- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
L-ascorbic acid (Vitamin C) can enhance the meiotic maturation and developmental competence of porcine oocytes, but the underlying molecular mechanism remains obscure. Here we show the role
of ascorbic acid in regulating epigenetic status of both nucleic acids and chromatin to promote oocyte maturation and development in pigs. Supplementation of 250 μM L-ascorbic acid
2-phosphate sesquimagnesium salt hydrate (AA2P) during in vitro maturation significantly enhanced the nuclear maturation (as indicated by higher rate of first polar body extrusion and
increased Bmp15 mRNA level), reduced level of reactive oxygen species, and promoted developmental potency (higher cleavage and blastocyst rates of parthenotes, and decreased Bax and Caspase3
mRNA levels in blastocysts) of pig oocytes. AA2P treatment caused methylation erasure in mature oocytes on nucleic acids (5-methylcytosine (5 mC) and N6-methyladenosine (m6A)) and histones
(Histone H3 trimethylations at lysines 27, H3K27me3), but establishment of histone H3 trimethylations at lysines 4 (H3K4me3) and 36 (H3K36me3). During the global methylation reprogramming
process, levels of TET2 (mRNA and protein) and Dnmt3b (mRNA) were significantly elevated, but simultaneously DNMT3A (mRNA and protein), and also Hif-1α, Hif-2α, Tet3, Mettl14, Kdm5b and Eed
(mRNA) were significantly inhibited. Our findings support that ascorbic acid can reprogram the methylation status of not only DNA and histone, but also RNA, to improve pig oocyte maturation
and developmental competence.
L-ascorbic acid (Vitamin C), a water-soluble antioxidant and electron donor, can be synthesized in the liver of many species, except for guinea pigs, human and other primates1. Ascorbic acid
can be actively transported into cells to reach a high concentration up to 1~10 mM1,2, by the high affinity sodium-dependent vitamin C transporters 1 and 2 (SVCT1 and SVCT2)3. Accumulating
evidences demonstrate that ascorbic acid plays an important role in multiple biological processes4,5 via its reductive form (ascorbate), to reduce free radicals and reactive oxygen species
by serving as powerful antioxidant (non-enzymatic function), and as an essential cofactor to modulate the family of ferrous ion- and 2-oxoglutarate (Fe2+ and 2-OG)-dependent dioxygenases
(enzymatic function)6. The dioxygenase enzyme family consists of several subgroups, exists widely in nature, and catalyzes epigenetic demethylation and hydroxylation reactions, to affect
multiple biological processes, such as collagen biosynthesis, hypoxic sensing, lipid metabolism7 and pluripotency8. Subgroup I, the ten-eleven-translocation 1–3 (Tet1–3) enzymes, is known to
catalyze the DNA demethylation by converting 5-methylcytosine (5 mC) to 5-hydroxymethylcytosine (5 hmC)9. Subgroup II, the AlkB dioxygenases, includes fat mass and obesity-associated (Fto)
and AlkB homologue 5 (Alkbh5) genes, to remove the methyl group from N6-methyladenosine (m6A) in DNA or RNA10,11. Subgroup III, the Jumonji C (JmjC) domain containing histone lysine
demethylases (JmjC-KDMs), erases the methyl group on lysine residues of histones12. Subgroup IV, the hypoxia-inducible factor (HIF) hydroxylases, catalyzes hydroxylation on specific proline
and asparagine residues of the transcription factor HIF-1α13. Studies confirmed that ascorbic acid regulates Tet, JmjC domain containing enzymes and HIF hydroxylases, to modulate dynamically
the epigenetic status of DNA/histone methylation and HIF-1α activity6,9,12,13,14. However, whether and how ascorbic acid act via AlkB dioxygenases to effect m6A modification are largely
unknown.
Mammalian oocyte development is coordinated by a complex molecular network, and dynamic epigenetic methylation regulation on DNA and histones is crucial for both oocyte meiosis15,16 and
early embryo development17,18,19. Histone H3 trimethylations at lysines 4 (H3K4me3) and 36 (H3K36me3) are associated with active chromatin status, and trimethylations at lysines 9 (H3K9me3)
and 27 (H3K27me3) represent repressive status20,21, which have critical epigenetic roles in regulating oogenesis and embryogenesis22. It is well known that the in vitro culture and
maturation system of mammalian oocytes is often far from optimization, and as a result, large amount of reactive oxygen species (ROS) will usually be induced. Normally, ROS can be
neutralized by the antioxidant defense system. However, when the net ROS level is above the physiological threshold, oxidative stress will occur, and thereby decrease oocyte quality and
hinder subsequent embryonic development23. Recently, RNA methylation was also found to play an important role in oocyte meiosis in Xenopus laevis24, and maternal-to-zygote transition of
early embryos in zebrafish25.
Furthermore, ascorbic acid treatment during in vitro maturation can enhance the nuclear maturation of porcine oocytes devoid of cumulus cells26, increase intracellular glutathione (GSH)
level, reduce ROS level of porcine oocytes enclosed with cumulus cells27, and improve developmental competence of porcine oocytes after parthenogenetic activation26,27, in vitro
fertilization28 and somatic cell nuclear transfer27. Supplementation of ascorbic acid during embryo culture can also improve blastocyst development of porcine hand-cloned embryos29 and mouse
embryos made by somatic cell nuclear transfer30. However, the underlying molecular mechanism remains unknown.
In the present study, we aimed to understand how ascorbic acid improves the maturation and developmental competence of porcine oocytes enclosed with cumulus cells, with a special focus on
epigenetic regulation. Here we showed that through regulating global epigenetic modifications at DNA, RNA and histone levels, supplementation of ascorbic acid during in vitro maturation can
benefit the meiotic maturation and subsequent development of pig oocytes.
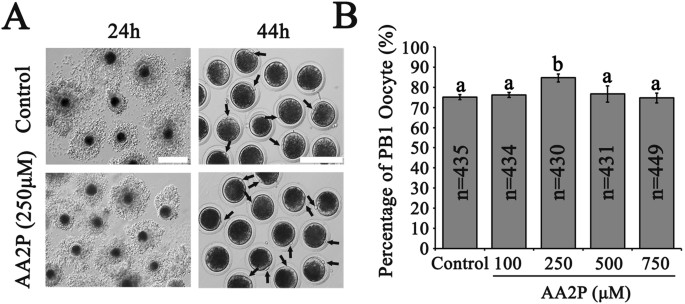
Addition of L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (AA2P) into the in vitro maturation (IVM) system to culture pig cumulus-oocyte complexes (COCs) for 44 h showed that the
rate of the first polar body (PB1) extrusion was significantly higher in 250 µM AA2P group (84.8%, n = 430) in comparison to the control (vs. 75.2%, n = 435; P










