org/10.1186/s12968-016-0302-x (2016). Article PubMed PubMed Central Google Scholar * Kellman, P.,
Arai, A. E. & Xue, H. T1 and extracellular volume mapping in the heart: estimation of error maps and the influence of noise on precision. _J Cardiovasc Magn Reson_ 15, 56,
https://doi.org/10.1186/1532-429X-15-56 (2013). Article PubMed PubMed Central Google Scholar * Pica, S. _et al_. Reproducibility of native myocardial T1 mapping in the assessment of
Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. _J Cardiovasc Magn Reson_ 16, 99, https://doi.org/10.1186/s12968-014-0099-4 (2014).
Article PubMed PubMed Central Google Scholar * Graham-Brown, M. P. _et al_. Native T1 mapping: inter-study, inter-observer and inter-center reproducibility in hemodialysis patients. _J
Cardiovasc Magn Reson_ 19, 21, https://doi.org/10.1186/s12968-017-0337-7 (2017). Article PubMed PubMed Central Google Scholar * Roy, C. _et al_. Age and sex corrected normal reference
values of T1, T2 T2* and ECV in healthy subjects at 3T CMR. _J Cardiovasc Magn Reson_ 19, 72, https://doi.org/10.1186/s12968-017-0371-5 (2017). Article PubMed PubMed Central Google
Scholar Download references ACKNOWLEDGEMENTS This study was supported by the National Amyloidosis Centre, University College London and the National Institute for Health Research University
College London Hospitals Biomedical Research Centre. AUTHOR INFORMATION Author notes * Tushar Kotecha and Ana Martinez-Naharro contributed equally. AUTHORS AND AFFILIATIONS * Institute of
Cardiovascular Science, University College London, London, UK Tushar Kotecha, Daniel S. Knight, Vivek Muthurangu & Roby D. Rakhit * Royal Free Hospital, London, UK Tushar Kotecha, Ana
Martinez-Naharro, Suree Yoowannakul, Tabitha Lambe, Tamer Rezk, Daniel S. Knight, Philip N. Hawkins, Roby D. Rakhit, Julian D. Gillmore, Paramjit Jeetley, Andrew Davenport & Marianna
Fontana * Division of Medicine, University College London, London, UK Ana Martinez-Naharro, Tamer Rezk, Philip N. Hawkins, Julian D. Gillmore & Marianna Fontana * Barts Heart Centre,
London, UK James C. Moon * National Heart, Lung and Blood Institute, National Institute of Health, Bethesda, Maryland, USA Peter Kellman Authors * Tushar Kotecha View author publications You
can also search for this author inPubMed Google Scholar * Ana Martinez-Naharro View author publications You can also search for this author inPubMed Google Scholar * Suree Yoowannakul View
author publications You can also search for this author inPubMed Google Scholar * Tabitha Lambe View author publications You can also search for this author inPubMed Google Scholar * Tamer
Rezk View author publications You can also search for this author inPubMed Google Scholar * Daniel S. Knight View author publications You can also search for this author inPubMed Google
Scholar * Philip N. Hawkins View author publications You can also search for this author inPubMed Google Scholar * James C. Moon View author publications You can also search for this author
inPubMed Google Scholar * Vivek Muthurangu View author publications You can also search for this author inPubMed Google Scholar * Peter Kellman View author publications You can also search
for this author inPubMed Google Scholar * Roby D. Rakhit View author publications You can also search for this author inPubMed Google Scholar * Julian D. Gillmore View author publications
You can also search for this author inPubMed Google Scholar * Paramjit Jeetley View author publications You can also search for this author inPubMed Google Scholar * Andrew Davenport View
author publications You can also search for this author inPubMed Google Scholar * Marianna Fontana View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS All the authors reviewed the manuscript. T.K., A.M.N., S.Y. and A.D. were involved in patient recruitment and CMR acquisition. T.K., A.M.N., T.L., D.S.K. and M.F. performed
image analysis. T.K. and A.M.N. performed statistical analysis. T.K., A.M.N., T.R., P.K., P.J., A.D. and M.F. prepared the manuscript. D.K., P.N.H., J.C.M., V.M., R.D.R. and J.D.G.
critically reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Marianna Fontana. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION DATASET RIGHTS
AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in
any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kotecha, T.,
Martinez-Naharro, A., Yoowannakul, S. _et al._ Acute changes in cardiac structural and tissue characterisation parameters following haemodialysis measured using cardiovascular magnetic
resonance. _Sci Rep_ 9, 1388 (2019). https://doi.org/10.1038/s41598-018-37845-4 Download citation * Received: 24 September 2018 * Accepted: 10 December 2018 * Published: 04 February 2019 *
DOI: https://doi.org/10.1038/s41598-018-37845-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








