- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT To develop effective therapies for advanced high grade serous ovarian cancer (HGSOC), understanding mechanisms of recurrence and metastasis is necessary. In this study, we define
the epithelial/mesenchymal status of cell lines that accurately model HGSOC, and evaluate the therapeutic potential of targeting Snai1 (Snail), a master regulator of the
epithelial/mesenchymal transition (EMT) _in vitro_ and _in vivo_. The ratio of Snail to E-cadherin (S/E index) at RNA and protein levels was correlated with mesenchymal morphology in four
cell lines. The cell lines with high S/E index (OVCAR8 and COV318) showed more CSC-like, motile, and chemoresistant phenotypes than those with low S/E index (OVSAHO and Kuramochi). We tested
the role of Snail in regulation of malignant phenotypes including stemness, cell motility, and chemotherapy resistance: shRNA-mediated knockdown of Snail reversed these malignant
phenotypes. Interestingly, the expression of let-7 tumour suppressor miRNA was upregulated in Snail knockdown cells. Furthermore, knockdown of Snail decreased tumour burden in an orthotopic
xenograft mouse model. We conclude that Snail is important in controlling HGSOC malignant phenotypes and suggest that the Snail/Let-7 axis may be an attractive target for HGSOC treatment.
SIMILAR CONTENT BEING VIEWED BY OTHERS ZEB2 FACILITATES PERITONEAL METASTASIS BY REGULATING THE INVASIVENESS AND TUMORIGENESIS OF CANCER STEM-LIKE CELLS IN HIGH-GRADE SEROUS OVARIAN CANCERS
Article Open access 01 July 2021 PNO1 ENHANCES OVARIAN CANCER CELL GROWTH, INVASION, AND STEMNESS VIA ACTIVATING THE AKT/WNT/Β-CATENIN PATHWAY Article Open access 20 March 2025 NOVEL ROLE OF
LNCRNA CHRF IN CISPLATIN RESISTANCE OF OVARIAN CANCER IS MEDIATED BY MIR-10B INDUCED EMT AND STAT3 SIGNALING Article Open access 08 September 2020 INTRODUCTION Metastatic
chemotherapy-resistant tumour recurrence is the primary cause of the 70% five-year mortality observed in patients with advanced high grade serous ovarian cancer (HGSOC). Despite successful
initial surgery and chemotherapy, most patients will experience recurrence of their disease, and of those who do, most will not respond to treatment with conventional chemotherapy
modalities1,2,3,4. We aim to understand mechanisms of recurrence and metastasis, toward the goal of developing targeted therapies. We have focused our efforts on HGSOC which causes 90% of
ovarian cancer deaths. Epithelial-mesenchymal transition (EMT), the process by which cells gain the ability to exit the epithelial layer and invade through the basement membrane, occurs as
part of normal embryonic development5. In cancer, transitions between epithelial and mesenchymal states are involved in processes leading to the aggressive phenotype that allows cells to
leave the primary tumour, invade secondary sites, and form metastases6. Of the transcription factors controlling EMT, we chose to focus on Snai1 (Snail) because its expression is sufficient
for EMT7, it transcriptionally activates pluripotency-related genes8 and its expression has been linked to stem cell characteristics in several cancers including breast9,10, liver11,
ovarian12, colorectal13, and squamous cell carcinoma of the head and neck14. Snail expression increases as HGSOC advances15,16, and correlates with poor prognosis17. Expression of the
transcription factor Snail (SNAI1) causes EMT by repressing the transcription of E-cadherin (CDH1), an adherens junction protein important for epithelial phenotype7, and other epithelial
factors. With the loss of E-cadherin there is a switch to N-cadherin (CDH2) production in the mesenchymal phenotype18. In addition to its actions leading to EMT, Snail has other effects in
normal epithelial cells, including growth arrest and resistance to apoptosis19. Partial EMTs, leading to cells expressing both mesenchymal and epithelial markers (E/M, also known as the
hybrid phenotype), may also be relevant for the acquisition of stem cell characteristics20. The hybrid state has been associated with aggressiveness and poor outcome in ovarian and other
cancers21,22,23. These E/M cells have been identified in primary tumour samples, and are the cells responsible for xenograft formation21. Expression of Snail has been linked to acquisition
of stem cell characteristics8,9,24,25. We have suggested that one mechanism by which Snail leads to stemness is by inhibiting let-7, a miRNA that maintains the differentiated state. We
demonstrated that Snail binds promoters of miRNA Let-7 family members during the process of reprogramming somatic cells to pluripotency25. Let-7 promotes differentiation and inhibits
self-renewal via its targets including HMGA2, LIN28, IMP-1, CDC34, and many others;26 it is expressed in somatic cells and absent in pluripotent cells. Let-7 acts as a tumour suppressor due
to its repression of targets such as c-Myc and Ras27. Let-7 is lost in many cancers, including ovarian28. Careful selection of cell lines facilitates their use as phenotypically accurate and
thus clinically useful _in vitro_ models of HGSOC. Very few publications have reported on the HGSOC cell lines with the highest degree of fidelity to patient samples29. In this work, we
describe HGSOC cell lines that accurately reflect the gene expression signature of HGSOC patient samples. Epithelial and mesenchymal characteristics of these cells are described, focusing on
the master EMT regulator Snail. Based on the EMT perspective, we correlate the presence of mesenchymal state with stem cell markers and function. MATERIALS AND METHODS CELL CULTURES OVSAHO,
Kuramochi, and COV318 human ovarian cancer cell lines were the kind gift of Gottfried Konecny (University of California Los Angeles), OVCAR8 human ovarian cancer cell line from Carlotta
Glackin (City of Hope), D2F human fibroblast cell line and NCCIT embryonal carcinoma cell line from George Daley (Harvard Medical School). OVSAHO, OVCAR8 and D2F cells were cultured in
Dulbecco’s Modification of Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS), 2 mM of L-Glutamine, 100 U/mL of penicillin, and 10 μg/mL of streptomycin. COV318 cells were cultured in
DMEM with 10% FBS, 2 mM of L-Glutamine, 100 U/mL of penicillin, 10 μg/mL of streptomycin, and 0.25 μg/mL of Gibco Amphotericin B. Kuramochi cells were cultured in Roswell Park Memorial
Institute Medium (RPMI) with 10% FBS, 2 mM of L-Glutamine, 0.25 U/mL of human insulin, 1x MEM non-essential amino acids (NEAA), 100 U/mL of penicillin, 10 μg/mL of streptomycin, and 0.25
μg/mL of Gibco Amphotericin B. NCCIT cells were cultured in RPMI with 10% FBS,2 mM of L-Glutamine, 1 mM of Sodium pyruvate, 1X NEAA, 100 U/mL of penicillin, and 10 μg/mL of streptomycin.
REAL-TIME QUANTITATIVE REVERSE-TRANSCRIPTION PCR (QRT-PCR) Total RNA from cell culture samples was isolated using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. For mRNA expression analysis, cDNA was synthesized with 1 μg of total RNA using Maxima First Strand cDNA Synthesis Kit (K1672; Thermo fisher scientific, Grand
Island, NY, USA). Real-time qRT-PCR for mRNA was performed using PowerUP SYBR Green master mix (Thermo fisher scientific, Grand Island, NY, USA) and specific primers on a Stratagene Mx3005P
instrument (Agilent Technology, Santa Clara, CA, USA). The sequence of primers for mRNA quantitation is shown in Table S1. For miRNA expression analysis, cDNA was synthesized with 100 ng of
total RNA using specific stem-loop RT primers and TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time qRT-PCR for miRNA was performed using TaqMan
Universal PCR Master Mix II (Applied Biosystems, Foster City, CA, USA) with specific primers and probes on a Stratagene Mx3005P instrument (Agilent Technology, Santa Clara, CA, USA). The
primers and probes for miRNA quantitation were supplied with the TaqMan microRNA Assay (Applied Biosystems, Foster City, CA, USA). The results were analysed using the ΔΔ cycles to threshold
(ΔΔCt) method. WESTERN BLOT Cells were lysed, and proteins were separated by SDS-PAGE and transferred to PVDF membrane. After blocking of non-specific binding, immunoblots were incubated
with primary antibodies for Snail (L70G2; Cell Signaling Technology, Danvers, MA, USA)30, E-cadherin (610182; BD Biosciences, San Jose, CA, USA)31, α/β-tubulin (2148 S; Cell Signaling
Technology, Danvers, MA, USA)32, and GAPDH (14C10; Cell Signaling Technology, Danvers, MA, USA)33 followed by incubation with an anti-mouse IgG conjugated with DyLight 800 (SA5-10176;
Invitrogen, Carlsbad, CA, USA)34 or anti-rabbit IgG antibody conjugated with DyLight 680 (35569; Invitrogen, Carlsbad, CA, USA)35. Immunoblots were scanned and visualized using Odyssey
Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Densitometry was performed on scanned immunoblots by ImageJ.software (National Institutes of Health, Bethesda, MD, USA).
SNAIL/E-CADHERIN INDEX Snail/E-cad index (S/E index) was determined based on protein expression levels quantified by Western Blot or mRNA expression levels measured by qRT-PCR. For
calculation, the following formula was used: $$S/E\,{\rm{Index}}={\rm{Snail}}\,{\rm{expression}}\,\mathrm{level}/E \mbox{-} {\rm{cadherin}}\,{\rm{expression}}\,{\rm{level}}$$ FLOW CYTOMETRY
Cells in FACS Stain (phosphate-buffered saline (PBS) with 1% FBS, 0.1% Sodium Azide, and 2 mM EDTA) were labeled with antibodies at 4 °C for 15 minutes, washed and then fixed in FACS Fix
(FACS Stain with 1% PFA). UltraComp eBeads (01-2222; Thermo fisher scientific, Grand Island, NY, USA) stained with each antibody were used for compensation. Flow cytometry was performed on
MACSQuant Analyzer 10 (Miltenyi Biotec, Auburn, CA, USA) and analysis of data was performed using FlowJo Version 10 (FlowJo LLC, Ashland, OR, USA). Fluorescent dye-conjugated antibodies for
CD44 (561292; BD Horizon, BD Biosciences, San Jose, CA, USA), CD117 (c-Kit) (130-099-325; Miltenyi Biotec, Auburn, CA, USA), CD133 (130-090-854, Miltenyi Biotec, Auburn, CA, USA), E-cadherin
(CD324) (130-099-141, Miltenyi Biotec, Auburn, CA, USA) and N-cadherin (CD325) (563435; BD Pharmingen, BD Biosciences, San Jose, CA, USA) were used. SPHEROID FORMATION ASSAY Cells were
plated at a density of 5 × 104 cells per well in 6-well non-tissue culture coated plates and maintained in serum-free medium for 7 days. The number of spheroids was counted and statistically
analysed. Phase contrast images of spheroids were taken and analysed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) to assess the size of spheroids. SCRATCH ASSAY
(WOUND-HEALING CELL MIGRATION ASSAY) Cells were grown to 90% confluency in 6-well tissue culture plates then treated with mitomycin C and scratched with a 200 μL micropipette tip. Pictures
of fixed positions in the wounds were taken every 4 hours for a 24-hour period with a bright field microscope with phase contrast. The wound area in each picture was measured by ImageJ
software (National Institutes of Health, Bethesda, MD, USA). CELL GROWTH INHIBITION ASSAY A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO,
USA) assay was used to determine cell viability. Cells were seeded at a density of 1 × 103 cells/well in 96-well plate and incubated overnight. The cells were then treated with increasing
concentrations of Cisplatin (0, 0.39, 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50 and 100 μM) for 3 days. After drug treatment, 10 μl of MTT solution was added to each well, and the plates were
incubated for 4 h at 37 °C. The formed formazan crystal was dissolved in dimethyl sulfoxide (DMSO), and the absorbance was measured at 570 nm using a SpectraMax i3x microplate reader
(Molecular Devices, Sunnyvale, CA, USA). The half-maximal inhibitory concentration (IC50) of cisplatin were analysed using the GraphPad Prism Version 7.0 (GraphPad Software, La Jolla, CA,
USA). LENTIVIRAL SHORT-HAIRPIN RNA (SHRNA) CONSTRUCTION AND CELL TRANSDUCTION Bacterial glycerol stock containing lentivirus plasmid vector pLKO.1-puro with shRNA specific for SNAI1
(shSnail; SHCLNG-NM_005985) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Scramble shRNA (shScr; Plasmid #1864) was purchased from Addgene (Addgene, Cambridge, MA, USA). Lentivirus
particles were produced in HEK293T cells after co-transfection of lentivirus plasmid vector shSnail or shScr with packaging plasmids using X-tremeGene9 (Sigma-Aldrich, St. Louis, MO, USA).
After 48 h and 72 h medium containing lentivirus was collected and filtered through 0.22 μM filter. Filtered virus containing medium was used for cell transduction or stored at −80 °C. Cells
were transduced with lentivirus in the presence of 6 μg/ml protamine sulfate and selected with puromycin for 4 days. MICE All animal procedures were conducted according to animal care
guidelines approved by the Institutional Animal Care and Use Committee at Loma Linda University. Nude mice (nu/nu) were obtained from Jackson Laboratory (Sacramento, CA, USA), were housed in
specific pathogen-free conditions, and were used for xenografts at 6–10 weeks of age. CELL PREPARATION FOR XENOGRAFT To allow _in vivo_ visualization, OVCAR8 cells were transduced with a
CMV-p:EGFP-ffluc pHIV7 lentiviral vector (eGFP-ffluc), which encodes a fusion protein of GFP and firefly luciferase36. The eGFP-ffluc-transduced OVCAR8 cells (OVCAR8-ffluc) were selectively
isolated based on GFP expression via FACSAria cell sorter (BD Biosciences, San Jose, CA, USA) and then transduced with lentivirus containing shRNA targeting Snail (shSnail) or scramble
control (shScr) for xenograft experiment. Lentivirus production and cell transduction were performed by the same procedure described in “Lentiviral short-hairpin RNA (shRNA) construction and
cell transduction” section. ORTHOTOPIC XENOGRAFT MODEL AND LIVE IMAGING shScr- or shSnail-expressing OVCAR8-ffluc cells were injected into the ovarian bursa of nude mice at 1:1 with
Matrigel (354248; Corning, Corning, NY, USA) at 2.5 × 105 cells per mouse (shScr group: n = 5, shSnail group: n = 4). After intraperitoneal injection of luciferin, the mice were imaged with
an IVIS Lumina Series III _In Vivo_ imaging system (PerkinElmer, Waltham, MA, USA). Live imaging was performed weekly and the bioluminescent images were analysed using Living Image _In Vivo_
Imaging Software (PerkinElmer, Waltham, MA, USA) to assess tumour burden at primary and metastatic sites. STATISTICS Graphical figures and statistical analysis were performed using GraphPad
Prism Version 7.0 (GraphPad Software, La Jolla, CA, USA). Detailed information on statistical analysis is described in figure legends. RESULTS CATEGORIZATION OF HGSOC CELL LINES BY
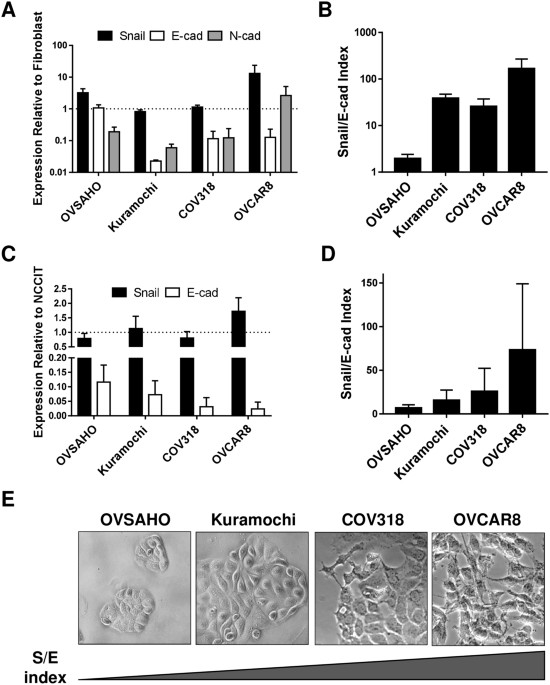
MESENCHYMAL/EPITHELIAL STATUS We characterized four of the best HGSOC cell line models, focusing on their epithelial and mesenchymal attributes. RNA and protein levels of Snail, CDH2 and
CDH1 varied widely among cell types, and all lines expressed all factors (Fig. 1A,C, Suppl. Figure 1). Because of CDH1 and Snail variability and coexpression, we developed an index that
considered relative levels of both factors, reasoning that both should be taken into consideration in determining the functional status of a cell population. On both the RNA and protein
level, the Snail/E-cadherin (S/E) index places OVCAR8 at the more mesenchymal end of the spectrum, OVSAHO is most epithelial, and COV318 and Kuramochi are intermediate (Fig. 1B,D).
Morphological differences between cell lines corroborated RNA and protein expression; OVCAR8 appeared most mesenchymal, with spindle-shaped cells extending projections, while OVSAHO grew in
more epithelial-like tightly-apposed colonies (Fig. 1E). CORRELATION BETWEEN SNAIL/E-CADHERIN INDEX AND MALIGNANT PHENOTYPE We examined the relationship between Snail expression and the
malignant phenotype of cell lines. First, the proportion of cancer stem-like cells in each line was evaluated. Using flow cytometry, percentage of cells expressing established ovarian CSC
markers CD117, CD133, and CD4437,38,39 was highest in OVCAR8, and lowest in OVSAHO (Fig. 2A, Suppl. Figure 2A). Because hybrid cells have been linked to the stem cell state23, we examined
the subset of cells positive for both CDH1 and CDH2 (Fig. 2B, Suppl. Figure 2B). These cells comprised between 2% (OVSAHO) and 22% (OVCAR8) of the population; cells positive for these
factors and the stem cell markers (CD117, CD133, CD44) were 0.1–6.75% of intact cells (Fig. 2C, Suppl. Figure 3A). Of note, over 95% of the CD117/CD133/CD44 triple positive population in all
lines were positive for both CDH1 and CDH2 (OVSAHO 100%, OVCAR8 97.2%), implying that the hybrid phenotype is necessary for the stem cell phenotype (Suppl. Figure 3B). However, between 5%
(OVSAHO) and 31.7% (OVCAR8) of the hybrid cells were positive for all three stem cell markers (Suppl. Figure 3B). Thus, the hybrid cells do not all attain the stem cell phenotype. Because
loss of let-7 corresponds with the stem cell state40, we determined let-7 levels in the HGSOC cell line panel. Let-7 levels were highest in the more epithelial, and lowest in the more
mesenchymal lines (Fig. 2D). To functionally assess the CSC phenotype, we assessed self-renewal ability of the HGSOC lines via growth in spheroids. Growth in non-adherent spheroid conditions
is used as a measure of stemness in both normal41,42,43 and cancer stem cells38,44,45,46,47,48,49. The frequency of cells triple positive for the markers associated with CSC was increased
after spheroid culture relative to cells grown in adherent conditions in cell lines assessed (Fig. 2E), confirming that spheroid culture enriches for stem cells. OVCAR8 produced the largest
spheroids (Fig. 2F), while COV318 and OVCAR8 produced the greatest number of spheroids (Fig. 2G). Thus, by both let-7 expression and spheroid formation, cells on the mesenchymal end of the
S/E ratio spectrum were more stem cell like. Mesenchymal cells are expected to be more invasive, and indeed a higher proportion of OVCAR8 than OVSAHO cells cross a basement membrane-like
barrier50. We assessed cell motility of lines by wound healing assay. Rate of wound recovery was greatest in OVCAR8, and least in OVSAHO (Fig. 2H). We examined cisplatin sensitivity by MTT
assay. Mesenchymal cells (COV318 and OVCAR8) were more resistant than epithelial cells (OVSAHO and Kuramochi) (Fig. 2I). Taken together, migratory ability and chemoresistance assays revealed
that more mesenchymal cells using the S/E index displayed a more malignant phenotype in HGSOC cell lines. KNOCKDOWN OF SNAIL EXPRESSION REVERSES MALIGNANT PHENOTYPE IN HGSOC CELLS We next
knocked down Snail through the use of virally-delivered shRNA in the most mesenchymal line, OVCAR8 (Suppl. Figures 4,5). We observed that CSC markers CD117 and CD133 both decreased, while
CD44 remained unchanged in shSnail relative to shScramble. CDH2 decreased as expected, but CDH1 levels remained unchanged (Fig. 3A). Snail knockdown (KD) resulted in a decrease in expression
of Nanog and Lin28 (Fig. 3B), and an increase in let-7 expression (Fig. 3C), both of which are consistent with disruption of the stem cell state. Size and number of spheroids formed with
Snail KD was also reduced showing a decrease in self renewal (Fig. 3D,E). Wound healing assays in OVCAR8 cells demonstrated a 30% decrease in migratory ability upon Snail KD (Fig. 3F).
Chemoresistance decreased in KD cells (Fig. 3G). These findings demonstrate that inhibiting Snail reverses functional measures of malignant phenotype in HGSOC cells. KNOCKDOWN OF SNAIL
EXPRESSION DECREASES TUMOUR BURDEN IN AN ORTHOTOPIC XENOGRAFT MOUSE MODEL We chose to develop an orthotopic xenograft model with OVCAR8, known to form clinically relevant tumours in immune
compromised mice, while Kuramochi and OVSAHO were shown to be poorly tumorigenic in xenografts51,52,53. Luciferized OVCAR8 cells in which Snail (or scrambled control) was knocked down by
lentiviral shRNA were injected into the ovarian bursae of Nude mice in an orthotopic xenograft model. Tumours were observed via bioluminescence imaging weekly for seven weeks, and quantified
via total flux. Although primary tumours often appear smaller in shSnail compared to shControl (Fig. 4A), quantitatively they were of similar size (Fig. 4B). This discrepancy may be due to
differences in three-dimensional size, or a more compact cellular organization in KD tumours. Metastatic tumours were significantly smaller in mice receiving shSnail cells (Fig. 4C). Upon
necropsy, primary and metastatic tumour weights showed a trend toward smaller tumours in shSnail mice (Suppl. Figure 6). Thus, we present evidence that decreasing levels of Snail reduces
metastatic tumour burden _in vivo_. DISCUSSION Our studies on acquisition of stemness in reprogramming25 led us to hypothesize that factors associated with EMT, specifically Snail, might
play pro-stemness roles in HGSOC. We investigated the role of Snail, known to control invasiveness54, in HGSOC stemness evolution. To do these studies, we characterized four of the ovarian
cancer lines shown to phenocopy HGSOC samples29. One unifying feature of these cells is their lack of a clearly defined epithelial or mesenchymal phenotype. On both RNA and protein levels,
all cell populations examined demonstrated the presence of both epithelial and mesenchymal factors (Fig. 1). This may reflect a dysregulation of stereotypical EMT pathways in these cells, or
could represent the activity of signals leading to the hybrid state specifically. The hybrid E/M state resulting from partial EMTs has been implicated in both normal development and in
cancer5,21. There is evidence for linkage between the hybrid state and stemness in both ovarian and breast cancer21,23. Although cells in all of these lines display a hybrid phenotype in
that they express both mesenchymal and epithelial markers, comparing levels of Snail and CDH1 allowed us to robustly categorize the lines along the E/M spectrum (Fig. 1). In comparison with
more epithelial lines (OVSAHO, Kuramochi) which grow in colonies, the more mesenchymal lines (COV318, OVCAR8) are morphologically more spindle-shaped, and the frequency of cells expressing
the established ovarian cancer stem cell surface markers CD133 and CD117 is higher. Culture in spheroid conditions resulted in enrichment of these stem cell markers. Mesenchymal lines formed
spheroids more efficiently, further correlating the mesenchymal with the stem cell phenotype. Importantly, cisplatin resistance was higher in the more mesenchymal lines, in agreement with
published results50,53. Let-7 levels, known to be high in differentiated cells and low in pluripotent stem cells, are lower in the mesenchymal lines COV318 and OVCAR8 (Fig. 2). Our studies,
while not designed to distinguish between stemness markers with regard to prediction of tumourigenesis, do suggest stemness in the CD117/CD133/CD44 triple positive population: it correlated
with spheroid-forming ability, and it nearly completely enriched for the E/M hybrid cells (Fig. 2, Suppl. Figure 3)55. However, similar to the observations of Strauss _et al_.21, the hybrid
state is diverse: not all hybrid cells express stem cell markers. Thus, our data are consistent with the hybrid state being necessary, but not sufficient, for the stem cell phenotype. Since
the majority of hybrid cells are apparently not stem cells, much remains to be learned about the signalling pathways leading to this state, and cell fate decisions leading to the presence or
absence of stemness markers in hybrid cells. Several signalling pathways have been proposed to play roles in partial EMTs, including the balance between Jagged/Notch signalling, regulated
by factors such as Numb56; and alternative splicing57,58. Inhibiting Snail is associated with an increase in levels of let-7 family members (Fig. 3), critical tumour suppressors with roles
in repressing stemness and proliferation. Snail positively regulates Nanog transcription, and associates with Nanog on the protein level leading to direct translational activation of
pluripotency genes8. The relationships between Let-7, Snail, and pluripotency, and the involvement of Snail in the EMT process, makes Snail an appealing target in cancer research. Our
studies suggest a connection between EMT, invasiveness, and traditionally defined stemness markers. These findings serve as _in vitro_ basis for a druggable model of tumour progression and
chemoresistance in HGSOC. Expanding our studies to animal models, we demonstrate that Snail inhibition leads to significant reduction of metastatic tumour burden _in vivo_ in an orthotopic
xenograft model. These data corroborate our cell line findings and provide preclinical evidence for Snail as a feasible target in HGSOC. Further exploration of Snail inhibition in synergy
with pharmacologic agents, such as platinum-based chemotherapy and targeted therapies such as PARP inhibitors, are underway. REFERENCES * Markman, M. _et al_. Duration of response to
second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. _Journal of clinical oncology: official journal of the American
Society of Clinical Oncology_ 22, 3120–3125, https://doi.org/10.1200/JCO.2004.05.195 (2004). Article CAS Google Scholar * Chang, S. J., Hodeib, M., Chang, J. & Bristow, R. E. Survival
impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. _Gynecologic oncology_ 130, 493–498,
https://doi.org/10.1016/j.ygyno.2013.05.040 (2013). Article PubMed Google Scholar * Coleman, R. L., Monk, B. J., Sood, A. K. & Herzog, T. J. Latest research and treatment of
advanced-stage epithelial ovarian cancer. _Nat Rev Clin Oncol_ 10, 211–224, https://doi.org/10.1038/nrclinonc.2013.5 (2013). Article PubMed PubMed Central Google Scholar * Aghajanian, C.
_et al_. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer.
_Gynecologic oncology_ 139, 10–16, https://doi.org/10.1016/j.ygyno.2015.08.004 (2015). Article PubMed PubMed Central CAS Google Scholar * Nieto, M. A., Huang, R. Y., Jackson, R. A.
& Thiery, J. P. Emt: 2016. _Cell_ 166, 21–45, https://doi.org/10.1016/j.cell.2016.06.028 (2016). Article PubMed CAS Google Scholar * Heerboth, S. _et al_. EMT and tumor metastasis.
_Clin Transl Med_ 4, 6, https://doi.org/10.1186/s40169-015-0048-3 (2015). Article PubMed PubMed Central Google Scholar * Cano, A. _et al_. The transcription factor snail controls
epithelial-mesenchymal transitions by repressing E-cadherin expression. _Nat Cell Biol_ 2, 76–83, https://doi.org/10.1038/35000025 (2000). Article PubMed CAS Google Scholar * Gingold, J.
_et al_. A Genome-Wide RNAi Screen Identifies Opposing Functions of Snai1 and Snai2 on the Nanog Dependency in Reprogramming. _Mol Cell_ 56, 140–152,
https://doi.org/10.1016/j.molcel.2014.08.014 (2014). Article PubMed PubMed Central CAS Google Scholar * Mani, S. A. _et al_. The epithelial-mesenchymal transition generates cells with
properties of stem cells. _Cell_ 133, 704–715 (2008). Article PubMed PubMed Central CAS Google Scholar * Morel, A. P. _et al_. Generation of breast cancer stem cells through
epithelial-mesenchymal transition. _PLoS ONE_ 3, e2888 (2008). Article ADS PubMed PubMed Central CAS Google Scholar * Dang, H., Ding, W., Emerson, D. & Rountree, C. B. Snail1
induces epithelial-to-mesenchymal transition and tumor initiating stem cell characteristics. _BMC cancer_ 11, 396, https://doi.org/10.1186/1471-2407-11-396 (2011). Article PubMed PubMed
Central CAS Google Scholar * Elloul, S. _et al_. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma.
_Cancer_ 103, 1631–1643, https://doi.org/10.1002/cncr.20946 (2005). Article PubMed CAS Google Scholar * Fan, F. _et al_. Overexpression of snail induces epithelial-mesenchymal transition
and a cancer stem cell-like phenotype in human colorectal cancer cells. _Cancer Med_ 1, 5–16, https://doi.org/10.1002/cam4.4 (2012). Article PubMed PubMed Central CAS Google Scholar *
Yang, M. H. _et al_. Overexpression of NBS1 induces epithelial-mesenchymal transition and co-expression of NBS1 and Snail predicts metastasis of head and neck cancer. _Oncogene_ 26,
1459–1467, https://doi.org/10.1038/sj.onc.1209929 (2007). Article PubMed CAS Google Scholar * Wang, Y. L. _et al_. Snail promotes epithelial-mesenchymal transition and invasiveness in
human ovarian cancer cells. _Int J Clin Exp Med_ 8, 7388–7393 (2015). PubMed PubMed Central CAS Google Scholar * Jin, H. _et al_. Snail is critical for tumor growth and metastasis of
ovarian carcinoma. _International journal of cancer. Journal international du cancer_ 126, 2102–2111, https://doi.org/10.1002/ijc.24901 (2010). Article PubMed CAS Google Scholar *
Blechschmidt, K. _et al_. The E-cadherin repressor Snail is associated with lower overall survival of ovarian cancer patients. _British journal of cancer_ 98, 489–495,
https://doi.org/10.1038/sj.bjc.6604115 (2008). Article PubMed CAS Google Scholar * Maeda, M., Johnson, K. R. & Wheelock, M. J. Cadherin switching: essential for behavioral but not
morphological changes during an epithelium-to-mesenchyme transition. _J Cell Sci_ 118, 873–887, https://doi.org/10.1242/jcs.01634 (2005). Article PubMed CAS Google Scholar * Vega, S. _et
al_. Snail blocks the cell cycle and confers resistance to cell death. _Genes Dev_ 18, 1131–1143, https://doi.org/10.1101/gad.294104 (2004). Article PubMed PubMed Central CAS Google
Scholar * Jolly, M. K. _et al_. Towards elucidating the connection between epithelial-mesenchymal transitions and stemness. _Journal of the Royal Society, Interface/the Royal Society_ 11,
20140962, https://doi.org/10.1098/rsif.2014.0962 (2014). Article CAS Google Scholar * Strauss, R. _et al_. Analysis of epithelial and mesenchymal markers in ovarian cancer reveals
phenotypic heterogeneity and plasticity. _PLoS ONE_ 6, e16186, https://doi.org/10.1371/journal.pone.0016186 (2011). Article ADS PubMed PubMed Central CAS Google Scholar * Schliekelman,
M. J. _et al_. Molecular portraits of epithelial, mesenchymal, and hybrid States in lung adenocarcinoma and their relevance to survival. _Cancer Res_ 75, 1789–1800,
https://doi.org/10.1158/0008-5472.CAN-14-2535 (2015). Article PubMed PubMed Central CAS Google Scholar * Grosse-Wilde, A. _et al_. Stemness of the hybrid Epithelial/Mesenchymal State in
Breast Cancer and Its Association with Poor Survival. _PLoS One_ 10, e0126522, https://doi.org/10.1371/journal.pone.0126522 (2015). Article PubMed PubMed Central CAS Google Scholar *
Lu, Z. Y. _et al_. SNAI1 overexpression induces stemness and promotes ovarian cancer cell invasion and metastasis. _Oncology reports_ 27, 1587–1591, https://doi.org/10.3892/or.2012.1685
(2012). Article PubMed CAS Google Scholar * Unternaehrer, J. J. _et al_. The epithelial-mesenchymal transition factor SNAIL paradoxically enhances reprogramming. _Stem cell reports_ 3,
691–698, https://doi.org/10.1016/j.stemcr.2014.09.008 (2014). Article PubMed PubMed Central CAS Google Scholar * Boyerinas, B. _et al_. Identification of let-7-regulated oncofetal
genes. _Cancer Res_ 68, 2587–2591, https://doi.org/10.1158/0008-5472.CAN-08-0264 (2008). Article PubMed CAS Google Scholar * Nimmo, R. A. & Slack, F. J. An elegant miRror: microRNAs
in stem cells, developmental timing and cancer. _Chromosoma_ 118, 405–418, https://doi.org/10.1007/s00412-009-0210-z (2009). Article PubMed PubMed Central CAS Google Scholar *
Boyerinas, B., Park, S. M., Hau, A., Murmann, A. E. & Peter, M. E. The role of let-7 in cell differentiation and cancer. _Endocrine-related cancer_ 17, F19–36,
https://doi.org/10.1677/ERC-09-0184 (2010). Article PubMed CAS Google Scholar * Domcke, S., Sinha, R., Levine, D. A., Sander, C. & Schultz, N. Evaluating cell lines as tumour models
by comparison of genomic profiles. _Nature communications_ 4, 2126, https://doi.org/10.1038/ncomms3126 (2013). Article ADS PubMed PubMed Central CAS Google Scholar * Kim, N. H. _et
al_. Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. _Nature communications_ 8, 14374,
https://doi.org/10.1038/ncomms14374 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Ma, L. _et al_. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer
metastasis. _Nat Cell Biol_ 12, 247–256, https://doi.org/10.1038/ncb2024 (2010). Article PubMed PubMed Central CAS Google Scholar * Yoshimaru, T. _et al_. A-kinase anchoring protein
BIG3 coordinates oestrogen signalling in breast cancer cells. _Nature communications_ 8, 15427, https://doi.org/10.1038/ncomms15427 (2017). Article ADS PubMed PubMed Central CAS Google
Scholar * Cole, A. J. _et al_. Assessing mutant p53 in primary high-grade serous ovarian cancer using immunohistochemistry and massively parallel sequencing. _Sci Rep_ 6, 26191,
https://doi.org/10.1038/srep26191 (2016). Article ADS PubMed PubMed Central CAS Google Scholar * Turchinovich, A., Surowy, H. M., Tonevitsky, A. G. & Burwinkel, B. Interference in
transcription of overexpressed genes by promoter-proximal downstream sequences. _Sci Rep_ 6, 30735, https://doi.org/10.1038/srep30735 (2016). Article ADS PubMed PubMed Central CAS
Google Scholar * Li, L. _et al_. Increased ROS production in non-polarized mammary epithelial cells induces monocyte infiltration in 3D culture. _J Cell Sci_ 130, 190–202,
https://doi.org/10.1242/jcs.186031 (2017). Article ADS PubMed CAS Google Scholar * Brown, C. E. _et al_. Recognition and killing of brain tumor stem-like initiating cells by
CD8+cytolytic T cells. _Cancer Res_ 69, 8886–8893, https://doi.org/10.1158/0008-5472.CAN-09-2687 (2009). Article PubMed PubMed Central CAS Google Scholar * Silva, I. A. _et al_.
Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. _Cancer Res_ 71, 3991–4001,
https://doi.org/10.1158/0008-5472.CAN-10-3175 (2011). Article PubMed PubMed Central CAS Google Scholar * Zhang, S. _et al_. Identification and characterization of ovarian
cancer-initiating cells from primary human tumors. _Cancer Res_ 68, 4311–4320, https://doi.org/10.1158/0008-5472.CAN-08-0364 (2008). Article PubMed PubMed Central CAS Google Scholar *
Foster, R., Buckanovich, R. J. & Rueda, B. R. Ovarian cancer stem cells: working towards the root of stemness. _Cancer letters_ 338, 147–157, https://doi.org/10.1016/j.canlet.2012.10.023
(2013). Article PubMed CAS Google Scholar * Peter, M. E. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. _Cell Cycle_ 8, 843–852 (2009). Article
PubMed PubMed Central CAS Google Scholar * Dontu, G. _et al_. _In vitro_ propagation and transcriptional profiling of human mammary stem/progenitor cells. _Genes Dev_ 17, 1253–1270,
https://doi.org/10.1101/gad.1061803 (2003). Article PubMed PubMed Central CAS Google Scholar * Bez, A. _et al_. Neurosphere and neurosphere-forming cells: morphological and
ultrastructural characterization. _Brain Res_ 993, 18–29 (2003). Article PubMed CAS Google Scholar * Shi, X., Gipp, J. & Bushman, W. Anchorage-independent culture maintains prostate
stem cells. _Dev Biol_ 312, 396–406, https://doi.org/10.1016/j.ydbio.2007.09.042 (2007). Article PubMed PubMed Central CAS Google Scholar * Cao, L. _et al_. Sphere-forming cell
subpopulations with cancer stem cell properties in human hepatoma cell lines. _BMC Gastroenterol_ 11, 71, https://doi.org/10.1186/1471-230X-11-71 (2011). Article ADS PubMed PubMed Central
CAS Google Scholar * Ponti, D. _et al_. Isolation and _in vitro_ propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. _Cancer Res_ 65, 5506–5511,
https://doi.org/10.1158/0008-5472.CAN-05-0626 (2005). Article PubMed CAS Google Scholar * Fang, D. _et al_. A tumorigenic subpopulation with stem cell properties in melanomas. _Cancer
Res_ 65, 9328–9337, https://doi.org/10.1158/0008-5472.CAN-05-1343 (2005). Article PubMed CAS Google Scholar * Gou, S. _et al_. Establishment of clonal colony-forming assay for
propagation of pancreatic cancer cells with stem cell properties. _Pancreas_ 34, 429–435, https://doi.org/10.1097/MPA.0b013e318033f9f4 (2007). Article PubMed Google Scholar * Fujii, H.
_et al_. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. _Int J Oncol_ 34, 1381–1386 (2009). PubMed CAS Google Scholar * Rappa, G. _et al_.
Growth of cancer cell lines under stem cell-like conditions has the potential to unveil therapeutic targets. _Exp Cell Res_ 314, 2110–2122, https://doi.org/10.1016/j.yexcr.2008.03.008
(2008). Article PubMed PubMed Central CAS Google Scholar * Haley, J. _et al_. Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative
experimental models of the disease. _Oncotarget_ 7, 32810–32820, https://doi.org/10.18632/oncotarget.9053 (2016). Article PubMed PubMed Central Google Scholar * Mitra, A. K. _et al_. _In
vivo_ tumor growth of high-grade serous ovarian cancer cell lines. _Gynecologic oncology_ 138, 372–377, https://doi.org/10.1016/j.ygyno.2015.05.040 (2015). Article PubMed PubMed Central
CAS Google Scholar * Roberts, C. M. _et al_. Nanoparticle delivery of siRNA against TWIST to reduce drug resistance and tumor growth in ovarian cancer models. _Nanomedicine:
nanotechnology, biology, and medicine_, https://doi.org/10.1016/j.nano.2016.11.010 (2016). * Elias, K. M. _et al_. Beyond genomics: critical evaluation of cell line utility for ovarian
cancer research. _Gynecologic oncology_ 139, 97–103, https://doi.org/10.1016/j.ygyno.2015.08.017 (2015). Article PubMed PubMed Central CAS Google Scholar * Lamouille, S., Xu, J. &
Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. _Nat Rev Mol Cell Biol_ 15, 178–196, https://doi.org/10.1038/nrm3758 (2014). Article PubMed PubMed Central CAS
Google Scholar * Jolly, M. K. _et al_. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. _Frontiers in oncology_ 5, 155, https://doi.org/10.3389/fonc.2015.00155
(2015). Article PubMed PubMed Central Google Scholar * Bocci, F. _et al_. Numb prevents a complete epithelial-mesenchymal transition by modulating Notch signalling. _Journal of the Royal
Society, Interface_/_the Royal Society_ 14, https://doi.org/10.1098/rsif.2017.0512 (2017). * Pradella, D., Naro, C., Sette, C. & Ghigna, C. EMT and stemness: flexible processes tuned by
alternative splicing in development and cancer progression. _Mol Cancer_ 16, 8, https://doi.org/10.1186/s12943-016-0579-2 (2017). Article PubMed PubMed Central CAS Google Scholar *
Shapiro, I. M. _et al_. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. _PLoS Genet_ 7, e1002218,
https://doi.org/10.1371/journal.pgen.1002218 (2011). Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to Gottfried Konecny, Ann
Klopp, Daniela Dinulescu, and Christine Brown for cell lines and reagents, Maria Filippova for expert advice, Kimberly Payne for flow cytometry support, Casiano lab for assistance with cell
viability assays, Marino DeLeon and the Center for Health Disparities and Molecular Medicine (CHDMM) for use of facilities, George Daley for laboratory equipment, and members of the
Unternaehrer and Glackin labs for helpful discussions. This work was supported by a Grant to Promote Collaboration and Translation from Loma Linda University (LLU) to J.U. and Y.I., and by
LLU startup funding. M.G. was supported by a CIRM Bridges grant, H.C. by the Apprenticeship Bridge to College program of the CHDMM. AUTHOR INFORMATION Author notes * N. Hojo and A. L.
Huisken contributed equally to this work. AUTHORS AND AFFILIATIONS * Division of Biochemistry, Department of Basic Sciences, Loma Linda University, Loma Linda, CA, USA N. Hojo, A. L.
Huisken, H. Wang, E. Chirshev, H. Campos & J. J. Unternaehrer * Department of Molecular Biology, Chonbuk National University, Dukjindong 664-14, Jeonju, Jeollabuk-do, 561-756, Republic
of Korea N. S. Kim * University of California, Riverside - School of Medicine, Riverside, CA, USA S. M. Nguyen * Center for Health Disparities and Molecular Medicine, Loma Linda University,
Loma Linda, CA, USA H. Campos * Beckman Research Institute, City of Hope, Duarte, CA, USA C. A. Glackin * Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Loma
Linda University Medical Center, Loma Linda, CA, USA Y. J. Ioffe Authors * N. Hojo View author publications You can also search for this author inPubMed Google Scholar * A. L. Huisken View
author publications You can also search for this author inPubMed Google Scholar * H. Wang View author publications You can also search for this author inPubMed Google Scholar * E. Chirshev
View author publications You can also search for this author inPubMed Google Scholar * N. S. Kim View author publications You can also search for this author inPubMed Google Scholar * S. M.
Nguyen View author publications You can also search for this author inPubMed Google Scholar * H. Campos View author publications You can also search for this author inPubMed Google Scholar *
C. A. Glackin View author publications You can also search for this author inPubMed Google Scholar * Y. J. Ioffe View author publications You can also search for this author inPubMed Google
Scholar * J. J. Unternaehrer View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.H., N.H., C.G., Y.I. and J.U. designed the experiments,
N.H., A.H., H.W., E.C., N.K., H.C., Y.I. and J.U. performed experiments, N.H., A.H., H.W., E.C., N.K., S.M.N., C.G., Y.I. and J.U. analysed and interpreted data, N.H., A.H., E.C. and J.U.
prepared figures and N.H., A.H., S.M.N., Y.I. and J.U. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to J. J. Unternaehrer. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hojo, N., Huisken, A.L., Wang, H. _et al._ Snail knockdown reverses stemness and
inhibits tumour growth in ovarian cancer. _Sci Rep_ 8, 8704 (2018). https://doi.org/10.1038/s41598-018-27021-z Download citation * Received: 29 December 2017 * Accepted: 23 May 2018 *
Published: 07 June 2018 * DOI: https://doi.org/10.1038/s41598-018-27021-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative