- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Flavonoids exert innumerable beneficial effects on cardiovascular health including the reduction of platelet activation, and thereby, thrombosis. Hence, flavonoids are deemed to be
a molecular template for the design of novel therapeutic agents for various diseases including thrombotic conditions. However, the structure-activity relationships of flavonoids with
platelets is not fully understood. Therefore, this study aims to advance the current knowledge on structure-activity relationships of flavonoids through a systematic analysis of
structurally-related flavones. Here, we investigated a panel of 16 synthetic flavones containing hydroxy or methoxy groups at C-7,8 positions on the A-ring, with a phenyl group or its
bioisosteres as the B-ring, along with their thio analogues possessing a sulfur molecule at the 4th carbon position of the C-ring. The antiplatelet efficacies of these compounds were
analysed using human isolated platelets upon activation with cross-linked collagen-related peptide by optical aggregometry. The results demonstrate that the hydroxyl groups in flavonoids are
important for optimum platelet inhibitory activities. In addition, the 4-C=O and B ring phenyl groups are less critical for the antiplatelet activity of these flavonoids. This
structure-activity relationship of flavonoids with the modulation of platelet function may guide the design, optimisation and development of flavonoid scaffolds as antiplatelet agents.
SIMILAR CONTENT BEING VIEWED BY OTHERS SYNTHESIS, PHYSICOCHEMICAL CHARACTERIZATION AND BIOLOGICAL ACTIVITY OF NOVEL PYRROLE FLAVONES Article Open access 03 March 2025 SYNTHESES OF
MONO-ACYLATED LUTEOLIN DERIVATIVES, EVALUATION OF THEIR ANTIPROLIFERATIVE AND RADICAL SCAVENGING ACTIVITIES AND IMPLICATIONS ON THEIR ORAL BIOAVAILABILITY Article Open access 15 June 2021
STRUCTURAL INVESTIGATION OF INTERACTIONS BETWEEN HALOGENATED FLAVONOIDS AND THE LIPID MEMBRANE ALONG WITH THEIR ROLE AS CYTOTOXIC AGENTS Article Open access 08 May 2024 INTRODUCTION
Platelets are small circulating blood cells that play pivotal roles in the regulation of haemostasis upon vascular injury through blood clotting1,2. However, unnecessary activation of
platelets within the vasculature leads to pathological conditions such as thrombosis, which results in blockage or reduction of blood flow to major organs including heart and brain
instigating heart attack and stroke, respectively3,4. The currently used therapeutic options that involve the use of antiplatelet drugs such as clopidogrel, aspirin, and prasugrel are often
linked with adverse side effects such as bleeding and are ineffective in some patients5,6,7,8,9,10. As cardiovascular diseases remain the leading cause of death worldwide11, the development
of improved therapeutic strategies to prevent and treat thrombotic diseases remains a pressing priority. Flavonoids, a group of polyphenolic plant metabolites, have been widely demonstrated
to possess beneficial effects in the prevention of cardiovascular diseases12,13,14. Epidemiological and clinical studies have established a prominent link between the regular consumption of
dietary flavonoids and decreased incidences of cardiovascular diseases or their risk markers15,16,17,18,19,20. Flavonoids have also been recognised as modulators of platelet function and
their inhibitory activities can be attributed to their ability to inhibit reactive oxygen species (ROS) production21,22, modify cytoskeletal proteins such as actin and tubulin that mediate
degranulation23,24, and to inhibit various kinases25,26,27,28,29 and receptors30,31 that play numerous roles in the regulation of platelet activation and thrombosis. The pharmacological
potential of flavonoids is strongly related to their molecular structure, hence, the identification of key structural elements that are prerequisites for antiplatelet activity has provoked
considerable interest in the area of drug discovery32,33. With a view to develop flavonoids as potential anti-thrombotic agents, some studies have been carried out to identify the key
structural features governing the antiplatelet activity of flavonoids34. However, the current knowledge of structure-activity relationships (SARs) has mainly resulted from analyses involving
different subclasses of natural flavonoids32,34. In order to translate flavonoids into potential molecular templates for drug design, a better understanding of the SAR of flavonoids, and a
careful comparison of substitution pattern within a flavonoid subclass is necessary. Hence, to gain greater insights into the SAR of flavonoids with human platelets, this study has focused
on assessing the effect of methoxylation, 4-C=S substitution, and different B-ring substitutions using a series of 16 synthetic flavones on the antiplatelet activities. MATERIALS AND METHODS
FLAVONES The synthetic flavones used in this study are grouped into four main classes namely; hydroxy flavones with free –OH and 4-C=O, hydroxy 4-thioflavones with free –OH and 4-C=S,
methoxy flavones with –OMe and 4-C=O and methoxy 4-thioflavones with–OMe and 4-C=S35. The hydroxy flavones include 7,8-dihydroxy-2-phenyl-4_H_-chromen-4-one (F-1),
7,8-dihydroxy-2(thiophen-2-yl)-4_H_-chromen-4-one (F-2), 2-(furan-2-yl)-7-8,dihydroxy-4_H_-chromen-4-one (F-3) and 7,8-dihydroxy-2(pyridine-3yl)-4_H_-chromen-4-one (F-4). The hydroxy
4-thioflavones include 7,8-dihydroxy-2-phenyl-4_H_-chromen-4-thione (TF-1), 7,8-dihydroxy-2(thiophen-2-yl)-4_H_-chromen-4-thione (TF-2), 2-(furan-2-yl)-7-8,dihydroxy-4_H_-chromen-4-thione
(TF-3) and 7,8-dihydroxy-2(pyridine-3yl)-4_H_-chromen-4-thione (TF-4). The methoxy flavones include 7,8-dimethoxy-2-phenyl-4_H_-chromen-4-one (CYC-1),
7,8-dimethoxy-2-(thiophen-2-yl)-4_H_-chromen-4 (CYC-2), 2-(furan-2-yl)-4_H_-chromen-4-one (CYC-3) and 7,8-dimethoxy-2-(pyridine-3-yl)-4_H_-chromen-4-one (CYC-4). The methoxy 4-thioflavones
include 7,8-dimethoxy-2-phenyl-4_H_-chromen-4-thione (TCYC-1), 7,8-dimethoxy-2-(thiophen-2-yl)-4_H_-chromen-4-thione (TCYC-2), 2-(furan-2-yl)-7,8-dimethoxy-4_H_-chromen-4-thione (TCYC-3) and
7,8-dimethoxy-2-(pyridine-3-yl)-4_H_-chromen-4-thione (TCYC-4). Stock solutions of these flavones were prepared in dimethyl sulfoxide (DMSO) (100%) at 10 mg/mL concentration and the final
required test concentrations were obtained by appropriately diluting these stocks. The final concentration of DMSO in platelets was maintained at 0.1% (v/v), which did not affect their
function. HUMAN BLOOD COLLECTION All the experiments in this study were conducted in line with the appropriate institutional and national guidelines and regulations. Human blood was
collected by venepuncture from aspirin free, healthy volunteers into vacutainers containing 3.2% (v/v) citrate after obtaining their informed consent. The procedures and consent forms used
in this study were approved by the University of Reading Research Ethics Committee. PREPARATION OF HUMAN ISOLATED PLATELETS Human isolated platelets were prepared by adding 7.5 mL of ACD
[(acid citrate dextrose) (20 g/L glucose, 25 g/L sodium citrate and 15 g/L citric acid)] to 50 mL of blood prior to centrifugation at 100 g for 20 minutes at room temperature. The
Platelet-Rich Plasma (PRP) was removed using wide bore pipette tips and mixed with 3 mL of ACD and centrifuged at 1400 g for 10 minutes at room temperature. The resulting platelet pellet was
resuspended in modified Tyrodes-HEPES buffer (2.9 mM KCl, 134 mM NaCl, 0.34 mM Na2HPO4.12H2O, 1 mM MgCl2, 12 mM NaHCO3, 20 mM HEPES, pH 7.3) and centrifuged again at 1400 g for 10
minutes28. The final platelet pellet obtained was suspended in modified Tyrodes-HEPES buffer at a density of 4 × 108 cells/mL for aggregation assays and rested at 30 °C for 30 minutes before
using in platelet functional assays. PLATELET AGGREGATION ASSAYS Platelet aggregation was performed using a platelet glycoprotein VI (GPVI)-selective agonist, CRP-XL (obtained from
Professor Richard Farndale, University of Cambridge) in the presence or absence of a vehicle control [0.1% (v/v) DMSO] or different concentrations of flavone derivatives by optical
aggregometry. Human isolated platelets (267 µL) taken in siliconised cuvettes were incubated with 3 µL of a vehicle control or various concentrations of flavone derivatives for 5 minutes at
37 °C. Following the incubation, 30 µL of CRP-XL (0.5 µg/mL) was added to platelets and the level of aggregation was measured for 5 minutes at 37 °C under constant stirring (1200 rpm). Data
were analysed by calculating the percentage of maximum platelet aggregation at 5 minutes, and the level of aggregation obtained with the vehicle control was considered as 100% to quantify
the impact of flavones on platelets. LACTATE DEHYDROGENASE ASSAY The lactate dehydrogenase (LDH) assay was performed using a LDH Cytotoxicity Assay Kit (Pierce, Thermo Fisher) according to
the manufacturer’s instructions. Briefly, to 50 µL of human isolated platelets, 1 µL of a positive control [1% (v/v) Triton-X 100, provided in the kit] or a vehicle control [0.1% (v/v) DMSO]
or various concentrations of flavone derivatives were added and incubated for 30 minutes at 37 °C. Then, 25 µL of the reaction mixture (provided in the kit) were added to the platelets and
further incubated for 30 minutes in dark. Finally, the reaction was stopped by adding 25 µL of stop solution (provided in the kit). The absorbance was measured at 490 and 650 nm using a
Fluostar Optima spectrofluorimeter (BMG Labtech, Germany). The level of LDH released with the positive control was considered as 100% to quantify the LDH release in flavone-treated samples.
STATISTICAL ANALYSIS The statistical significance between the vehicle controls and flavones-treated platelet samples was analysed by one-way ANOVA followed by Bonferroni _post-hoc_ test. All
the statistical analyses were performed using GraphPad Prism 7 (GraphPad Software Inc., USA). RESULTS To determine the relationship between the specific functional groups in the structures
of flavones and their antiplatelet activity, a series of hydroxy flavones, hydroxy 4-thioflavones, methoxy flavones and methoxy 4-thioflavones containing different B-ring (Figs 1A, 2A, 3A
& 4A) were used in this study. These 16 flavones were synthesised based on the molecular template of 7,8-hydroxy flavone to systematically determine the influence of hydroxyl (-OH),
methoxy (-OMe) and 4-thiocarbonyl (4-C=S) groups as well as the effects of phenyl group and its bioisosteres such as thiofuran, furanyl and pyridinyl moieties as B-ring on platelet
activation/function. The synthesis of these compounds from our laboratories has been previously reported35 and the purities of compounds were analysed by reverse phase HPLC, and they were
found to be >95%. In order to determine the SAR of flavones with human platelets, the effects of 16 selected synthetic flavones on CRP-XL-stimulated platelet aggregation were evaluated by
optical aggregometery. Human isolated platelets were treated with a vehicle [0.1% (v/v) DMSO] or diverse concentrations of flavones (3.125, 6.25, 12.5, 25, 50 and 100 μM) for 5 minutes
prior to activation with 0.5 μg/mL CRP-XL for 5 minutes. None of the flavones exhibited activatory effects on platelets on their own, and the vehicle control containing 0.1% (v/v) DMSO did
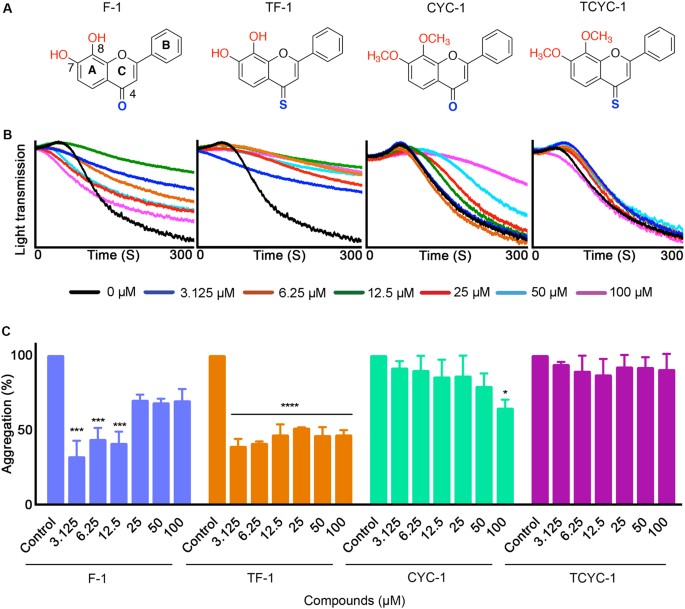
not affect platelet activation. FLAVONES WITH PHENYL B-RING Hydroxy flavone (F-1) with free hydroxyls and carbonyl moiety significantly inhibited CRP-XL-stimulated platelet aggregation at
lower concentrations such as 3.125, 6.25 and 12.5 μM but no significant inhibition was observed at concentrations higher than 12.5 μM. The hydroxy 4-thioflavone (TF-1) showed significant
inhibitory effects at all the concentrations tested. However, the methoxy flavone (CYC-1) inhibited the aggregation significantly only at 100 μM and the methoxy 4-thioflavone (TCYC-1) did
not show any inhibitory effects at any of the concentrations tested (Fig. 1A–C). FLAVONES WITH THIOFURAN B-RING Hydroxy flavone (F-2) displayed a similar inhibitory trend to F-1 with
significant inhibition at lower concentrations up to 12.5 μM, whereas the hydroxy 4-thioflavone (TF-2) and the methoxy flavone (CYC-2) inhibited aggregation only at 100 μM. The methoxy
4-thioflavone (TCYC-2) of this group was found to possess no inhibitory activity on platelet aggregation (Fig. 2A–C). FLAVONES WITH FURAN B-RING Hydroxy flavone (F-3) and hydroxy
4-thioflavone (TF-3) inhibited the CRP-XL-induced platelet activation at all the concentrations tested, however, the methoxy flavone (CYC-3) and the methoxy 4-thioflavone (TCYC-3) failed to
inhibit the aggregation (Fig. 3A–C). FLAVONES WITH PYRIDINE B-RING None of the flavones with a pyridine B-ring displayed inhibitory effects on CRP-XL-stimulated platelet aggregation (Fig.
4A–C) at concentrations up to 100 μM. It is interesting to note the impact of altering the B-ring on the platelet activity among the same class of flavones. For hydroxy flavones (with –OH
and 4-C=O), changing the phenyl group (F-1) to a thiofuran group (F-2) did not affect the inhibitory potential as both compounds elicited the inhibitory activity up to 12.5 μM. Consistent
inhibition (~65–70%) across the tested concentrations (3.125–100 μM) was observed when the phenyl group was replaced with a furan group (F-3). In contrast, substitution of the phenyl group
with a pyridine group led to a complete abolition of inhibitory activity in platelets (F-4). A similar trend was observed for the hydroxy 4-thioflavones (with –OH and 4-C=S) when the B-ring
phenyl group was replaced with its bioisosteres furan and pyridine. However, the substitution of thiofuran led to a reduction in the activity, for example, TF-2 showed ~50% inhibition only
at 100 μM (Fig. 2) where its phenyl analogue, TF-1 elicited ~50% inhibition at all the concentrations used (Fig. 1). The influence of B-ring modifications was not conspicuous among the
methoxy flavones (with –OMe and 4-C=O) and methoxy 4-thioflavones (with –OMe and 4-C=S) as these two classes of flavones did not display any significant inhibitory activity on platelets in
comparison to their hydroxyl analogues. Nevertheless, amongst the methoxy flavones, flavones with a B ring phenyl group (CYC-1) or thiofuran group (CYC-2) showed similar activities with
inhibition only at 100 μM. Methoxy 4-thioflavones (with –OMe and 4-C=S) did not exert any inhibitory effects on platelets. To corroborate the above results, the effects of these flavones on
another platelet activation marker, specifically fibrinogen binding (a marker for inside-out signalling to integrin αIIbβ3), were measured by flow cytometry. The results obtained from this
experiment concur with the aggregation data where hydroxy flavones F-1 (at 3.125–12.5 μM), F-2 (at 3.125–12.5 μM) and F-3 (at all concentrations tested), as well as thiohydroxy flavones TF-1
(at all concentrations tested), TF-2 (at 100 μM) and TF-3 (at all concentrations tested) significantly inhibited fibrinogen binding (Fig. S1), which is critical for subsequent platelet
aggregation. Finally, to determine whether the platelet inhibition observed was the result of a specific pharmacological effect of flavones rather than due to their cytotoxic effects, an LDH
cytotoxicity assay was performed. For this, platelets were treated with a vehicle control [0.1% (v/v) DMSO] or diverse concentrations of flavones (3.125, 6.25, 12.5, 25, 50 and 100 μM) and
the release of LDH, a cytosolic enzyme, which is an indicator of cellular toxicity was measured. As shown in Fig. 5, a positive control showed the maximum level of cytotoxicity with a higher
LDH release, whereas the flavones did not exert cytotoxic effects at the concentrations (3.125–100 μM) used. These observations suggest that the inhibitory effects of flavones demonstrated
in this study were not due to their cytotoxic effects on platelets. DISCUSSION Understanding the relationship between distinct functional groups within the structures of flavones and their
influence on antiplatelet activity is critical for further development and modification of flavones in order to make them as more potent lead compounds for drug design. Such knowledge will
aid in the development of improved therapeutic strategies for the prevention and treatment of cardiovascular disesaes, specifically thrombosis. In the present study, the analysis and
comparison of the inhibitory activities of a series of 16 structurally-related synthetic flavones on CRP-XL-stimulated human platelet activation highlighted the key structural features that
are required for the inhibition of platelet function. In general, comparison between different classes of flavones with the same B-ring moiety suggested that the hydroxy flavones (with free
-OH groups) were more effective than their corresponding methoxy flavones (with -OCH3 group). This highlighted that hydroxy groups, which are hydrogen bond donors, are essential for their
inhibitory activities in platelets and that methoxy groups with hydrogen bond accepting profiles are less effective in this regard. However, the study by Bojić _et al_.34 reports increased
anti-aggregatory potencies of _O_-methylated derivatives in comparison to their hydroxy analogues. This disparity could possibly be due to the difference in the hydroxyl substitution pattern
of the flavones studied which would affect the interaction with the molecular target. In addition, several previous studies reported the loss of biological activity of flavonoids upon
complete methylation of their active hydroxy groups29,35,36. Together with these previous studies, our data suggest that hydroxy groups are a key descriptor for the biological activity of
flavonoids. It is worth highlighting that the hydroxy flavones used in this study possess hydroxyl groups at the C-7,8 position as opposed to the C-5,7 position in chrysin, a natural flavone
that was previously reported to negatively modulate platelet activity37. When comparing the activity between chrysin and its 7,8-hydroxy analogue (F-1), it can be deduced that the position
of the hydroxyl groups also influences platelet function as chrysin exhibited dose-dependent inhibition of platelet activity (6.25–100 μM)38, whereas, 7,8- hydroxyl flavone showed inhibition
only between 3.25–12.5 μM. Further studies are required to determine the reasons for the low inhibitory effects obtained from higher concentrations of hydroxyl flavones with phenyl and
thiofuran B-rings. A number of previous studies have reported the significance of 4-C=O in the C-ring of flavonoids for antiplatelet activities based on the comparison between flavonoids
with and without 4-C=O32,34,39. In this study, the influence of modification of 4-C=O to 4-C=S was also evaluated. Indeed, the replacement of 4-C=O with 4-C=S was well tolerated for flavones
with free hydroxy groups (hydroxy flavones and hydroxy 4-thioflavones), however, no beneficial effects were observed for flavones with methoxy groups (methoxy flavones and methoxy
4-thioflavones). It is interesting to note that the influence of 4-C=S was also found to be dependent on the B-ring functionality as moderate loss of inhibitory activity was observed upon
the replacement of 4-C=O with 4-C=S for flavones with a thiofuran B-ring. This demonstrates that the systematic analysis of flavones through careful correlation of effect of each
substitution with respect to other functional groups is important for better optimisation of these compounds as molecular templates for drug design and discovery. Natural flavonoids contain
a phenyl group as the B-ring, hence, previous reports have focused on the influence of the position of the B-ring and its hydroxylation patterns. The present study involving synthetic
flavonoids allowed the determination of the effect of incorporating bioisosteres of the phenyl group. It was found that replacing the phenyl group with a furan group was well tolerated for
hydroxy flavones and hydroxy 4-thioflavones, whereas replacement of a phenyl group with a thiofuran group led to loss of inhibitory activities in platelets for hydroxy 4-thioflavones but was
tolerated for hydroxy flavones. Conversely, replacement of the phenyl group with a pyridine group led to complete loss of inhibition in platelets. These observations suggest that the B-ring
phenyl group is not critical for the antiplatelet activity, but the B-ring heteroatoms largely influence the activity. Furthermore, these observations suggest that the orientation and
binding modes of the B-ring moieties might influence the interaction with their molecular targets. Hence, identification of the molecular targets for these flavones, and careful optimisation
of the nature of the B-ring could lead to more efficacious flavone scaffolds for the development of novel antiplatelet agents. Furthermore, the hydroxy flavones and thiohydroxy flavones
showed significant inhibitory effects on fibrinogen binding, a key marker for platelet activation via inside-out signalling to integrin αIIbβ3. This suggests that these flavones specifically
with free hydroxy groups may modulate distinctive functions of platelets. The LDH cytotoxicity assay showed that these flavones are not cytotoxic to platelets at the concentrations tested
and hence the inhibitory effects observed are due to their pharmacological effects on platelet function. In conclusion, a panel of 16 structurally-related hydroxy flavones, methoxy flavones
and their 4-thio analogues were screened for their antiplatelet activity upon CRP-XL-induced activation in human platelets. SAR analysis of these flavones suggested that the free hydroxyl
group is essential for antiplatelet activity. Moreover, the modification at 4-C=O to 4-C=S in the C-ring, and B-ring modifications of phenyl group into specific bioisostere such as furanyl
group, are well tolerated without any significant loss of their inhibitory activities. The molecular targets and the impact of these synthetic flavones on specific signalling pathways in
platelets were not investigated in this study. Since the natural flavonoids posses broad spectrum of binding affinities and inhibitory activities against numerous cellular targets, the
synthetic flavones with higher specificity for selective targets may be beneficial in achieving targeted effects. Therefore, further studies will be required to underpin the impact of these
synthetic flavones with specific functional groups on various molecular targets in platelets. Together, the results obtained in this study with synthetic flavones enhance the current
understanding of the SAR of flavones with human platelets and may aid in the design and development of novel anti-thrombotic strategies using flavones as potential molecular templates.
REFERENCES * George, J. N. Platelets. _Lancet_ 355, 1531–1539 (2000). Article PubMed CAS Google Scholar * Ghoshal, K. & Bhattacharyya, M. Overview of platelet physiology: Its
hemostatic and nonhemostatic role in disease pathogenesis. _Sci. World J._ 2014, 16 (2014). Article Google Scholar * Ruggeri, Z. M. Platelets in atherothrombosis. _Nat. Med._ 8, 1227–1234
(2002). Article PubMed CAS Google Scholar * Willoughby, S., Holmes, A. & Loscalzo, J. Platelets and cardiovascular disease. _Eur. J. Cardiovasc. Nurs._ 1, 273–288 (2002). Article
PubMed Google Scholar * Vane, J. R. & Botting, R. M. The mechanism of action of aspirin. _Thromb. Res._ 110, 255–258 (2003). Article PubMed CAS Google Scholar * Mackman, N.
Triggers, targets and treatments for thrombosis. _Nature_ 451, 914–918 (2008). Article ADS PubMed PubMed Central CAS Google Scholar * Michelson, A. D. P2Y12 Antagonism: Promises and
Challenges. _Arterioscler. Thromb. Vasc. Biol._ 28, s33–s38 (2008). Article PubMed CAS Google Scholar * Cattaneo, M. New P2Y12 blockers. _Journal of Thrombosis and Haemostasis_ 7,
262–265 (2009). Article PubMed CAS Google Scholar * Angiolillo, D. J., Bates, E. R. & Bass, T. A. Clinical profile of prasugrel, a novel thienopyridine. _Am. Heart J_. 156 (2008). *
Jakubowski, J. A., Winters, K. J., Naganuma, H. & Wallentin, L. Prasugrel: A novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic
basis for its distinct antiplatelet profile. _Cardiovascular Drug Reviews_ 25, 357–374 (2007). Article PubMed CAS Google Scholar * http://www.who.int/mediacentre/factsheets/fs317/en/;
Date accessed: 2017-06-17. * Hertog, M. G., Feskens, E. J., Hollman, P. C., Katan, M. B. & Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen
Elderly Study. _Lancet_ 342, 1007–1011 (1993). Article PubMed CAS Google Scholar * Wang, C.-Z., Mehendale, S. R., Calway, T. & Yuan, C.-S. Botanical flavonoids on coronary heart
disease. _Am. J. Chin. Med._ 39, 661–71 (2011). Article PubMed PubMed Central CAS Google Scholar * Hollenberg, N. K., Schmitz, H., McDonald, I. & Poulter, N. Cocoa, flavanols and
cardiovascular risk. _Br. J. Cardiol._ 11, 379–386 (2004). Google Scholar * Knekt, P., Jarvinen, R., Reunanen, A. & Maatela, J. Flavonoid intake and coronary mortality in Finland: a
cohort study. _BMJ_ 312, 478–481 (1996). Article PubMed PubMed Central CAS Google Scholar * Marjorie, M. _et al_. Flavonoid intake and cardiovascular disease mortality in a prospective
cohort of {US} adults. _Am. J. Clin. Nutr._ 95, 454–464 (2012). Article CAS Google Scholar * Ponzo, V. _et al_. Dietary flavonoid intake and cardiovascular risk: a population-based cohort
study. _J. Transl. Med._ 13, 218 (2015). Article PubMed PubMed Central CAS Google Scholar * McCullough, M. L. _et al_. Flavonoid intake and cardiovascular disease mortality in a
prospective cohort of US adults. _Am. J. Clin. Nutr._ 95, 454–64 (2012). Article PubMed PubMed Central CAS Google Scholar * Chiva-Blanch, G. _et al_. Differential effects of polyphenols
and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: A randomized clinical trial. _Am. J. Clin. Nutr._ 95, 326–334 (2012).
Article PubMed CAS Google Scholar * Estruch, R. _et al_. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: A prospective randomized
crossover trial: Effects of wine on inflammatory markers. _Atherosclerosis_ 175, 117–123 (2004). Article PubMed CAS Google Scholar * Pignatelli, P. _et al_. The flavonoids quercetin and
catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. _Am. J. Clin. Nutr._ 72, 1150–1155 (2000). Article PubMed CAS Google
Scholar * Pignatelli, P. _et al_. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine. _Atherosclerosis_ 188, 77–83 (2006). Article PubMed CAS
Google Scholar * Gupta, K. & Panda, D. Perturbation of microtubule polymerization by quercetin through tubulin binding: A novel mechanism of its antiproliferative activity.
_Biochemistry_ 41, 13029–13038 (2002). Article PubMed CAS Google Scholar * Böhl, M., Czupalla, C., Tokalov, S. V., Hoflack, B. & Gutzeit, H. O. Identification of actin as
quercetin-binding protein: An approach to identify target molecules for specific ligands. _Anal. Biochem._ 346, 295–299 (2005). Article PubMed CAS Google Scholar * Peluso, M. R.
Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue and liver. _Exp. Biol. Med. (Maywood)._ 231, 1287–1299 (2006).
Article PubMed CAS Google Scholar * Maeda-Yamamoto, M. _et al_. O-Methylated Catechins from Tea Leaves Inhibit Multiple Protein Kinases in Mast Cells. _J. Immunol._ 172, 4486–4492
(2004). Article PubMed CAS Google Scholar * Vaiyapuri, S. _et al_. Pharmacological actions of nobiletin in the modulation of platelet function. _Br. J. Pharmacol._ 172, 4133–4145 (2015).
Article PubMed PubMed Central CAS Google Scholar * Vaiyapuri, S. _et al_. Tangeretin regulates platelet function through inhibition of phosphoinositide 3-kinase and cyclic nucleotide
signaling. _Arterioscler. Thromb. Vasc. Biol._ 33, 2740–2749 (2013). Article PubMed CAS Google Scholar * Kumar, S. & Pandey, A. K. Flavonoids: Reviews Chemistry and Biological
Activities of Flavonoids: An Overview. _Sci. world J._ 2013, 17 (2013). MathSciNet Google Scholar * Hubbard, G. P. _et al_. Quercetin inhibits collagen-stimulated platelet activation
through inhibition of multiple components of the glycoprotein VI signaling pathway. _J. Thromb. Haemost._ 1, 1079–1088 (2003). Article PubMed CAS Google Scholar * Holt, R. R.,
Actis-Goretta, L., Momma, T. Y. & Keen, C. L. Dietary flavanols and platelet reactivity. _Journal of Cardiovascular Pharmacology_ 47 (2006). * Wright, B., Spencer, J. P. E., Lovegrove,
J. A. & Gibbins, J. M. Insights into dietary flavonoids as molecular templates for the design of anti-platelet drugs. _Cardiovasc. Res._ 97, 13–22 (2013). Article PubMed CAS Google
Scholar * Wright, B. _et al_. A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids. _Br. J. Pharmacol._ 159,
1312–1325 (2010). Article PubMed PubMed Central CAS Google Scholar * Bojić, M., Debeljak, Ž., Tomičić, M., Medić-Šarić, M. & Tomić, S. Evaluation of antiaggregatory activity of
flavonoid aglycone series. _Nutr. J._ 10, 73 (2011). Article PubMed PubMed Central CAS Google Scholar * Ravishankar, D., Watson, K. A., Greco, F. & Osborn, H. M. I. Novel
synthesised flavone derivatives provide significant insight into the structural features required for enhanced anti-proliferative activity. _RSC Adv._ 6, 64544–64556 (2016). Article CAS
Google Scholar * Xie, Y., Yang, W., Tang, F., Chen, X. & Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. _Curr. Med. Chem._ 22, 132–149
(2014). Article CAS Google Scholar * Liu, G. _et al_. Antiplatelet activity of chrysin via inhibiting platelet αIIbβ3-mediated signaling pathway. _Mol. Nutr. Food Res._ 60, 1984–1993
(2016). Article PubMed CAS Google Scholar * Ravishankar, D. _et al_. Ruthenium-conjugated chrysin analogues modulate platelet activity, thrombus formation and haemostasis with enhanced
efficacy. _Sci. Rep_. 7 (2017). * Navarro-Núñez, L. _et al_. Thromboxane A2 receptor antagonism by flavonoids: Structure-activity relationships. _J. Agric. Food Chem._ 57, 1589–1594 (2009).
Article PubMed CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors would like to thank the British Heart Foundation (Grant reference: PG/16/64/32311) and the Felix Trust
(PhD studentship for Dr Ravishankar) for their funding support. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Pharmacy, University of Reading, Reading, UK Divyashree Ravishankar,
Maryam Salamah, Angela Akimbaev, Harry F. Williams, Dina A. I. Albadawi, Francesca Greco, Helen M. I. Osborn & Sakthivel Vaiyapuri * School of Pharmacy, University of Reading Malaysia,
Johor, Malaysia Rajendran Vaiyapuri Authors * Divyashree Ravishankar View author publications You can also search for this author inPubMed Google Scholar * Maryam Salamah View author
publications You can also search for this author inPubMed Google Scholar * Angela Akimbaev View author publications You can also search for this author inPubMed Google Scholar * Harry F.
Williams View author publications You can also search for this author inPubMed Google Scholar * Dina A. I. Albadawi View author publications You can also search for this author inPubMed
Google Scholar * Rajendran Vaiyapuri View author publications You can also search for this author inPubMed Google Scholar * Francesca Greco View author publications You can also search for
this author inPubMed Google Scholar * Helen M. I. Osborn View author publications You can also search for this author inPubMed Google Scholar * Sakthivel Vaiyapuri View author publications
You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.R. and S.V. designed the biological aspects of this study, performed experiments, analysed data and wrote the
paper; H.M.I.O., F.G. and D.R. designed the chemical aspects of this study; M.S., A.A., H.W., D.A.I.A. and R.V. have performed experiments and analysed data. CORRESPONDING AUTHOR
Correspondence to Sakthivel Vaiyapuri. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ravishankar, D., Salamah,
M., Akimbaev, A. _et al._ Impact of specific functional groups in flavonoids on the modulation of platelet activation. _Sci Rep_ 8, 9528 (2018). https://doi.org/10.1038/s41598-018-27809-z
Download citation * Received: 02 March 2018 * Accepted: 05 June 2018 * Published: 22 June 2018 * DOI: https://doi.org/10.1038/s41598-018-27809-z SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative