- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Emitting ultrasound upon hearing an attacking bat is an effective defence strategy used by several moth taxa. Here we reveal how _Yponomeuta_ moths acquire sophisticated acoustic
protection despite being deaf themselves and hence unable to respond to bat attacks. Instead, flying _Yponomeuta_ produce bursts of ultrasonic clicks perpetually; a striated patch in their
hind wing clicks as the beating wing rotates and bends. This wing structure is strikingly similar to the thorax tymbals with which arctiine moths produce their anti-bat sounds. And indeed,
_Yponomeuta_ sounds closely mimic such arctiine signals, revealing convergence in form and function. Because both moth taxa contain noxious compounds, we conclude they are mutual Müllerian
acoustic mimics. _Yponomeuta_’s perpetual clicking would however also attract bat predators. In response, their click amplitude is reduced and affords acoustic protection just as far as
required, matching the distance over which bat biosonar would pick up _Yponomeuta_ echoes anyway – advanced acoustic defences for a deaf moth. SIMILAR CONTENT BEING VIEWED BY OTHERS SILENCE
AND REDUCED ECHOLOCATION DURING FLIGHT ARE ASSOCIATED WITH SOCIAL BEHAVIORS IN MALE HOARY BATS (_LASIURUS CINEREUS_) Article Open access 20 September 2021 FLEXIBLE CONTROL OF VOCAL TIMING IN
_CAROLLIA PERSPICILLATA_ BATS ENABLES ESCAPE FROM ACOUSTIC INTERFERENCE Article Open access 13 November 2023 ECHOLOCATION AT HIGH INTENSITY IMPOSES METABOLIC COSTS ON FLYING BATS Article 13
July 2020 INTRODUCTION Bats and moths have been involved in a 65-million-year evolutionary arms race since the advent of biosonar in Chiroptera1. As a result, moths have evolved a plethora
of defences against their chiropteran adversaries. In addition to hearing structures tuned to the echolocation frequencies of sympatric bats2,3 providing an early warning system and allowing
time for evasive manoeuvres, sound production as a defence against bats has evolved independently in at least three moth families4,5,6. Many bat species detect and localise prey by the
sounds they generate7,8, so sound production is only adaptive when it creates protection with the sounds startling attacking bats, warning them of a (chemical) defence or jamming their
biosonar9,10,11. These sounds’ acoustic properties such as duty cycle and number of clicks may be used to classify them by function1,4. In addition to the anti-bat sounds of some moth
species, others produce sound as a courtship song12 or for territory defence13. Several moth taxa produce sound by stridulation5,14,15,16, however, the majority do so with tymbals17: thin
areas of cuticle, almost exclusively on the body, backed by an air cavity, which are buckled by a dedicated muscle. It is this buckling that produces sounds, generally ultrasonic clicks18.
Alternative sound producing structures in moths are modified genital structures5,14,19, percussive ‘castanets’20,21, and tymbals placed in the tegula22 or forewing23. _Yponomeuta_ Latreille,
[1796] (Lepidoptera; Yponomeutidae) is a genus of likely over 100 species (Agassiz, D. Personal Communication, Mar 2018) of small (‘microlepidoptera’) to medium sized, mostly nocturnal
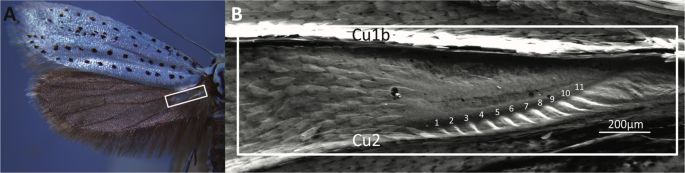
moths24,25, characterised by the presence of a translucent patch devoid of scales at the hindwing base between Cu1b and Cu2 veins (Fig. 1)26. Such a patch is also known from related genera
of the subfamily Yponomeutinae, _Teinoptila_, _Ptiloteina_, _Trisophista_, _Eumonopyta_27,28. Agassiz27 found that these translucent patches contain a row of ridges adjacent to the Cu2 vein
(Fig. 1B), and proposed sound production as their function by stridulation, naming the structure itself a _stridularium_. Very little is known about the evolutionary (acoustic) arms race
between bats and the ‘microlepidoptera’, let alone _Yponomeuta_ specifically29,30, though one observation exists of _Yponomeuta evonymella_ and _Y. padella_ producing ultrasound during
flight in the field31. In this study we investigate the acoustic ecology of some Yponomeutinae and address the following questions: firstly, is the translucent patch a sound producing
structure, and if so, what are the acoustic properties of the sounds it produces? Secondly, if the translucent patch is a sound producing structure, how does it function? And finally, what
is the adaptive value of any sound produced by these moths, in particular with respect to the acoustic arms race with bats as auditory specialist predators? RESULTS _YPONOMEUTA_ PRODUCE
ULTRASONIC CLICKS IN FLIGHT USING THEIR TRANSLUCENT PATCHES We recorded _Yponomeuta evonymella_ and _Y. cagnagella_ in free and tethered flight. All tested individuals (15 tethered and two
free flying _Y. evonymella_ and nine tethered _Y. cagnagella_) produced two bursts of a similar number of broadband ultrasonic clicks for every wingbeat cycle (Fig. 2). One burst was
produced at the beginning of the upstroke (lower burst) and the other at its end (upper burst), with the clicks emitted in a more rapid succession during the former. The number of clicks per
burst appears to be similar to the number of striations on the translucent patch. In _Y. evonymella_ the mean number of clicks per burst was 12.6 ± 1.7 (mean ± SD, n = 14) and the number of
striations was 11 + (Fig. 1B). Note however that these recordings are a superposition of the click bursts created by the two hindwings, as proven by almost identical sounds recorded with
microphones on either side of the moth. We removed both tymbals (area 260 × 800 µm; see Fig. 1) in 12 tethered _Y. evonymella_, recorded their flight sounds pre- and post-ablation, and
determined the number of clicks produced per 100 ms (about three wingbeat cycles) as this is the duration used in the literature for calculating parameters such as maximum duty cycle4.
Post-ablation, seven individuals produced no clicks, the eighth individual produced one click, and the remaining four produced fewer clicks with lower amplitudes. Microscopic examination
showed that in these four individuals ablation of the translucent patch had been incomplete, so these were excluded from further analysis. A paired-samples t-test revealed a highly
significant difference (n = 8, _t_(7) = 20.3, _p_ < 0.001) between the two treatments. _YPONOMEUTA_ DO NOT RESPOND TO ULTRASOUND Twenty _Y. evonymella_ and four _Y. cagnagella_ were used
in hearing experiments. While in flight, no individual of either species reacted to the playback of an ultrasonic pulse known to elicit reactions of moths possessing ultrasonic hearing32.
There was no flight cessation, or even alteration in flight direction. The 20 _Y. evonymella_ individuals were exposed to the stimulus while resting as a group within a flight cage, as were
the four _Y. cagnagella_, in a separate cage. None of these resting individuals showed any response, such as twitching, movement cessation, or flight initiation to ultrasound playback. Ten
_Y. evonymella_ were also left in a flight cage and their responses to each other observed. As with the playbacks, no individuals showed any change in resting behaviour in response to
take-off or flight, and therefore sound production, of any other moth. SOUND PRODUCTION DOES NOT INVOLVE STRIDULATION BUT WING MOTION High-speed infrared videos of _Y. evonymella_ and _Y.
cagnagella_ in tethered flight revealed that there was no contact of any body part (potentially serving as scraper) with the translucent patch during sound production or any other phase of
the wingbeat cycle. So _Yponomeuta_ do not produce sound by stridulation. Instead, clicks exclusively and always occur while the hindwing rotates (pronates or supinates) along its
base-to-tip axis during the upper and lower turning phases of a wing stroke (Fig. 2 and see Supplementary Video S1). More detailed analysis showed that during supination at the beginning of
the upstroke the posterior anal and jugal areas of the hindwing fold downwards relative to its anterior remigium, along what is likely the claval furrow. This folding progresses from the tip
to the base of the wing including the translucent patch, and its folding coincides with the production of the lower click burst (see Supplementary Video S2). During pronation at the top of
the upstroke the upper click burst is produced, but no equally obvious folding of the hindwing occurs. DURATION, SPECTRUM, SOURCE LEVEL, DIRECTIONALITY, DETECTION DISTANCE AND DUTY CYCLE OF
CLICKS Ten clicks recorded laterally (90°) from each of 14 _Y. evonymella_ and nine _Y. cagnagella_ were analysed for duration, temporal, amplitude and spectral parameters (Table 1). To
measure horizontal emission directionality eight individuals (only six for 45°) were recorded from 0°, 45°, 90°, and 180° and five clicks analysed each (n = 150). In terms of the horizontal
directionality of _Y. evonymella_ clicks, pairwise comparisons following a nested ANOVA (n = 264, F(1,3) = 7145.475, p < 0.001) showed that the sounds recorded laterally were
significantly louder than those recorded at the three other angles (0° and 90°, Z = 6.6, p < 0.001, 45° and 90°, Z = 5.8, p < 0.001, and 180° and 90°, Z = −9.0, p < 0.001) (Fig. 3).
There were no differences between the other three orientations (0° and 45°, Z = 0.3, p = 1.0, 0° and 180°, Z = −2.3, p = 0.13, 45° and 180°, Z = −2.4, p = 0.11) (Fig. 3). Mean calculated
distances over which bats can detect these clicks were 6.0 ± 0.4 m (n = 8, 40) at 0°, 6.5 ± 0.4 m (n = 6, 30) at 45°, 7.9 ± 0.7 m at 90°, and 5.6 ± 0.4 m at 180° (n = 8, 40) (Fig. 4B). ECHO
DETECTION DISTANCES OF _YPONOMEUTA_ BY BAT BIOSONAR Echoes of five _Y. evonymella_ were measured to determine over what distances they would be detectable to the biosonar of insectivorous
bats. Spectral target strength (the signal amplitude reflected back to the receiver compared to the incident amplitude at each frequency) ranged between −35 and −43 dB at all frequencies
between 20 and 160 kHz (Fig. 4A). Total target strength was highest when the moth was at 90° to the bat corresponding to a mean echo detection distance of 7.1 ± 1.1 m (n = 5) for frequencies
between 20–30 kHz, and at its lowest when it was 177° to the bat with a mean echo detection distance of 4.3 ± 0.8 m (n = 5) at these frequencies (Fig. 4B). DISCUSSION THE TRANSLUCENT
HINDWING PATCHES OF _YPONOMEUTA_ LIKELY ACT AS BUCKLING TYMBALS Several lines of evidence corroborate the translucent patch as a buckling tymbal. First, translucent patch structure is
strikingly convergent to the ultrasound emitting tymbals found on the thorax of many arctiine moths18,33. In both, similarly sized thin areas of cuticle, with air on either side, consist of
a larger smooth area (window) with a series of parallel striations of increasing length (band of microtymbals) running alongside it (Fig. 1). In arctiines, an inward muscular pull buckles
the microtymbals in sequence and creates a burst of individual clicks. When the muscle relaxes, elastic forces buckle the microtymbals back in the reverse order creating a similar second
burst of clicks18,34. The peak frequency of individual clicks increases during one burst and then decreases in the other, which is in agreement with the reverse order of buckling in and out
(Fig. S4). Almost identically, _Yponomeuta_ sounds also consist of two alternating click bursts with concurrent increases and decreases in individual click peak frequencies (Fig. 2A;
Supplementary Audio S3, Fig. S4). Additionally, the mean number of clicks per burst (Table 1) is just above the number of striations (Fig. 1). Note though that the tymbals in the two
hindwings operate in parallel, thereby theoretically creating twice as many clicks per burst than there are striations. Our recordings show that the moth body does not cast an effective
sound shadow such that the clicks from both hindwings reach to either side. We propose that the observed mean number of clicks per burst is only about half the theoretical maximum for both
wings combined because many clicks will coincide between sides, some neighbouring striations might buckle together, and some clicks may be too faint to be detected amongst other louder
clicks. In summary, we conclude that the striations act as microtymbals, and that they are convergent in structure and mechanism to the sound production by microtymbals of arctiines. It can
even be speculated that the tymbal deformations leading to microtymbal buckling might be similar. The actuation mechanisms creating these tymbal deformations are however fundamentally
different. In arctiines, direct muscle actuation deforms the tymbal, while in _Yponomeuta_, flight muscles at the base of the wing are the actuators, and the tymbal is deformed by the
rotation and aeroelastic folding of the hindwing along the claval furrow (directly adjacent to the microtymbals of the translucent patch) during the wingbeat cycle. Because the actuation of
the translucent patch is due to aerodynamic forces and the aeroelastic properties of the wing, we are terming it the ‘aeroelastic tymbal’. The evidence supports that the claval furrow is
integral to the actuation of tymbal buckling, the exact biomechanical buckling mechanism is still unclear though. _YPONOMEUTA_ ACOUSTICALLY MIMIC ARCTIINE ANTI-BAT WARNING SOUNDS Both
species of _Yponomeuta_ produced two bursts of ultrasonic clicks similar to those of the Arctiinae, with peak frequencies within both the hearing range of bats and the range of frequencies
produced by arctiines, including sympatric species _Arctia caja_ and _Phragmatobia fuliginosa_ (16.6 to 109.5 kHz; Fig. 2A)4,35,36. The sounds even show similar increases and decreases in
frequency associated with the two bursts (Fig. 2A, Supplementary Audio S3, Fig. S4). In conjunction with the lack of hearing, and therefore the lack of acoustic intraspecific communication,
this suggests an anti-bat function. However, the remarkable acoustic difference to arctiines is that _Yponomeuta_ sounds are produced constantly during flight. All other Lepidoptera produce
sound only at specific times, for example during courtship, territory defence, or in response to the perceived presence of bats1,21,37. Perpetually casting its protective sound signal is
inevitable for a deaf moth unable to detect and react to approaching bats. _Yponomeuta_ sounds appear to be directed at bats, but to what effect? The constant nature of _Yponomeuta_ sound
production eliminates the possibility of startle being the main mechanism of defence, as bats would habituate to these sounds and even use them as cues to find prey38. Arctiine anti-bat
sounds used for aposematism/mimicry differ characteristically from those for sonar jamming in their maximum duty cycle (the percentage of time a signal is ‘on’) and the number of clicks per
modulation cycle (the number of clicks per two bursts i.e. per buckling in and out of a tymbal)4. Whilst _Y. evonymella_ and _Y. cagnagella_ produce more clicks per modulation cycle than
typical aposematic signalling arctiines, their duty cycles of 1.9 and 3.4% respectively place them exactly within the aposematic range. These low duty cycle anti-bat sounds are unable to jam
biosonar, as a duty cycle of 20% or more is essential1,4. We hence conclude that _Yponomeuta_ are acoustically mimicking the aposematic anti-bat sounds of tiger moths. The efficiency of
these sounds as a defence should however be quantified through behavioural tests with bats. _YPONOMEUTA_ EMPLOY ACOUSTIC MÜLLERIAN MIMICRY The toxicity or unpalatability of an organism
indicates whether their mimicking warning signals are truly aposematic (Müllerian mimicry) or impostors (Batesian mimicry). _Yponomeuta_ larvae tend to be monophagous or at least limited to
only a few species of food plant, something often associated with Lepidoptera that sequester specific toxins39. Principally, their hosts tend to be from Celastraceae, Rosaceae, Salicaceae
and Crassulaceae40. Celastraceae and Crassulaceae contain butenolides41, secondary metabolites the derivatives of which are reported to have cytotoxic activity42, and Salicaceae contain
salicin, a secondary metabolite known to act as a deterrent to insects and mammals40,43,44. _Yponomeuta cagnagella_ larvae feed on _Euonymus europaeus_ (European spindle tree; Celastraceae)
which contains two butenolides, siphonodin and to a lesser extent isosiphonodin41. Isosiphonodin is found in _Y. cagnagella_ and is either synthesised or sequestered by the insect41.
Interestingly, isosiphonodin is also found in adults of _Yponomeuta_ species that do not feed on butenolide-containing plants41, providing evidence that at least these species synthesise
butenolides. Presence of Isosiphonodin in several species of _Yponomeuta_ suggests that the compound is important in the ecology of these insects. Additionally, _Prunus padus_ (Bird cherry,
Rosaceae), the food plant of _Y. evonymella_, contains glucosides that can release hydrogen cyanide upon digestion, which has led to cattle poisoning45. Unpalatability to predators is an
obvious proposal for a function of containing butenolides and other noxious compounds. In fact, birds became drowsy when force-fed _Yponomeuta_ adults40. However, as Menken _et al_.40
observed, neither larvae nor adults of _Yponomeuta_ are obviously visually aposematic. We show that they use acoustic aposematism instead. These moths produce sounds with properties
extremely similar to the aposematic signals of larger moths (particularly arctiines), and are mostly nocturnal and therefore at low risk of predation by birds, explaining the lack of visual
aposematism. Similarly, highly nocturnal Arctiinae tend to be acoustically aposematic but visually cryptic46. So these butenolides, and probably other compounds such as glucosides and
salicin, are likely a defence against bats. We believe that _Yponomeuta_ sounds are warning bats of the presence of distasteful and potentially toxic compounds in these moths. Thus, at least
the species of _Yponomeuta_ containing such compounds are aposematic signallers and therefore Müllerian not Batesian mimics of arctiines. _YPONOMEUTA_ SOUNDS DO NOT INCREASE THEIR
CONSPICUOUSNESS TO HUNTING BATS The continuous nature of _Yponomeuta_ sound production might render them more vulnerable to bats because they will be able to eavesdrop on and be attracted to
the warning sounds. So reduced click amplitude might be adaptive, and _Yponomeuta evonymella_ clicks are indeed on average around 22 dB fainter than those of arctiines4. On the other hand,
too low an amplitude and bats might detect the warnings too late to avoid fatal interactions. Therefore, the most adaptive warning click would be perceivable over the exact distance that a
bat would detect the insect’s echoes anyway, and this is what we found for all orientations we tested (mean differences of 0.7, 0.5, 0.6 and 0.3 m for 0°, 45°, 90°, and 180° respectively;
see Fig. 4B). _Yponomeuta_’s zone of acoustic protection has evolved to be just large enough to cover their zone of detectability by echolocation. In conclusion, the aeroelastic tymbal of
_Yponomeuta_ is a completely novel sound-producing structure in Lepidoptera. Whilst wing-based sound production exists within the order13,23,47, all are evolutionarily independent of the
aeroelastic tymbal and none are used to produce anti-bat ultrasound. This tymbal is a striking example of both structural and acoustic convergent evolution in the bat-moth evolutionary arms
race, as well as being remarkable as a passive acoustic defence mechanism that bypasses the need for predator detection. The use of acoustic Müllerian mimicry by a deaf moth in the bat-moth
evolutionary arms race shows again how little we know of the complex acoustic war raging in the night skies. MATERIALS AND METHODS SPECIMENS Live specimens of two British _Yponomeuta_
species (Yponomeutidae, Lepidoptera), _Y. evonymella_ and _Y. cagnagella_ were used during the investigation. All specimens were wild caught as larvae and reared to pupation by donors. Pupae
were kept in the laboratory until eclosion within 297 × 159 × 102 mm plastic rearing boxes (WorldwideButterflies, Lulworth, United Kingdom) at 21 °C. Due to initially having much higher
numbers of the former than the latter, ablation and directionality experiments were performed using _Y. evonymella_ only while sound production was documented and analysed in both species.
As numbers of available individuals were limited, all individuals were flown until they became exhausted and would no longer fly. TETHERING Moths were tethered for most high-speed video
recordings as well as all ablation experiments. Due to their small size, standard methods of tethering such as adhesives failed, so we inserted a size 000 insect pin dorsally into the
mesothorax/prothorax of the moth until it just protruded ventrally. The pinhead was attached to a piece of dowel (5 mm in diameter) which itself was clamped so the moth was suspended in the
centre of the flight arena. Although this is obviously an invasive tethering method, tethered specimens continued to fly for prolonged periods of time. Both audio and video recordings showed
no obvious difference in the sounds produced by moths or their behaviour between tethered and free flight, so we continued with this as our tethering method. SOUND PLAYBACK Twenty _Y.
evonymella_, and four _Y. cagnagella_ were free flown in a semi-anechoic chamber with and without exposure to an ultrasonic stimulus. Two human observers documented the behaviour of each
individual under both conditions. A reaction was defined as any typical anti-bat escape manoeuvre including sudden cessation of flight or change in flight direction. Each moth was flown
twice and one observer chose the order of stimulus exposure for each individual, while the other observer was kept blind to the stimulus condition. A Dazer II Ultrasonic Dog Deterrent (Dazer
International, London, UK) was used as the stimulus, between one and two metres from the subject. The Dazer II produces a 25 kHz tone at 118.1 dB SPL (at 0.1 m). All 24 individuals
(separated by species) were also ensonified at rest within a 24 × 24 × 24″ BugDorm-1 Insect Rearing Cage (Megaview Science Co., Ltd., Taichung City, Taiwan), at a distance of around 1 m from
the centre of the cage. AUDIO RECORDINGS All recordings were made using a Type 4954 ¼″ free-field microphone (grid on) with a Type 2669-L preamplifier, connected to a Type 2690 NEXUS
conditioning amplifier (all Brüel & Kjær Sound and Vibration Measurements A/S, Nærum, Denmark), run through National Instruments NI-USB-6251 BNC sound card (National Instruments, Austin,
Texas, United States). For audio and high-speed video recordings insects were released or tethered within a 24 × 24 × 24″ BugDorm-1 Insect Rearing Cage (Megaview Science Co., Ltd., Taichung
City, Taiwan) lined on the base, back and one wall with ultrasound absorbing foam (Studiofoam 4″ Pyramids, Auralex Acoustics Inc., Indianapolis, IN) to reduce echoes and reverberation. The
recording microphone was positioned through a small circular hole cut into the mesh on the unlined side of the cage. The front panel (facing the camera) and the right-hand panel of the
BugDorm-1 were removed for synchronous audio and video recordings in order to facilitate the activation of the synchronisation click. The software used to make the recordings was AviSoft
Ni-Daq Recorder (Avisoft Bioacoustics, Berlin, Germany). All audio recordings were 16 bit, recorded at a sampling rate of 300 kHz, with the microphone between 7 and 13.5 cm from the moth for
tethered flight. HIGH-SPEED VIDEO RECORDINGS Video recordings were made in the same set-up as above with the camera (Photron FASTCAM SA1.1, Phtoron, Tokyo, Japan) lens (Nikon Micro-NIKKOR
105 mm prime lens, Nikon, Tokyo, Japan) positioned through a sleeve opening of the BugDorm-1 and pointing perpendicular to the microphone axis. Video recordings were made at 3000 fps with a
resolution of 1024 × 1024 pixels and the subject was illuminated using infrared (IR, 850 nm) lighting from four LED light sources. Video and audio recordings were synchronised with the use
of a pair of pliers. The pliers were kept in frame and when closed they produced an extremely short broadband click which allowed for very accurate synchronisation of frame (video) and
sample (audio) number. Synchronisation frames and samples were those that contained the collision of the jaws of the pliers and the beginning of the click respectively. ABLATION Twelve _Y.
evonymella_ were tethered, flown, and recorded with their hindwings intact. The moths were positioned in the flight set-up and left, holding a small piece of foam to simulate being sat on a
surface for 15–30 minutes or until they initiated flight themselves. If they had not initiated flight by then, it was elicited by removing the piece of foam they were holding, which reliably
triggered flight. Under a 50x magnification dissection microscope (Leica EZ5 Stereo Microscope, Leica Microsystems, Wetzlar, Germany) the translucent patches in both hindwings were then
removed using microdissection scissors from the wing joint to the point where scales began to appear. All ablated individuals were alive after that treatment and continued to fly on a tether
with no noticeable difference in their flight pattern and readiness. Their sounds were then recorded again using the same procedure. SOUND EMISSION DIRECTIONALITY The directionality of the
click amplitude of eight, tethered Y. evonymella was quantified by recording the sounds using the same setup with the microphone facing the moth from four orientations with respect to the
longitudinal axis of the moths from distances between 11.5 and 13.3 cm. Moth sounds were recorded from anterior (microphone at 0° facing the moth), posterior (microphone at 180°, behind the
moth), lateral (at 90° from the right side of the moth) and anterio-lateral (microphone at 45° to the moth’s right side) directions. For each individual at each orientation the loudest click
was isolated from the upper click burst for five consecutive wingbeats. Full bursts of clicks could not be detected from anterio-lateral recordings from two individuals and so the number of
clicks at this orientation was 30 from six individuals as opposed to 40 from eight for the other orientations. ACOUSTIC ANALYSIS All sound recordings were analysed using Avisoft SASLab Pro
(version 5.2.07, Avisoft Bioacoustics, Berlin, Germany). For each individual, click bursts from ten consecutive wingbeats were analysed, either counting all clicks or further analysing the
loudest click from each upper click burst. Click number was determined by totalling the number of clicks discernible in waveform and spectrogram for each of the two click bursts. Individual
click duration was measured manually from the waveform. Click amplitude was calculated as peak-to-peak sound pressure values using the waveform of individual clicks, and was then converted
to dB peSPL using a calibrated 40 kHz signal generator (Avisoft Bioacoustics, Berlin, Germany) and using the following formula: $$CA+20\ast lo{g}_{10}(\frac{TS}{CS})$$
\(CA={Calibration}\,{Tone}\,{Amplitude}\,({dB})\) \(TS={Test}\,{Signal}\,{Pressure}\,({\rm{Pa}})\) \(CS={Calibration}\,{Signal}\,{Sound}\,{Pressure}\,({\rm{Pa}})\). For spectral analysis,
individual clicks were isolated from the waveform including a linear ramp of 0.05 ms of noise on either side. Silence was then added (zero padding) on either side before analysis. Peak
frequency was determined from a power spectrum (Hamming window size 1024). High and low frequency values are the frequencies ± 15 dB below the amplitude of the peak frequency. CALCULATION OF
DETECTION DISTANCE OF _YPONOMEUTA_ SOUNDS Click detection distances were calculated for 14 _Yponomeuta evonymella_ individuals. For each individual the loudest click from the upper burst of
ten consecutive wingbeats was analysed. The peak frequencies and source level (dB peSPL) of each click were used to calculate the distance at which these sounds could be detected by bats,
using a hearing threshold of 10 dB SPL. The following formula, an adaptation of the sonar equation48, was used to calculate these distances. $$CSL-20\ast lo{g}_{10}(\frac{\delta -\delta
ref}{\delta ref})-FDA\ast (\delta -\delta ref)=HT$$ \(HT={\rm{hearing}}\,{\rm{threshold}}=10\,{\rm{dB}}\,{\rm{SPL}}\)
\(CSL={\rm{Click}}\,{\rm{Source}}\,{\rm{Level}}\,({\rm{dB}}\,{\rm{peSPL}}\,{\rm{at}}\,{\rm{\delta }}\mathrm{ref})\) \(\delta =\mathrm{Distance}\,({\rm{m}})\) \(\delta
ref={\rm{Reference}}\,{\rm{Distance}}=0.1\,{\rm{m}}\) \(FDA={\rm{Frequency}}\,{\rm{Dependent}}\,{\rm{Attenuation}}\,(\mathrm{dB}\,{{\rm{m}}}^{-1}){\rm{.}}\) _Yponomeuta evonymella_
directional click detection distance was calculated in the same manner, but using five consecutive wingbeats per angle (0°, 45°, 90°, and 180°) from eight individuals. MOTH ECHO DETECTION
DISTANCES Echo strength of whole insect specimens was measured as spectral target strength (the fraction of the impinging sound energy returned from the target). Live specimens were killed
by freezing and set with their wings in an upwards direction with the leading edge of the forewing perpendicular to the longitudinal axis of the body. The target specimen was positioned on a
vertical tower (27.8 cm high, 2.5 × 5 cm wide) of ultrasound absorbing foam (Basotect W, BASF, Ludwigshafen, Germany) placed on a turntable (LT360, LinearX Systems Inc., Battle Ground, WA).
A sonar measurement head mounted on a lever arm faced the target from a lateral distance of 31 cm. The sonar measurement head consisted of a ¼″ ultrasound microphone (type 26AB without
protective grid), pre-amplifier (type 2669 L, both GRAS Sound & Vibration A/S, Holte, Denmark), microphone power supply (type 5935-L, Brüel & Kjær, Nærum, Denmark) and a custom-made
ferro-electret foil loudspeaker (33 × 14 mm, Emfit Ltd., Vaajakoski, Finland) powered by a PZD350 M/S high-voltage amplifier (TREK Inc., Lockport, NY). The centres of the microphone and
speaker were separated by 15 mm, roughly replicating the distance between the mouth and ears of a bat. The turntable, speaker, and microphone were connected to a soundcard (NI-DAQ BNC-2110)
controlled using custom-programmes (LabVIEW v.16.0; both National Instruments, Austin, TX). For detailed methods see49,50. Five individual _Yponomeuta evonymella_ were analysed using this
technique. Each moth was scanned from 0–180° in 0.5° steps in the horizontal plane. A frequency modulated sweep from 15 to 250 kHz was used to ensonify the moth and for each position four
echoes were recorded and averaged. Detection distances of moth echoes were calculated analogous to click detection distances, but for two-way spherical transmission losses and with FDA for
bat call frequencies with the highest detection range in the UK, i.e. at 20–30 kHz: $$BSL-2\ast 20\ast lo{g}_{10}(\frac{\delta -\delta ref}{\delta ref})-2\ast FDA\ast (\delta -\delta
ref)+TS=HT$$ \(TS={\rm{spectral}}\,{\rm{target}}\,\mathrm{strength}\,\,{\rm{of}}\,{\rm{moth}}\,{\rm{echo}}\,({\rm{dB}}\,{\rm{at}}\,\delta ref)\)
\(BSL={\rm{Source}}\,{\rm{Level}}\,{\rm{of}}\,{\rm{bat}}\,{\rm{call}}\,({\rm{dB}}\,{\rm{peSPL}}\,{\rm{at}}\,\delta ref)\) STATISTICS All statistical tests were performed using R studio (R
version 3.1.2.). A two-tailed paired samples t-test was performed to compare the number of clicks produced before and after ablation of the aeroelastic tymbals. A two-tailed nested ANOVA run
as a mixed effects model, with moth individual as the random effect, was used to test for differences between the amplitudes of _Yponomeuta_ sounds recorded at different angles. Moth
individual was nested within the angle at which it was recorded. This was followed by a Tukey post-hoc test with Bonferroni correction. REFERENCES * Conner, W. E. & Corcoran, A. J. Sound
strategies: the 65-million-year-old battle between bats and insects. _Annu. Rev. Entomol._ 57, 21–39 (2012). Article CAS Google Scholar * Fullard, J. H. Auditory habitat changes in
noctuid moths endemic to a bat-free habitat. _J. Evol. Biol._ 7, 435–445 (1994). Article Google Scholar * ter Hofstede, H. M., Goerlitz, H. R., Ratcliffe, J. M., Holderied, M. W. &
Surlykke, A. The simple ears of noctuoid moths are tuned to the calls of their sympatric bat community. _J. Exp. Biol._ 216, 3954–3962 (2013). Article Google Scholar * Corcoran, A. J.,
Conner, W. E. & Barber, J. R. Anti-bat tiger moth sounds: Form and function. _Curr. Zool._ 56, 358–369 (2010). Google Scholar * Barber, J. R. & Kawahara, A. Y. Hawkmoths produce
anti-bat ultrasound. _Biol. Lett._ 9, 1–5 (2013). Article Google Scholar * Corcoran, A. J. & Hristov, N. I. Convergent evolution of anti-bat sounds. _J. Comp. Physiol. A_ 200, 811–821
(2014). Article Google Scholar * Holderied, M., Korine, C. & Moritz, T. Hemprich’s long-eared bat (Otonycteris hemprichii) as a predator of scorpions: Whispering echolocation, passive
gleaning and prey selection. _J. Comp. Physiol. A_ 197, 425–433 (2011). Article Google Scholar * Fenton, M. B., Gaudet, C. L. & Leonard, M. L. Feeding behaviour of the bats Nycteris
grandis and Nycteris thebaica (Nycteridae) in captivity. _J. Zool._ 200, 347–354 (1983). Article Google Scholar * Corcoran, A. J., Barber, J. R., Hristov, N. I. & Conner, W. E. How do
tiger moths jam bat sonar? _J. Exp. Biol._ 214, 2416–2425 (2011). Article Google Scholar * Hristov, N. I. & Conner, W. E. Sound strategy: acoustic aposematism in the bat-tiger moth
arms race. _Naturwissenschaften_ 92, 164–169 (2005). Article ADS CAS Google Scholar * Corcoran, A. J., Barber, J. R. & Conner, W. E. Tiger moth jams bat biosonar. _Science (80-.)._
325, 325–328 (2009). Article ADS CAS Google Scholar * Nakano, R. _et al_. Moths produce extremely quiet ultrasonic courtship songs by rubbing specialized scales. _Proc. Natl. Acad. Sci.
USA_ 105, 11812–11817 (2008). Article ADS CAS Google Scholar * Alcock, J., Gwynne, D. T. & Dadour, I. R. Acoustic signaling, territoriality, and mating in whistling moths, Hecatesia
thyridion (Agaristidae). _J. Insect Behav._ 2, 27–37 (1989). Article Google Scholar * Gwynne, D. T. & Edwards, E. D. Ultrasound production by genital stridulation in Syntonarcha
iriastis (Lepidoptera: Pyralidae): long-distance signalling by male moths? _Zool. J. Linn. Soc._ 88, 363–376 (1986). Article Google Scholar * Surlykke, A. & Gogala, M. Stridulation and
hearing in the noctuid moth Thecophora fovea (Tr.). _J. Comp. Physiol. A_ 159, 267–273 (1986). Article Google Scholar * Lees, D. C. Foreleg stridulation in male Urania moths (Lepidoptera:
Uraniidae). _Zool. J. Linn. Soc._ 106, 163–170 (1992). Article Google Scholar * Skals, N. & Surlykke, A. Sound production by abdominal tymbal organs in two moth species: the green
silver-line and the scarce silver-line (Noctuoidea: Nolidae: Chloephorinae). _J. Exp. Biol._ 202, 2937–2949 (1999). CAS PubMed Google Scholar * Blest, A. D., Collett, T. S. & Pye, J.
D. The generation of ultrasonic signals by a New World arctiid moth. _Proc. R. Soc. B_ 158, 196–207 (1963). ADS Google Scholar * Heller, K. & Krahe, R. Sound Production and Hearing in
the Pyralid Moth Symmoracma Minoralis. _J. Exp. Biol._ 187, 101–11 (1994). CAS PubMed Google Scholar * Matthews, M. The African Species of Heliocheilus Grote (Lepidoptera: Noctuidae).
_Syst. Entomol._ 12, 459–473 (1987). Article Google Scholar * Bailey, W. J. Resonant wing systems in the Australian whistling moth Hecatesia (Agarasidae, Lepidoptera). _Nature_ 272,
444–446 (1978). Article ADS Google Scholar * Spangler, H. G., Greenfield, M. D. & Takessian, A. Ultrasonic mate calling in the lesser wax moth. _Physiol. Entomol._ 9, 87–95 (1984).
Article Google Scholar * Heller, K. & Achmann, R. The ultrasonic song of the moth Amyna natalis (Lepidoptera: Noctuidae: Acontiinae). _Bioacoustics_ 5, 89–97 (1993). Article Google
Scholar * Turner, H., Lieshout, N., Van Ginkel, W. E. & Menken, S. B. J. Molecular phylogeny of the small ermine moth genus Yponomeuta (Lepidoptera, Yponomeutidae) in the Palaearctic.
_PLoS One_ 5, 15–19 (2010). Article Google Scholar * Heppner, J. B. In _Encyc_lopedia _o_f Ent_omology_ (ed. Capinera, J. L.) 1360–1361, https://doi.org/10.1007/978-1-4020-6359-6_3661
(Springer Netherlands, 2008). * Meyrick, E. _A handbook of British Lepidoptera_. (London, Macmillan, 1895). * Agassiz, D. J. L. Do small ermine moths sing? Possible stridulatory sound
production in Yponomeutidae (Lepidoptera). _J. Nat. Hist_. 1–8, https://doi.org/10.1080/00222933.2017.1324063 (2017). * Sohn, J. C. Review of the genus Eumonopyta (Lepidoptera:
Yponomeutidae) with descriptions of two new species. _Entomol. Sci._ 19, 155–160 (2016). Article Google Scholar * Waters, D. A., Rydell, J. & Jones, G. Echolocation call design and
limits on prey size: a case study using the aerial-hawking bat Nyctalus leisleri. _Behav. Ecol. Sociobiol._ 37, 321–328 (1995). Article Google Scholar * Gonsalves, L., Bicknell, B., Law,
B., Webb, C. & Monamy, V. Mosquito Consumption by Insectivorous Bats: Does Size Matter? _PLoS One_ 8, 1–11 (2013). Google Scholar * Ahlen, I. Fältobservationer av ultraljud hos flygande
fjärilar. _Entomol. Tidskr_. 118 (1997). * St. Juliana, J. R. _et al_. Note: A Field Assessment of the Defensive Responses of Moths to an Auditory Stimulus. _Isr. J. Ecol. Evol._ 53,
173–177 (2007). Article Google Scholar * Fenton, M. B. & Roeder, K. D. The microtymbals of Arctiidae. _J. Lepid. Soc._ 28, 205–211 (1974). Google Scholar * Fullard, J. H. &
Heller, B. Functional Organization of the Arctiid Moth Tymbal (Insecta, Lepidoptera). _J. Morphol._ 204, 57–65 (1990). Article CAS Google Scholar * Surlykke, A. & Miller, L. A. The
influence of arctiid moth clicks on bat echolocation; jamming or warning? _J. Comp. Physiol. A_ 156, 831–843 (1985). Article Google Scholar * Koay, G., Heffner, H. E. & Heffner, R. S.
Audiogram of the big brown bat (Eptesicus fuscus). _Hear. Res._ 105, 202–210 (1997). Article CAS Google Scholar * Nakano, R. _et al_. Moths are not silent, but whisper ultrasonic
courtship songs. _J. Exp. Biol._ 212, 4072–4078 (2009). Article CAS Google Scholar * Bates, D. L. & Fenton, M. B. Aposematism or startle? Predators learn their responses to the
defenses of prey. _Can. J. Zool._ 68, 49–52 (1990). Article Google Scholar * Engler-Chaouat, H. S. & Gilbert, L. E. De novo Synthesis vs. Sequestration: Negatively Correlated Metabolic
Traits and the Evolution of Host Plant Specialization in Cyanogenic Butterflies. _J. Chem. Ecol._ 33, 25–42 (2007). Article CAS Google Scholar * Menken, S. B. J., Heerebout, W. M. &
Wiebes, J. T. Small ermine moths (Yponomeuta): Their Host Relations and Evolution. _Annu. Rev. Entomol._ 37, 41–66 (1992). Article Google Scholar * Fung, S. Y., Herrebout, W. M.,
Verpoorte, R. & Fischer, F. C. Butenolides in small ermine moths, Yponomeuta spp. (Lepidoptera: Yponomeutidae), and spindle-tree, Euonymus europaeus (Celastraceae). _J. Chem. Ecol._ 14,
1099–1111 (1988). Article CAS Google Scholar * Wagner, H., Flitsch, K. & Jurcic, K. Cytotoxizität von Siphonosid und aliphatischen Estern des Siphonodins. _Planta Med._ 43, 249–251
(1981). Article CAS Google Scholar * Bernays, E. A., Oppenheim, S., Chapman, R. F., Kwon, H. & Gould, F. Taste sensitivity of insect herbivores to deterrents is greater in specialists
than in generalists: A behavioral test of the hypothesis with two closely related caterpillars. _J. Chem. Ecol._ 26, 547–563 (2000). Article CAS Google Scholar * Pass, G. J. & Foley,
W. J. Plant secondary metabolites as mammalian feeding deterrents: separating the effects of the taste of salicin from its post-ingestive consequences in the common brushtail possum
(Trichosurus vulpecula). _J. Comp. Physiol. B_ 170, 185–192 (2000). Article CAS Google Scholar * Sargison, N. D., Williamson, D. S., Duncan, J. R. & McCance, R. W. Prunus padus (bird
cherry) poisoning in cattle. _Vet. Rec._ 138, 188 (1996). Article CAS Google Scholar * Ratcliffe, J. M. & Nydam, M. L. Multimodal warning signals for a multiple predator world.
_Nature_ 455, 96 (2008). Article ADS CAS Google Scholar * Agee, H. R. Ultrasound produced by wings of adults of Heliothis zea. _J. Insect Physiol._ 17, 1267–1273 (1971). Article Google
Scholar * Møhl, B. In _Animal Sonar: Processes and Performance_ (eds Nachtigall, P. E. & Moore, P. W. B.) 435–450, https://doi.org/10.1007/978-1-4684-7493-0_43 (Springer US, 1988). *
Clare, E. L. & Holderied, M. W. Acoustic shadows help gleaning bats find prey, but may be defeated by prey acoustic camouflage on rough surfaces. _Elife_ 4, 1–14 (2015). Article Google
Scholar * Balleri, A., Griffiths, H. D., Woodbridge, K., Baker, C. J. & Holderied, M. W. Bat-inspired ultrasound tomography in air. In _Radar Conference, 2010 IEEE_ 44–47 (2010).
Download references ACKNOWLEDGEMENTS We thank Ray Barnett and the Bristol and District Moth Group for putting us in contact with Dr. Agassiz and providing us with live specimens.
Additionally, we thank Dr John Langmaid for providing _Yponomeuta evonymella_ specimens. We also thank the Aerospace Engineering department at the University of Bristol for use of their
high-speed camera. Liam O’Reilly’s PhD studies are funded through the University of Bristol’s Graduate Teaching Assistant scheme. Thomas Neil is employed under the BBSRC funded BB/N009991/1
Diffraction of Life project, with Marc Holderied as principal investigator. Marc Holderied was also supported by Leverhulme Research Fellowship RF-2017-717\2. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * School of Biological Sciences, University of Bristol, Bristol, UK Liam J. O’Reilly, Thomas R. Neil & Marc W. Holderied * Department of Life Sciences, Insect Division,
Natural History Museum, London, UK David J. L. Agassiz Authors * Liam J. O’Reilly View author publications You can also search for this author inPubMed Google Scholar * David J. L. Agassiz
View author publications You can also search for this author inPubMed Google Scholar * Thomas R. Neil View author publications You can also search for this author inPubMed Google Scholar *
Marc W. Holderied View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.J.O. and M.W.H. conceived the study. L.J.O. led the data collection for
the study and T.R.N. also participated in behavioural, and acoustic tomography data collection. L.J.O. and M.W.H. shared writing and L.J.O. led data analysis. M.W.H. offered advice and
MATLAB code for acoustic tomography analysis, and T.R.N. participated in acoustic tomography analysis and produced Figure 4. D.J.L.A. provided expert advice on Yponomeutidae as well as live
specimens for acoustic and video recordings, and dead specimens for SEM data. CORRESPONDING AUTHOR Correspondence to Marc W. Holderied. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S4 SUPPLEMENTARY VIDEO S1 SUPPLEMENTARY VIDEO S2 SUPPLEMENTARY AUDIO S3 RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE O’Reilly, L.J., Agassiz, D.J.L., Neil, T.R. _et al._
Deaf moths employ acoustic Müllerian mimicry against bats using wingbeat-powered tymbals. _Sci Rep_ 9, 1444 (2019). https://doi.org/10.1038/s41598-018-37812-z Download citation * Received:
10 July 2018 * Accepted: 19 November 2018 * Published: 05 February 2019 * DOI: https://doi.org/10.1038/s41598-018-37812-z SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative






