- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Schizophrenia (SZ) and bipolar I disorder (BD-I) share genetic risk factors and cognitive impairments, but these conditions may exhibit differences in cortical functioning
associated with inhibitory control. We measured hemodynamic responses during a stop-signal task using near-infrared spectroscopy (NIRS) in 20 patients with SZ, 21 patients with BD-I and 18
healthy controls (HCs). We used stop-signal reaction time (SSRT) to estimate behavioural inhibition. Compared with HCs, patients with either SZ or BD-I exhibited significantly reduced
activation in the bilateral inferior, middle and superior frontal gyri. Furthermore, patients with BD-I showed inactivation of the right superior temporal gyri compared with patients with SZ
or HCs. Patients with SZ or BD-I demonstrated significant negative correlations between SSRT and hemodynamic responses of the right inferior frontal gyrus. Moreover, patients with SZ
exhibited correlations in the middle and superior frontal gyri. Our findings suggest that right inferior frontal abnormalities mediate behavioural inhibition impairments in individuals with
SZ or BD-I. Differential patterns of orbitofrontal or superior temporal functional abnormalities may reflect important differences in psychopathological features between these disorders.
SIMILAR CONTENT BEING VIEWED BY OTHERS REDUCED TEMPORAL VARIABILITY OF CORTICAL EXCITATION/INHIBITION RATIO IN SCHIZOPHRENIA Article Open access 18 February 2025 AN ERP STUDY ON PROACTIVE
AND REACTIVE RESPONSE INHIBITION IN INDIVIDUALS WITH SCHIZOTYPY Article Open access 16 April 2021 REDUCTION OF INTRACORTICAL INHIBITION (ICI) CORRELATES WITH COGNITIVE PERFORMANCE AND
PSYCHOPATHOLOGY SYMPTOMS IN SCHIZOPHRENIA Article Open access 14 September 2024 INTRODUCTION Although the traditional diagnostic classification in psychiatry cuts across the natural
boundaries between schizophrenia (SZ) and bipolar I disorder (BD-I), recent epidemiological and molecular evidence supports shared genetic contributions between these disorders1. In
addition, these psychiatric disorders share cognitive impairments that may be linked to structural and functional alterations in the frontotemporal cortex2,3. Deficits in inhibitory control,
a major subcomponent of executive function, have been described in both SZ and BD-I. Inhibitory control is the ability to override, interrupt, or abort ongoing processes, especially when
these processes are well engrained3. The stop-signal task can estimate the time required by an individual to withhold ongoing responses, as measured by stop-signal reaction time (SSRT)4.
Compared with healthy controls (HCs), SSRTs are longer (i.e. suggesting impaired behavioural inhibition) in individuals with SZ5 or BD-I6,7. Neuroimaging studies indicate that inhibitory
processes involve the frontal cortex, particularly the right inferior frontal gyrus and presupplementary motor area8,9,10,11. Previous neuroimaging studies have found structural and
functional abnormalities in these regions in individuals with SZ12,13,14 or BD-I7,15,16. In addition, functional magnetic resonance imaging (fMRI) studies have reported associations between
behavioural inhibition and functional abnormalities of these brain regions in individuals with SZ13,14 or BD-I17,18. These neuroimaging studies suggest overlapping neural abnormalities in
inhibitory processes between SZ or BD-I; however, it remains unclear to what extent these abnormalities have similar profiles or to what degree they differ between the two psychiatric
disorders. Near-infrared spectroscopy (NIRS) is a non-invasive optical technique for monitoring hemodynamic changes related to cortical neural activity by measuring relative changes in
haemoglobin (Hb). Previous NIRS studies have reported functional abnormalities in the prefrontal cortex during a cognitive task in patients with SZ19,20 and BD-I (mainly individuals with
bipolar II disorder)21. Recent studies have also reported that, compared with HCs, individuals with SZ show functional abnormalities of the bilateral prefrontal cortex during Go/No Go22 and
stop-signal tasks23. Additionally, patients with bipolar II disorder show decreased oxygenated haemoglobin (oxy-Hb) changes in the bilateral orbitofrontal and left prefrontal cortices during
the Iowa Gambling Task24. However, to our knowledge, no NIRS studies to date have directly compared functional abnormalities associated with inhibitory control in patients with SZ or BD-I
relative to HCs. In the current study, we investigated differences in cortical frontotemporal functional abnormalities associated with inhibitory control between patients with SZ or BD-I and
HCs. We hypothesised that patients with SZ or BD-I possess shared–as well as different–cortical function patterns. These functional abnormalities appear to be directly associated with
observed cognitive and clinical features, and these associations may characterise important differences in psychopathology between the two disorders. RESULTS DEMOGRAPHIC CHARACTERISTICS The
study participants consisted of patients with SZ (n = 20), BD-I (n = 21) and 18 HCs. Participants’ demographic data are shown in Table 1. TASK PERFORMANCE SSRTs differed significantly across
the SZ, BD-I and HC groups (P = 0.015; Table 1). Patients with SZ demonstrated longer SSRTs than HCs (P = 0.011). No significant differences were observed between with patients BD-I and HCs
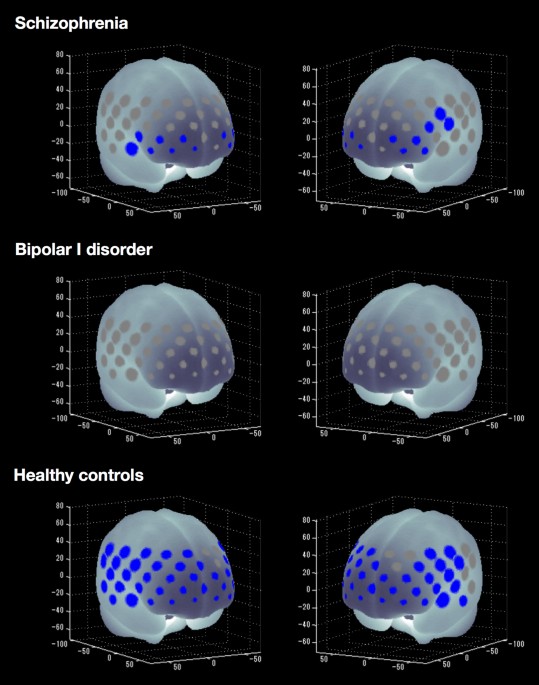
(P = 0.26) or between patients with SZ and BD-I (P = 0.30). COGNITIVE ACTIVATION WITHIN GROUPS HCs showed significantly increased mean oxy-Hb levels between the pre-task baseline and the
inhibitory control task period in 45 of 52 channels (‘ch’) (ch1–5, ch8–9, ch11–16, ch18–20, ch22–30, ch32–41 and ch43–52; t = −8.27 to −2.28; maximum false discovery rate (FDR)-corrected P
< 0.05, corrected for 52 channels). Thus, widespread frontotemporal cortical activation related to oxy-Hb was induced by the inhibitory control task in HCs. Patients with SZ showed
significantly increased mean oxy-Hb levels in 14 of 52 channels (ch19, ch29–30, ch34–36, ch38–39, ch44–47 and ch49–50; t = −4.22 to −2.76; FDR-corrected P < 0.05, corrected for 52
channels). By contrast, patients with BD-I did not show significant changes in oxy-Hb levels in any channel (FDR-corrected P < 0.05, corrected for 52 channels). Figure 1 summarises the
cognitive activation results for all three groups. REGIONAL DIFFERENCES IN TASK-RELATED OXY-HB CHANGES AMONG GROUPS Task-related oxy-Hb changes differed significantly among the SZ, BD-I and
HC groups in 21 channels (ch4–5, ch12, ch14–15, ch18, ch24–25, ch34–37, ch39, ch43–46, ch48–51; F = 4.23–11.18; FDR-corrected P < 0.05, corrected for 52 channels). Compared with HCs, the
SZ group exhibited significantly smaller task-related oxy-Hb changes in 11 channels (ch5, ch14–15, ch18, ch25, ch36–37, ch39, ch46 and ch48–49; p = 0.009–0.049), whereas the BD group
exhibited significantly smaller changes in 21 channels (ch4–5, ch12, ch14–15, ch18, ch24–25, ch34–37, ch39, ch43–46 and ch48–51; P = 0.000–0.044; Fig. 2). Thus, both patient groups produced
significantly smaller task-related changes in oxy-Hb levels in the bilateral middle frontal, inferior frontal and superior frontal gyri compared with HCs (Table 2). Furthermore, patients
with BD-I demonstrated significantly smaller task-related changes in oxy-Hb levels than patients with SZ or HCs in three channels (ch43–45; P = 0.015–0.036) located in the middle temporal
and superior temporal gyri. In addition, analysis of covariance (ANCOVA) revealed that task-related oxy-Hb changes differed significantly among the SZ, BD-I and HC groups in 9 channels (ch5,
ch34–35, ch43–46, ch49 and ch51; F = 5.41–11.59; FDR-corrected P < 0.05, corrected for 52 channels). The BD group exhibited significantly smaller task-related oxy-Hb changes in 9
channels in comparison to HCs (ch5, ch34–35, ch43–46, ch49 and ch51; P = 0.000–0.010) and in 3 channels in comparison to the SZ group (ch43–45; P = 0.004–0.021). However, ANCOVA did not show
any significance between the SZ and HC groups after FDR correction. Figure 3 summarises the results of regional differences in task-related changes in oxy-Hb levels in patients with SZ or
BD-I. In brief, reduced hemodynamic responses in the bilateral middle frontal, inferior frontal and superior frontal (including orbitofrontal region) gyri are a shared feature in SZ and
BD-I. However, the reduction in the right superior temporal gyrus is specific to patients with BD-I. CORRELATION ANALYSIS Significant negative correlations between individual SSRT and
task-related oxy-Hb changes were observed in the SZ group at four channels (ch36 and ch46–48; r = −0.71 to −0.64; FDR-corrected P < 0.05, corrected for 52 channels) located in the right
middle frontal, inferior frontal and left superior frontal gyri, as well as in the BD-I group at one channel (ch45; r = −0.70; FDR-corrected P < 0.05, corrected for 52 channels) located
in the right inferior frontal gyrus. There were no significant correlations between SSRT and task-related oxy-Hb changes in HCs. Figure 4 summarises the correlation analysis in patients with
SZ or BD-I. We observed significant negative correlations between the Global Assessment of Functioning (GAF) scale score and task-related oxy-Hb changes in the BD-I group at two channels
(ch43–44; r = −0.72 to −0.68; FDR-corrected P < 0.05, corrected for 52 channels) located in the right middle and superior temporal gyri. No significant correlations were observed between
task-related oxy-Hb changes and any other clinical variable in the SZ, BD-I, or HC groups, including antipsychotic or antidepressant dosage. No significant correlations were observed between
task-related oxy-Hb changes and HAMD or YMRS scores in the BD-I group. DISCUSSION To our knowledge, this is the first NIRS study to directly compare regional cortical hemodynamic responses
associated with inhibitory control among patients with SZ or BD-I and HCs. Patients with SZ or BD-I exhibited functional abnormalities associated with inhibitory control in the right
inferior frontal gyrus. The differential abnormalities associated with behavioural inhibition were observed in the right middle and superior frontal gyri in patients with SZ and in the right
superior temporal gyrus of patients with BD-I. Our findings suggest that differential patterns of frontotemporal functional abnormalities may reflect an important difference in
psychopathology between schizophrenia and bipolar disorder. Compared with HCs, patients with SZ or BD-I showed significantly reduced hemodynamic responses in the bilateral inferior frontal,
middle frontal and superior frontal gyri. These results are consistent with previous SZ or BD-I neuroimaging studies that reported functional abnormalities in these regions7,12,13,14,15,16.
The right inferior frontal gyrus is known to play a general role in attentional control, rapidly adapting inhibitory control responses to current salient stimuli25,26,27. Previous
neuroimaging studies of HCs reported activation of the right inferior frontal and superior temporal gyri during a response inhibition task28. By contrast, studies have reported significant
associations between poor inhibitory control and the volume of damage to grey matter in the right inferior frontal gyrus in patients with lesions of the right frontal lobe29 and reduced
white matter fractional anisotropy in this region in patients with methamphetamine dependence30. Our results indicate that poor inhibitory control is associated with reduced hemodynamic
responses in the right inferior frontal gyrus of patients with SZ or BD-I. These individuals have specific patterns of cerebral alterations in the right inferior frontal gyrus; this region
may therefore be a neural substrate of impaired behavioural inhibition. Our findings suggest that right inferior frontal abnormalities mediate deficits in inhibitory control that are shared
across patients with either SZ or BD-I. In addition, the results of the current study suggest that patients with SZ exhibited deficits in inhibitory control associated with reduced
hemodynamic responses in the right middle frontal and left superior frontal gyri, including the orbitofrontal region (channels 47–48). Previous studies on individuals with SZ indicated that
structural or functional abnormalities in the orbitofrontal cortex were strongly associated with impulsive aggression or suicidal behavior31,32,33. Furthermore, alterations of orbitofrontal
areas associated with SZ have been linked to positive and negative symptom profiles in previous structural neuroimaging studies34,35. Taken together, these studies suggest that functional
abnormalities in the orbitofrontal region impair inhibition during impulsive or aggressive expression of symptoms in patients with SZ. Further, the alteration in this region may be related
to the specific psychopathology of SZ. Our findings suggest that orbitofrontal functional abnormalities related to impaired behavioural inhibition are a neural substrate of the impulsive or
aggressive clinical features associated with SZ. By contrast, patients with BD-I demonstrated reduced hemodynamic responses in the right superior temporal gyrus, which was unique to this
group. Moreover, patients with BD-I exhibited negative associations between SSRT and hemodynamic responses in this region. The superior temporal cortex (gyrus and sulcus) is part of a
complex face-processing system involved in the perception of emotions36 and regulating responses to negative visual social stimuli37. Recent structural neuroimaging studies in patients with
BD-I have found abnormally reduced thickness of the right superior temporal cortex38 or abnormally reduced fractional anisotropy in the right temporal lobe39. One investigation suggested
that reduced activation in the superior temporal sulcus may indicate disturbances in affect-processing circuitry, leading to abnormalities in mood and social cognition40. These findings
suggest that the superior temporal gyrus is an important neural substrate of emotion regulation and reward processing, and that alterations in this region may produce some of the cognitive
symptoms and social impairments associated with BD-I. Moreover, functional abnormalities in this region may result in impaired emotional control inhibition in individuals with BD-I. This
hypothesis is partly supported by the results of the current study, which showed an association between impaired global functioning and abnormal right temporal region hemodynamic responses
in patients with BD-I. Our findings suggest that the functional abnormalities related to inhibitory control in the right superior temporal gyrus are characteristic of BD-I. In addition,
patients with SZ showed significantly longer SSRTs compared to patients with BD-I or HCs. This is consistent with previous evidence associating SZ with response inhibition deficits2,5. By
contrast, no such difference in mean SSRT was observed patients with BD-I or HCs, consistent with the notion that neurocognitive functioning in individuals with BD-I is impaired relative to
HCs but better than in patients with SZ41,42. An alternative explanation for this difference between SZ and BD-I groups is that the current mood state in patients with BD-I influenced SSRT,
whereas the deficits persisted across all phases of BD-I6,7,43. A further explanation is that neuroimaging techniques, including the NIRS signal, are more sensitive than SSRT at detecting
differences between the BD-I and HC groups, especially given small sample sizes. This observation has been reported in previous neuroimaging studies17,18. In the current study, we considered
observed differences in hemodynamic responses among patients with SZ or BD-I and HCs to be activated by the stop-signal task. There are several potential limitations to our study. First,
our sample size was small, which may have limited the statistical power to produce reliable findings, i.e. ANCOVA did not show any significant differences between the SZ and HC groups after
FDR correction. This could also explain why associations between SSRT and task-related oxy-Hb changes were not observed in HCs. In addition, the NIRS signal might be more sensitive, allowing
greater detection of cortical functional abnormalities in patients with psychiatric diagnoses. This speculation is partly supported by previous NIRS studies that did not detect brain
regions associated with task performances in HCs during Go/No Go22 and stop-signal tasks23. Further studies with larger sample sizes are needed to verify our findings. Second, we cannot
exclude the possibility of medication effects on hemodynamic responses in patients with SZ and BD-I. However, it should be noted that the dosage of antidepressants or antipsychotics was not
correlated with hemodynamic responses in any channels, suggesting a minimal confounding effect of medication. In addition, previous NIRS studies have reported no significant effect of
psychotropic medications on neural activity in patients with SZ or BD19,20,21,22,23. Lastly, NIRS has lower spatial resolution to detect cortical activity from the scalp surface than other
neuroimaging techniques such as fMRI. However, this limitation should be within an acceptable range because differences in hemodynamic responses in patients with SZ or BD-I and HCs were
clearly observed in our study, as they have been in other NIRS studies23,24,44. In addition, we used a virtual spatial registration method to define the spatial information for each channel
using data45,46,47. This method was used in most recent NIRS studies and may be useful for replicating our findings. CONCLUSION This study demonstrated shared and differential patterns of
hemodynamic responses in the frontotemporal cortex among patients with SZ or BD-I and HCs. Our findings suggest that differential patterns in frontotemporal functional abnormalities may
reflect important differences in the psychopathological features of SZ or BD-I. NIRS is a non-invasive neuroimaging modality with easy applicability and high ecological validity. This makes
NIRS particularly suitable for use with patients with psychiatric diagnoses who may be afraid of enclosed spaces or exhibit motor restlessness that interferes with motion-sensitive imaging
methods45. Therefore, NIRS has the potential to be a powerful and specific diagnostic tool for use among individuals with SZ or BD-I. METHODS PARTICIPANTS The diagnosis of SZ or BD-I was
made according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition48 criteria using the Mini International Neuropsychiatric Interview (MINI)49. HCs were also
screened using the MINI and excluded if there was any history of psychiatric disorders or heritable neurological diseases among first- or second-order relatives. Exclusion criteria for the
study groups were as follows: a history of head trauma with loss of consciousness for more than 5 min, current or previous neurological disease, current or previous endocrine disease, a
history of electroconvulsive therapy and/or alcohol/substance abuse or addiction. Psychiatric symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS)50. Global
functioning was assessed with GAF48. Depression severity was evaluated using the 17-item Hamilton depression rating scale administered using a structured interview guide51. Manic symptoms
were assessed using the Young mania rating scale (YMRS)52. Premorbid IQ was estimated using the Japanese version of the National Adult Reading Test53. Handedness was evaluated according to
the Edinburgh Inventory54. The chlorpromazine-equivalent dose of antipsychotics was calculated for each patient55. After providing a complete description of the study, written informed
consent was obtained from all participants. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Kindai University Faculty of Medicine. All
data generated or analysed during this study are included in this article. NIRS METHODOLOGY We used a 52-channel NIRS device (ETG-4000 Optical Topography System; Hitachi Medical Co., Tokyo,
Japan) to measure relative changes in oxy-Hb and deoxygenated haemoglobin (deoxy-Hb) at two wavelengths (694 and 830 nm) of near-infrared light (indicated as mM) based on the modified
Beer–Lambert law56. The NIRS probes were fixed using 3 × 11 thermoplastic shells with 17 emitters and 16 detectors. The distance between each source and detector probe was 30 mm and the area
analysed between the probes was defined as a ‘channel’. The probes of the NIRS device were placed on the frontotemporal region of each participant, with the lowermost probes located along
the T3-Fp1-Fpz-Fp2-T4 line, in accordance with the International 10–20 Placement System used for electroencephalography. The NIRS device can measure Hb values bilaterally from the prefrontal
and temporal surface regions at depths of 20–30 mm from the scalp, which correspond with the surface of the cerebral cortex. The spatial information for each channel was estimated using
data from the Functional Brain Science Laboratory at Jichi Medical University, Japan45,46. According to the LONI Probabilistic Brain Atlas (LPBA40)47, NIRS channels can record functional
hemodynamics within the bilateral frontal, temporal and parietal cortices. We anatomically labelled NIRS channels only after the LPBA40 region of highest probability was determined (Fig. 5).
We recorded relative mean changes in Hb concentrations from baseline in mM because NIRS cannot measure the absolute path length from the emitter to the detector. NIRS signals were acquired
with a time resolution of 0.1 s. We set the moving average window to 5 s to remove high frequency noise such as heartbeat and small bodily movements. Channel records with low signal-to-noise
ratios or motion artefacts were excluded, and there was no difference in the number of samples at each channel, including the channels that indicated significant differences among study
groups. In the present study, we focused on changes in oxy-Hb. Animal studies indicate that oxy-Hb is the most sensitive indicator of regional cerebral blood flow because the direction of
change in deoxy-Hb is determined by the degree of change in venous blood oxygenation and volume57. Pre-task baseline was determined as the mean across the last 10 s of the 30 s pre-task
segment. The obtained oxy-Hb data were averaged for each participant during each baseline and task period. The task-related oxy-Hb changes (task minus baseline) were used for statistical
analysis. STOP-SIGNAL TASK We used a variation on the classic stop-signal task4 (SST) to assess inhibitory control58. The original task design has been described in detail58,59. In brief,
across the task period, participants viewed the shape of a ‘go’ stimulus (circle or square) that appeared on screen in rapid succession. Occasionally, an auditory stop signal was presented
at a short, variable delay after the onset of the go stimulus. PROCEDURE The cognitive activation task used in this study had a one-block design and included 1) a pre-task baseline period
and 2) a stop-signal task period (Fig. 6A). During a 30 s pre-task period, participants were instructed to alternatively tap with their right and left index fingers every 1 s as accurately
as they could, using the left and right buttons on a keyboard. The task consisted of a block of 64 trials; stop-signal trials (75% of trials) and non-stop-signal trials (25% of trials) were
randomly presented. Each trial started with the presentation of a fixation sign on a computer monitor, which was replaced by the primary task stimulus after 250 ms. Stop-signal delay was
initially set at 250 ms and continuously adjusted with the staircase tracking procedure (range of 250–1050 ms). When inhibition was successful, the stop-signal delay increased by 50 ms; when
inhibition was unsuccessful, it decreased by 50 ms. Response registration continued during stop-signal presentation. In stop-signal trials, the go stimulus was followed by a stop signal
(750 Hz, 75 ms) after a variable stop-signal delay. In both no-stop-signal trials and stop-signal trials, the go stimulus remained on screen until subjects responded or the maximal reaction
time (RT; 1,250 ms) had elapsed. The inter-trial interval was 2,000 ms and independent of RT. Participants were instructed to respond as quickly and accurately as possible to the go stimulus
in non-stop-signal trials. In stop-signal trials, participants were instructed to try to withhold their response as required until the auditory stop signal occurred (Fig. 6B). STATISTICAL
ANALYSIS Demographic and clinical variables were compared among the study groups using the χ2 test, _t_-test and one-way ANOVA followed by Tukey’s _post hoc_ test. Statistical significance
was assumed at P < 0.05 (two-tailed). To identify regional differences in frontotemporal hemodynamic activation associated with the inhibitory control task, we compared mean oxy-Hb
changes during the pre-task (baseline) and task periods for every channel within individual subjects using the paired _t_-test. Task-related oxy-Hb changes were compared among the study
groups using ANOVA followed by Tukey’s _post hoc_ test. Because Tukey’s _post hoc_ test focused on NIRS channels where the hemodynamic response was shown to be significant after FDR
correction, no further correction for multiple comparisons was applied for the post hoc test and responses were considered significant at _P_ < 0.0523. Complementary analyses were
performed to identify between-group differences in activation associated with inhibitory control using ANCOVA with age, gender (dummy parameterised, male = 0, female = 1) and SSRT as
covariates. Furthermore, to examine the relationships between task-related oxy-Hb changes and clinical variables, we calculated Pearson’s correlation coefficients. We set the value of q
(i.e. FDR) to 0.05 such that the false-positive rate was not greater than 5% on average when processing oxy-Hb data obtained from multiple channels60. REFERENCES * Craddock, N. & Sklar,
P. Genetics of bipolar disorder. _Lancet_ 381, 1654–1662, https://doi.org/10.1016/s0140-6736(13)60855-7 (2013). Article CAS PubMed Google Scholar * Barch, D. M., Braver, T. S., Carter,
C. S., Poldrack, R. A. & Robbins, T. W. CNTRICS final task selection: executive control. _Schizophr. Bull._ 35, 115–135, https://doi.org/10.1093/schbul/sbn154 (2009). Article PubMed
Google Scholar * Banich, M. T. & Depue, B. E. Recent advances in understanding neural systems that support inhibitory control. _Curr. Opin. Behav. Sci._ 1, 17–22,
https://doi.org/10.1016/j.cobeha.2014.07.006 (2015). Article Google Scholar * Logan, G. D. & Cowan, W. B. On the ability to inhibit thought and action: A theory of an act of control.
_Psychol. Rev._ 91, 295–327, https://doi.org/10.1037/0033-295X.91.3.295 (1984). Article Google Scholar * Lipszyc, J. & Schachar, R. Inhibitory control and psychopathology: a
meta-analysis of studies using the stop signal task. _J. Int. Neuropsychol. Soc._ 16, 1064–1076, https://doi.org/10.1017/S1355617710000895 (2010). Article PubMed Google Scholar *
Ethridge, L. E. _et al_. Behavioral response inhibition in psychotic disorders: diagnostic specificity, familiality and relation to generalized cognitive deficit. _Schizophr. Res._ 159,
491–498, https://doi.org/10.1016/j.schres.2014.08.025 (2014). Article PubMed PubMed Central Google Scholar * Hajek, T., Alda, M., Hajek, E. & Ivanoff, J. Functional neuroanatomy of
response inhibition in bipolar disorders–combined voxel based and cognitive performance meta-analysis. _J. Psychiatr. Res._ 47, 1955–1966, https://doi.org/10.1016/j.jpsychires.2013.08.015
(2013). Article PubMed Google Scholar * Hampshire, A., Chamberlain, S. R., Monti, M. M., Duncan, J. & Owen, A. M. The role of the right inferior frontal gyrus: inhibition and
attentional control. _Neuroimage_ 50, 1313–1319, https://doi.org/10.1016/j.neuroimage.2009.12.109 (2010). Article PubMed PubMed Central Google Scholar * Sharp, D. J. _et al_. Distinct
frontal systems for response inhibition, attentional capture, and error processing. _Proc. Natl. Acad. Sci. USA_ 107, 6106–6111, https://doi.org/10.1073/pnas.1000175107 (2010). Article ADS
CAS PubMed PubMed Central Google Scholar * Wager, T. D. _et al_. Common and unique components of response inhibition revealed by fMRI. _Neuroimage_ 27, 323–340,
https://doi.org/10.1016/j.neuroimage.2005.01.054 (2005). Article PubMed Google Scholar * Aron, A. R. & Poldrack, R. A. Cortical and subcortical contributions to stop signal response
inhibition: role of the subthalamic nucleus. _J. Neurosci._ 26, 2424–2433, https://doi.org/10.1523/JNEUROSCI.4682-05.2006 (2006). Article CAS PubMed Google Scholar * Minzenberg, M. J.,
Laird, A. R., Thelen, S., Carter, C. S. & Glahn, D. C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. _Arch. Gen. Psychiatry_ 66, 811–822,
https://doi.org/10.1001/archgenpsychiatry.2009.91 (2009). Article PubMed PubMed Central Google Scholar * Zandbelt, B. B., van Buuren, M., Kahn, R. S. & Vink, M. Reduced proactive
inhibition in schizophrenia is related to corticostriatal dysfunction and poor working memory. _Biol. Psychiatry_ 70, 1151–1158, https://doi.org/10.1016/j.biopsych.2011.07.028 (2011).
Article PubMed Google Scholar * Hughes, M. E., Fulham, W. R., Johnston, P. J. & Michie, P. T. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential
and functional neuroimaging data. _Biol. Psychol._ 89, 220–231, https://doi.org/10.1016/j.biopsycho.2011.10.013 (2012). Article PubMed Google Scholar * Hanford, L. C., Nazarov, A., Hall,
G. B. & Sassi, R. B. Cortical thickness in bipolar disorder: a systematic review. _Bipolar Disord._ 18, 4–18, https://doi.org/10.1111/bdi.12362 (2016). Article PubMed Google Scholar *
Piguet, C., Fodoulian, L., Aubry, J. M., Vuilleumier, P. & Houenou, J. Bipolar disorder: Functional neuroimaging markers in relatives. _Neurosci. Biobehav. Rev._ 57, 284–296,
https://doi.org/10.1016/j.neubiorev.2015.08.015 (2015). Article PubMed Google Scholar * Weathers, J. D. _et al_. A developmental study of the neural circuitry mediating motor inhibition
in bipolar disorder. _Am. J. Psychiatry_ 169, 633–641, https://doi.org/10.1176/appi.ajp.2012.11081244 (2012). Article PubMed PubMed Central Google Scholar * Strakowski, S. M. _et al_.
Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. _Early Interv. Psychiatry_ 2, 225–233,
https://doi.org/10.1111/j.1751-7893.2008.00082.x (2008). Article PubMed PubMed Central Google Scholar * Marumo, K. _et al_. Functional abnormalities in the left ventrolateral prefrontal
cortex during a semantic fluency task, and their association with thought disorder in patients with schizophrenia. _Neuroimage_ 85(Pt 1), 518–526,
https://doi.org/10.1016/j.neuroimage.2013.04.050 (2014). Article PubMed Google Scholar * Takizawa, R. _et al_. Reduced frontopolar activation during verbal fluency task in schizophrenia:
a multi-channel near-infrared spectroscopy study. _Schizophr. Res._ 99, 250–262, https://doi.org/10.1016/j.schres.2007.10.025 (2008). Article PubMed Google Scholar * Kameyama, M. _et al_.
Frontal lobe function in bipolar disorder: a multichannel near-infrared spectroscopy study. _Neuroimage_ 29, 172–184, https://doi.org/10.1016/j.neuroimage.2005.07.025 (2006). Article
PubMed Google Scholar * Nishimura, Y. _et al_. Prefrontal cortex activity during response inhibition associated with excitement symptoms in schizophrenia. _Brain Res._ 1370, 194–203,
https://doi.org/10.1016/j.brainres.2010.11.003 (2011). Article CAS PubMed Google Scholar * Okada, N. _et al_. Characterizing prefrontal cortical activity during inhibition task in
methamphetamine-associated psychosis versus schizophrenia: a multi-channel near-infrared spectroscopy study. _Addict. Biol._ 21, 489–503, https://doi.org/10.1111/adb.12224 (2016). Article
CAS PubMed Google Scholar * Ono, Y. _et al_. Reduced prefrontal activation during performance of the Iowa Gambling Task in patients with bipolar disorder. _Psychiatry Res._ 233, 1–8,
https://doi.org/10.1016/j.pscychresns.2015.04.003 (2015). Article PubMed Google Scholar * Dehaene, S., Kerszberg, M. & Changeux, J. P. A neuronal model of a global workspace in
effortful cognitive tasks. _Proc. Natl. Acad. Sci. USA_ 95, 14529–14534 (1998). Article ADS CAS PubMed PubMed Central Google Scholar * Duncan, J. An adaptive coding model of neural
function in prefrontal cortex. _Nature Reviews Neuroscience_ 2, 820–829, https://doi.org/10.1038/35097575 (2001). Article CAS PubMed Google Scholar * Corbetta, M. & Shulman, G. L.
Control of goal-directed and stimulus-driven attention in the brain. _Nat. Rev. Neurosci._ 3, 201–215, https://doi.org/10.1038/nrn755 (2002). Article CAS PubMed Google Scholar * Horn, N.
R., Dolan, M., Elliott, R., Deakin, J. F. W. & Woodruff, P. W. R. Response inhibition and impulsivity: an fMRI study. _Neuropsychologia_ 41, 1959–1966,
https://doi.org/10.1016/s0028-3932(03)00077-0 (2003). Article CAS PubMed Google Scholar * Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J. & Robbins, T. W. Stop-signal
inhibition disrupted by damage to right inferior frontal gyrus in humans. _Nat. Neurosci._ 6, 115–116, https://doi.org/10.1038/nn1003 (2003). Article CAS PubMed Google Scholar * Ersche,
K. D. _et al_. Abnormal brain structure implicated in stimulant drug addiction. _Science_ 335, 601–604, https://doi.org/10.1126/science.1214463 (2012). Article ADS CAS PubMed Google
Scholar * Richard-Devantoy, S., Orsat, M., Dumais, A., Turecki, G. & Jollant, F. Neurocognitive vulnerability: suicidal and homicidal behaviours in patients with schizophrenia. _Can. J.
Psychiatry_ 59, 18–25 (2014). Article PubMed PubMed Central Google Scholar * Soyka, M. Neurobiology of aggression and violence in schizophrenia. _Schizophr. Bull._ 37, 913–920,
https://doi.org/10.1093/schbul/sbr103 (2011). Article PubMed PubMed Central Google Scholar * Naudts, K. & Hodgins, S. Neurobiological correlates of violent behavior among persons
with schizophrenia. _Schizophr. Bull._ 32, 562–572, https://doi.org/10.1093/schbul/sbj036 (2006). Article PubMed Google Scholar * Nakamura, M. _et al_. Orbitofrontal volume deficit in
schizophrenia and thought disorder. _Brain_ 131, 180–195, https://doi.org/10.1093/brain/awm265 (2008). Article PubMed Google Scholar * Wolkin, A. _et al_. Inferior frontal white matter
anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. _Am. J. Psychiatry_ 160, 572–574, https://doi.org/10.1176/appi.ajp.160.3.572 (2003). Article PubMed
Google Scholar * Campbell, R., Heywood, C. A., Cowey, A., Regard, M. & Landis, T. Sensitivity to eye gaze in prosopagnosic patients and monkeys with superior temporal sulcus ablation.
_Neuropsychologia_ 28, 1123–1142 (1990). Article CAS PubMed Google Scholar * Buchheim, A. _et al_. Neural correlates of attachment trauma in borderline personality disorder: a functional
magnetic resonance imaging study. _Psychiatry Res._ 163, 223–235, https://doi.org/10.1016/j.pscychresns.2007.07.001 (2008). Article PubMed Google Scholar * Rimol, L. M. _et al_. Cortical
thickness and subcortical volumes in schizophrenia and bipolar disorder. _Biol. Psychiatry_ 68, 41–50, https://doi.org/10.1016/j.biopsych.2010.03.036 (2010). Article PubMed Google Scholar
* Mahon, K. _et al_. Abnormal temporal lobe white matter as a biomarker for genetic risk of bipolar disorder. _Biol. Psychiatry_ 73, 177–182, https://doi.org/10.1016/j.biopsych.2012.07.033
(2013). Article PubMed Google Scholar * Pavuluri, M. N., O’Connor, M. M., Harral, E. & Sweeney, J. A. Affective neural circuitry during facial emotion processing in pediatric bipolar
disorder. _Biol. Psychiatry_ 62, 158–167, https://doi.org/10.1016/j.biopsych.2006.07.011 (2007). Article PubMed Google Scholar * Hill, S. K. _et al_. Neuropsychological impairments in
schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. _Am. J. Psychiatry_ 170, 1275–1284,
https://doi.org/10.1176/appi.ajp.2013.12101298 (2013). Article PubMed PubMed Central Google Scholar * Barrett, S. L., Mulholland, C. C., Cooper, S. J. & Rushe, T. M. Patterns of
neurocognitive impairment in first-episode bipolar disorder and schizophrenia. _Br. J. Psychiatry_ 195, 67–72, https://doi.org/10.1192/bjp.bp.108.054874 (2009). Article PubMed Google
Scholar * Malhi, G. S. _et al_. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. _Bipolar Disord._ 9, 114–125,
https://doi.org/10.1111/j.1399-5618.2007.00324.x (2007). Article PubMed Google Scholar * Yamamuro, K. _et al_. Differential patterns of blood oxygenation in the prefrontal cortex between
patients with methamphetamine-induced psychosis and schizophrenia. _Sci. Rep._ 5, 12107, https://doi.org/10.1038/srep12107 (2015). Article ADS CAS PubMed PubMed Central Google Scholar
* Singh, A. K., Okamoto, M., Dan, H., Jurcak, V. & Dan, I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. _Neuroimage_ 27, 842–851,
https://doi.org/10.1016/j.neuroimage.2005.05.019 (2005). Article PubMed Google Scholar * Tsuzuki, D. _et al_. Virtual spatial registration of stand-alone fNIRS data to MNI space.
_Neuroimage_ 34, 1506–1518, https://doi.org/10.1016/j.neuroimage.2006.10.043 (2007). Article PubMed Google Scholar * Shattuck, D. W. _et al_. Construction of a 3D probabilistic atlas of
human cortical structures. _Neuroimage_ 39, 1064–1080, https://doi.org/10.1016/j.neuroimage.2007.09.031 (2008). Article PubMed Google Scholar * American Psychiatric Association.
_Diagnostic and Statistical Manual of Mental Disorders_. 4th edn, (American Psychiatric Association, 1994). * Otsubo, T. _et al_. Reliability and validity of Japanese version of the
Mini-International Neuropsychiatric Interview. _Psychiatry Clin. Neurosci._ 59, 517–526, https://doi.org/10.1111/j.1440-1819.2005.01408.x (2005). Article PubMed Google Scholar * Kay, S.
R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. _Schizophr. Bull._ 13, 261–276 (1987). Article CAS PubMed Google Scholar *
Williams, J. B. A structured interview guide for the Hamilton Depression Rating Scale. _Arch. Gen. Psychiatry_ 45, 742–747 (1988). Article CAS PubMed Google Scholar * Young, R. C.,
Biggs, J. T., Ziegler, V. E. & Meyer, D. A. A rating scale for mania: reliability, validity and sensitivity. _Br. J. Psychiatry_ 133, 429–435 (1978). Article CAS PubMed Google Scholar
* Matsuoka, K., Uno, M., Kasai, K., Koyama, K. & Kim, Y. Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words:
Japanese version of National Adult Reading Test. _Psychiatry Clin. Neurosci._ 60, 332–339, https://doi.org/10.1111/j.1440-1819.2006.01510.x (2006). Article PubMed Google Scholar *
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. _Neuropsychologia_ 9, 97–113 (1971). Article CAS PubMed Google Scholar * Inada, T. & Inagaki, A.
Psychotropic dose equivalence in Japan. _Psychiatry Clin. Neurosci._ 69, 440–447, https://doi.org/10.1111/pcn.12275 (2015). Article PubMed Google Scholar * Cope, M. _et al_. Methods of
quantitating cerebral near infrared spectroscopy data. _Adv. Exp. Med. Biol._ 222, 183–189 (1988). Article CAS PubMed Google Scholar * Hoshi, Y., Kobayashi, N. & Tamura, M.
Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. _J. Appl. Physiol._ 90, 1657–1662 (2001). Article CAS PubMed Google Scholar
* Verbruggen, F., Logan, G. D. & Stevens, M. A. STOP-IT: Windows executable software for the stop-signal paradigm. _Behav. Res. Methods_ 40, 479–483,
https://doi.org/10.3758/brm.40.2.479 (2008). Article PubMed Google Scholar * Verbruggen, F. & Logan, G. D. Response inhibition in the stop-signal paradigm. _Trends Cogn. Sci._ 12,
418–424, https://doi.org/10.1016/j.tics.2008.07.005 (2008). Article PubMed PubMed Central Google Scholar * Singh, A. K. & Dan, I. Exploring the false discovery rate in multichannel
NIRS. _Neuroimage_ 33, 542–549, https://doi.org/10.1016/j.neuroimage.2006.06.047 (2006). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was partly supported
by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 25461792 and No. 16K10229). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Neuropsychiatry, Kindai University Faculty of Medicine, Osaka, Japan Noa Tsujii, Wakako Mikawa, Toru Adachi, Tomoyuki Hirose & Osamu Shirakawa Authors * Noa Tsujii View author
publications You can also search for this author inPubMed Google Scholar * Wakako Mikawa View author publications You can also search for this author inPubMed Google Scholar * Toru Adachi
View author publications You can also search for this author inPubMed Google Scholar * Tomoyuki Hirose View author publications You can also search for this author inPubMed Google Scholar *
Osamu Shirakawa View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.T. designed the study, wrote the protocol, collected the data, undertook
the statistical analyses and wrote the manuscript. W.M. and T.A. collected the data. T.H. critically revised the text for important intellectual content. O.S. is the head of our laboratory
and designed the study, wrote the protocol and was involved in working on all drafts of the manuscript. All authors contributed to and have approved the final manuscript. CORRESPONDING
AUTHOR Correspondence to Noa Tsujii. ETHICS DECLARATIONS COMPETING INTERESTS Dr. Shirakawa has received honoraria from Pfizer, GlaxoSmithKline and Eli Lilly. The other authors report no
financial relationships with commercial interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Tsujii, N., Mikawa, W., Adachi, T. _et al._ Shared and differential cortical functional abnormalities associated with inhibitory control in patients with
schizophrenia and bipolar disorder. _Sci Rep_ 8, 4686 (2018). https://doi.org/10.1038/s41598-018-22929-y Download citation * Received: 26 April 2017 * Accepted: 05 March 2018 * Published: 16
March 2018 * DOI: https://doi.org/10.1038/s41598-018-22929-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




:max_bytes(150000):strip_icc():focal(719x0:721x2)/jameela-2-5fd373397ba6432f85173932ad0a7355.jpg)