- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Excitotoxicity leads to the activation of a cytotoxic cascade that causes neuronal death. In the retina, retinal ganglion cells (RGCs) die after an excitotoxic insult. Multiple
pathways have been proposed to contribute to RGC death after an excitotoxic insult, including TNF signaling, JNK activation, and ER stress. To test the importance of these pathways in RGC
death after excitotoxic injury, the excitotoxin N-methyl-D-aspartate (NMDA) was intravitreally injected into mice deficient in components of these pathways. Absence of _Tnf_ or its canonical
downstream mediator, _Bid_, did not confer short- or long-term protection to RGCs. Despite known activation in RGCs and a prominent role in mediating RGC death after other insults,
attenuating JNK signaling did not prevent RGC death after excitotoxic insult. Additionally, deficiency of the ER stress protein DDIT3 (CHOP), which has been shown to be involved in RGC
death, did not lessen NMDA induced RGC death. Furthermore, absence of both _Jun_ (JNK’s canonical target) and _Ddit3_, which together provide robust, long-term protection to RGC somas after
axonal insult, did not lessen RGC death. Collectively, these results indicate that the drivers of excitotoxic injury remain to be identified and/or multiple cell death pathways are activated
in response to injury. SIMILAR CONTENT BEING VIEWED BY OTHERS MOLECULAR MECHANISMS OF NMDA EXCITOTOXICITY IN THE RETINA Article Open access 27 October 2023 INTERCELLULAR COMMUNICATION ATLAS
REVEALS OPRM1 AS A NEUROPROTECTIVE FACTOR FOR RETINAL GANGLION CELLS Article Open access 11 March 2024 Σ2R/TMEM97 IN RETINAL GANGLION CELL DEGENERATION Article Open access 01 December 2022
INTRODUCTION Excessive stimulation of glutamate receptors has been shown to disrupt the intracellular environment and lead to activation of cytotoxic cascades culminating in neuronal death1.
In the retina, excitotoxic insult is thought to contribute to several diseases, including diabetic retinopathy and retinal ischemia2,3,4. Multiple cell types, including both neurons and
glia, are affected by excitotoxic insult. In the retina, excitotoxic insult is known to cause the death of retinal ganglion cells (RGCs) and at least some types of amacrine cells5,6,7,8,9.
Despite excitotoxicity’s importance in disease, the required cell death pathways controlling excitotoxic induced RGC death are not well-defined. Multiple intrinsic and extrinsic cell death
signaling pathways have been implicated in neuronal death after excitotoxic injury. Recently, TNFR1 activation has been shown to initiate the process of RGC death after excitotoxic insult7.
A key mediator downstream of TNFR1 activation, the pro-apoptotic protein BID, has also been shown to be important in mediating neuronal damage after glutamate toxicity10 including in RGCs11.
Interestingly, if BID is important for NMDA induced RGC death, it is not acting through BAX activation, since _Bax_ deficiency does not prevent RGC death after excitotoxic insult8,12.
Additionally, the JNK (c-Jun N-terminal kinases) pathway has been proposed as a regulator of neuronal death after excitotoxic insult. Canonical JNK signaling leads to activation of the
transcription factor JUN, a member of the activation protein-1 (AP-1) family of transcription factors13. In the eye, JNK and JUN activation in RGCs is present after induced ischemia, axonal
injury, increased intraocular pressure, and excitotoxicity14,15,16,17,18,19. Pan inhibitors against JNK, as well as _Jun_ antisense oligodeoxynucleotides, provided some protection to inner
retinal neurons after an excitotoxic insult20,21,22. Furthermore, ER stress has been proposed to mediate excitotoxic cell death23,24,25,26. In the retina, an increase in expression of the ER
stress gene _Ddit3_ (_Chop_) has been found in RGCs after intravitreal injection of the excitotoxin NMDA25. _Ddit3_ deficiency provided a modest, but significant level of protection of
ganglion cell layer neurons (which consists of RGCs and amacrine cells) after and intravitreal injection of NMDA23. It has also been observed that lack of _Ddit3_ significantly reduces RGC
death after axonal injury27. Thus, there appears to be numerous cell death pathways contributing to RGC death after an excitotoxic insult. Here, the importance of these pathways in
excitotoxic RGC death is critically tested using mice deficient in critical molecular components of these pathways. RESULTS INTRAVITREAL NMDA INJECTION KILLS RGCS Administration of
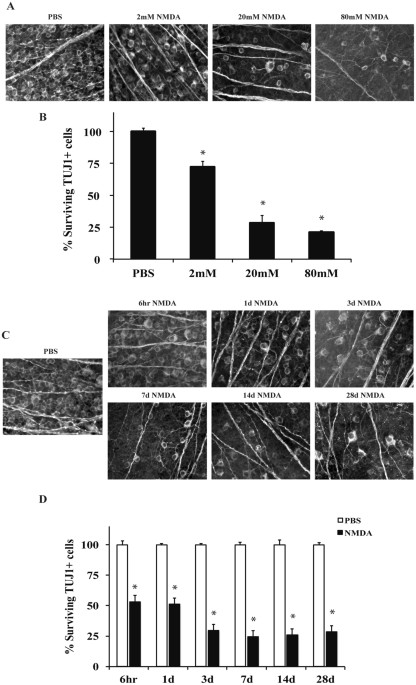
excitotoxins to the retina affects multiple cell types, including RGCs28. In order to establish a relevant concentration of NMDA to study NMDA-induced RGC death, the effect on RGC survival
of different concentrations of NMDA was examined. 2 μl of three concentrations of NMDA (2 mM, 20 mM, or 80 mM) were intravitreally injected into C57BL/6 J mice. For controls, the
contralateral eye was injected with 2 μl of PBS. The number of TUJ1+ RGCs remaining 7 days after injection was counted. All concentrations of NMDA (2 mM, 20 mM and 80 mM) resulted in a
significant decrease in the number of TUJ1+ RGCs compared to control eyes (_p_ < 0.001 for all concentrations compared to control; Fig. 1A,B). There was also significant decrease in cell
number between 2 mM and 20 mM injections of NMDA (_p_ < 0.001, for all comparisons); however, there was no significant decrease in cell number between 20 mM and 80 mM NMDA injection (_p_
= 0.819). The 20 mM NMDA concentration was chosen for future experiments based upon this concentration producing a significant amount of retinal neuron loss and relevance to previously
published studies7,29,30,31. To determine the window of RGC loss, TUJ1+ cells were counted 6hrs, 1d, 3d, 7d, 14d, and 28d after intraocular injection of 20 mM NMDA (Fig. 1C,D). There was a
significant loss of approximately 50% of TUJ1+ cells 6 hr and 1d after NMDA insult (_p_ < 0.001 comparing either 6 hour or 1 day after NMDA injection to control eyes). Between 1 day and 3
days after injection there was a further loss of RGCs (_p_ = 0.007, comparing 1d to 3d). However, after 3 days, there was not a further, significant decrease in TUJ1+ cell number _(p_ >
0.05 comparing 3d to either 7d, 14d, or 28d). Thus, cell loss appears to be complete by 3 days after 20 mM NMDA insult. TNF AND BID ARE NOT REQUIRED FOR NMDA-INDUCED RGC DEATH TNF has been
suggested to be critical for inducing RGC death 6 hours after excitotoxic injury7. In order to determine if TNF is required for long-term NMDA induced RGC death, 2 µl of PBS (control,
contralateral eye) or 20 mM of NMDA was injected into the vitreous of _Tnf_ deficient (_Tnf_−/−) mice. The number of surviving TUJ1+ RGCs was counted at 6 hours and 7 days after intravitreal
NMDA injection, two time points that encompass when RGCs are dying and after the time window of cell death (see Fig. 1). _Tnf_ deficiency did not lessen RGC loss at either the 6 hour or 7
day time point compared to wildtype mice (_p_ = 0.9694 comparing WT to _Tnf_−/− NMDA; Fig. 2). These data suggest that _Tnf_ is not required nor an important contributor to RGC death after
excitotoxic injury. Extrinsic death receptors, including TNFR1, may recruit the intrinsic pathway in order to amplify the cell death cascade. This is mediated through BID cleavage and
fragment translocation to the mitochondria where it activates BAX and/or BAK, two pro-apoptotic BCL2 proteins that are critical for apoptotic cell death32. To determine if BID is required
for NMDA induced RGC death, 2 μl of either 20 mM NMDA or PBS was injected into the vitreous of _Bid_ deficient (_Bid_−/−) and wildtype (_Bid_+/+) mice. The number of surviving RGCs (TUJ1+
cells) was counted at 6 hours and 7 days after insult. Significant RGC loss in both wildtype and _Bid_ deficient eyes was observed at both time points. Furthermore, _Bid_ deficiency did not
lessen RGC death (Fig. 3; _p_ ≥ 0.719 comparing WT to _Bid_−/− NMDA). These data suggest BID is not a critical mediator of short- and long-term excitotoxic RGC death. JUN IS ACTIVATED IN
MULTIPLE DIFFERENT RETINAL CELL TYPES AFTER EXCITOTOXIC INSULT The transcription factor JUN is activated by JNK signaling. JNK inhibitors have lessened RGC death after an NMDA
insult20,21,22. However, there was variability in RGC protection between inhibitors and it is probable that the inhibitors are not completely specific to JNKs, therefore it is important to
test the JNK pathway in excitotoxicity induced RGC death using genetics. In order to determine if JUN was activated (phosphorylated) in retinal cells after an excitotoxic insult, retinal
flat mounts were examined for pJUN at 6 hours after intravitreal injections of either NMDA or PBS. Similar to previous findings, pJUN was detected in TUJ1+ RGCs after NMDA insult (Fig. 4)22.
These results demonstrate that JUN dependent MAPK signaling is active in RGCs after an NMDA insult. MAPK SIGNALING DOES NOT APPEAR TO BE IMPORTANT FOR RGC DEATH AFTER NMDA INSULT DLK is a
MAP3K upstream of JNK and JUN that has been shown to be critical for excitotoxic-induced neuronal death. Deficiency of _Dlk_ significantly protects CA1 hippocampal neurons after excitotoxic
injury33. In order to assess the involvement of DLK in excitotoxic mediated RGC loss, DLK was conditionally deleted in the retina using a floxed allele of _Dlk_ and the retinal cre,
_Six3-cre_. Previously, we have shown that _Six3_-_cre_ successfully deletes _Dlk_ (and _Jun_, discussed below) in approximately 85% of RGCs34,35. Seven days post-NMDA injection, RGC loss
was comparable between wildtype and _Dlk_ deficient (_Dlk__fl/fl__; Six3-cre_+) mice (Fig. 5; _p_ ≥ 0.1843 comparing WT to _Dlk__fl/fl__; Six3-cre_+ NMDA injected eyes). These results
suggest that DLK is not critical for NMDA-induced RGC death. There are three JNKs in vertebrates, _Jnk1, Jnk2_, and _Jnk3_36. Deficiency of _Jnk3_ has been reported to confer protection to
hippocampal neurons in the brain after excitotoxic injury37. Inhibitors against JNK have produced mixed results, showing minimal or very significant protection for RGCs after
excitotoxicity20,22. After axonal injury, combined absence of _Jnk2_ and _Jnk3_ provides significant protection from RGC loss, showing that _Jnk2_ and _Jnk3_ can mediate pro-death signaling
in injured RGCs38. Therefore, in order to examine the role of JNK in NMDA mediated RGC death using a genetic approach, mice deficient for both _Jnk2_ and _Jnk3_ (_Jnk2_−/− _Jnk3_−/−) were
intravitreally injected with 2 μl of 20 mM NMDA. Compared to wildtype mice, the number of RGCs lost 7 days post-intravitreal injection of NMDA was similar in _Jnk2_−/− _Jnk3_−/− mice (Fig.
6; _p_ ≤ 0.001 for comparison between PBS and NMDA for each genotype, _p_ ≥ 0.9769 comparing WT to _Jnk2_−/− _Jnk3_−/− NMDA). This result shows that the JNK involved in excitotoxic death in
other neurons (JNK3) and the JNKs that have been shown to be important in RGC death after an insult (JNK2 and JNK3) are not required for NMDA-mediated RGC death. This suggests that _Jnk1_
could have a role in the excitotoxic injury response, in the future it would be interesting to look at a conditional knockout of all three JNK isoforms. JUN is a canonical injury response
factor downstream of JNK and DLK that is known to mediate RGC death. JUN has been shown to be a mediator of RGC death after other injuries, for example inhibiting _Jun_ significantly lessens
RGC death after mechanical axonal injury or axotomy38,39. The role of JUN in excitotoxic-mediated RGC death is unclear, though protection of RGCs was found using a c-Jun antisense
oligodeoxynucleotide after excitotoxic insult21. In order to critically test the importance of JUN in NMDA induced RGC death, NMDA was injected into the vitreous of _Jun_ deficient mice.
Deletion of _Jun_ is embryonically lethal, therefore a conditional retinal deletion of JUN was achieved using a floxed allele of JUN and the early retinal deleter cre, _Six3-cre_
(_Jun__fl/fl__; Six3-cre_+; referred to as _Jun_ deficient mice)40,41,42,43. The number of surviving RGCs (TUJ1+ cells) were counted 7 days after intravitreal injection, a time point when
the majority of RGCs have died in wildtype retinas. There was a significant loss of RGCs in both the wildtype and _Jun_ deficient retinas after NMDA insult (Fig. 7; _p_ = 0.9846, comparing
WT to _Jun__fl/fl__; Six3-cre_+ NMDA). This result shows that the injury response factor JUN is not required for NMDA-mediated RGC death. THE ER STRESS PROTEIN DDIT3 (CHOP) IS NOT REQUIRED
FOR RGC EXCITOTOXIC DEATH ER stress has been suggested to mediate excitotoxic RGC death and an increase in expression of the ER stress protein _Ddit3_ has been found in RGCs after NMDA
injury25. _Ddit3_ has been shown to be involved in RGC death after several insults and modest protection of ganglion cell layer cells was seen in _Ddit3_ null mice after intraocular
injection of NMDA23. Thus, this ER stress pathway could play an important role in excitotoxic RGC death. Therefore, in order to determine the extent of involvement of DDIT3 in mediating RGC
death after excitotoxic injury, mice deficient in _Ddit3_ were injected with NMDA and RGC loss analyzed 7 days post-injection. Compared to wildtype, _Ddit3_ deficient mice had no significant
change in surviving TUJ1+ RGCs after NMDA insult (Fig. 8; _p_ = 0.8105 comparing WT to _Ddit3_−/− NMDA). This result suggests the ER stress pathway involving DDIT3 does not play a major
role in mediating RGC death after NMDA injury. _Jun_ or _Ddit3_ deficiency alone did not prevent RGC loss after excitotoxic injury, however it has been shown that deficiency in _Jun_ and
_Ddit3_ together provide increased protection to RGC somas after an axonal injury44. To test whether these two pathways together mediate RGC loss after NMDA insult, surviving RGCs were
counted in mice deficient in both _Jun_ and _Ddit3_ (_Jun__fl/fl__Ddit3_−/−_; Six3-cre_+) after an excitotoxic injury. Mice deficient in both _Jun_ and _Ddit3_ had a similar amount of RGC
loss as wildtype mice 7 days post-NMDA injection (Fig. 9; _p_ = 0.8582 between WT and _Jun__fl/fl__Ddit3_−/−_; Six3-cre_+ NMDA). KAINIC ACID INDUCED RGC DEATH IS NOT MEDIATED BY JUN OR DDIT3
There are differences in the severity and specificity of cell death based upon the type of excitotoxin45. Another excitotoxin that is used to induce excitotoxic cell death is kainic
acid45,46,47. Kainic acid, along with NMDA, is used for investigating neuroprotective strategies to limit RGC loss in glaucoma, retinal ischemia, and diabetic retinopathy48,49,50. _Jun_ and
_Ddit3_ have been shown to be important in KA-mediated death26,37,51, thus it is possible that in the retina these molecules are necessary for RGC death induced by KA, but not NMDA. In order
to find a concentration that produces consistent and reproducible RGC death, different concentrations of KA were intravitreally injected into wildtype mice (10 mM, 12 mM, 15 mM, or 20 mM;
Fig. 10). These KA concentrations are similar to what has been previously used to induce retinal injury50,52. All concentrations of KA produced significant RGC loss compared to PBS injection
(Fig. 10; _p_ < 0.001 for all concentrations compared to control), though the RGC loss achieved was not statistically significant between KA concentrations. Therefore, to assess the
involvement of _Jun_ and _Ddit3_ in KA-induced RGC death, 15 mM KA was intravitreally injected into mice deficient in both _Jun_ and _Ddit3_ (_Jun__fl/fl__Ddit3_−/−_; Six3-cre_+). Surviving
RGCs (TUJ1+ cells) were assessed 7 days post injection and there was no statistical difference between KA-injected wildtype and _Jun/Ddit3_ deficient mice (Fig. 11; _p_ = 0.9962 between WT
and _Jun__fl/fl__Ddit3_−/−_; Six3-cre_+ KA). This result is similar to what was described above with NMDA-mediated RGC injury. Overall, these data suggest that the JUN/JNK signaling pathway
does not play a role in either NMDA or KA-induced excitotoxic RGC death. DISCUSSION In this study, the importance of key components of the extrinsically activated mitochondrial cell death
pathway for RGC death after excitotoxic injury were tested. The absence of _Tnf_ or _Bid_ did not lessen RGC death after excitotoxic insult. The importance of intrinsic cell death signaling
pathways was also tested using mouse mutants of various components of the JNK-JUN signaling pathway (_Dlk_, _Jnk2_, _Jnk3_ and _Jun_) and ER stress pathway (_Ddit3_). Finally, we tested
whether combined deficiency in _Jun_ and _Ddit3_ provided additive protection to RGCs after excitotoxic insult. Collectively, our data show key extrinsic and intrinsic pathways that have
been implicated in RGC death do not appear to be necessary for RGC death after excitotoxic injury. TNF has been implicated in RGC death after excitotoxic insult7. Surprisingly, in our hands
_Tnf_ deficiency did not provide long-term protection to RGCs after excitotoxic injury nor did it prevent RGC loss at an early time point after insult. Additionally, absence of _Bid_ failed
to prevent RGC loss after NMDA insult. Together, these data show a key extrinsic pathway that has been implicated in RGC death does not appear to be necessary for mediating RGC death after
excitotoxic injury. Lebrun-Julien and colleagues previously showed a significant, but incomplete protection against RGC death in _Tnf_ deficient mice six hours after an intravitreal NMDA
injection7. These experiments are inconsistent with the work presented here showing no protection provided by _Tnf_ deficiency at six hours after intravitreal injection of NMDA7. It is
unclear why the experiments presented here, performed with similar concentrations of NMDA, similar procedures, and both using TNF deficient mice obtained different results compared to
Lebrun-Julien and colleagues previous work7. However, the longest time point examined by Lebrun-Julien _et al_. was only 6 hours after injury, and at this time point only a partial
protection was observed (about half of the cells that had died in the control were protected). It is important to note that Lebrun-Julien and colleagues7 found similar protection at this
time point using a TNF neutralizing drug, Etanercept. Therefore, it is possible that slight differences in the kinetics of cell loss between the different experiments explain the observed
differences. Nevertheless, the experiments described here show that TNF signaling is not required for RGC death after an excitotoxic insult. Multiple intrinsic pro-death signaling pathways
have been implicated in excitotoxic induced RGC death. JNK/JUN signaling has been shown to be involved in excitotoxic neuronal death in multiple systems. Manipulating upstream regulators of
JUN have been shown to reduce RGC death after excitotoxic injury, for example _Jnk3_ deficiency lessened neuronal loss after a kainic acid-induced excitotoxic injury37. Also, an upstream
kinase of JNK which is known to activate JNK in injured RGCs35,53,54, DLK, has been shown to be involved in excitotoxic insult induced neuronal death33,35. In RGCs, multiple lines of
evidence point to JNK/JUN signaling having a role in cell death after an excitotoxic insult. JNK and JUN are activated in RGCs after induced excitotoxicity (Fig. 4)18,22. Also, inhibiting
JNK signaling by an intravitreal injection of a JNK inhibitor decreased RGC loss after NMDA insult22. However, despite the potential importance of JNK signaling in excitotoxic injured
neurons, experiments herein which critically tested key components of JNK/JUN signaling showed that this pathway may not be a primary driver of excitotoxic RGC death. Specifically, we showed
that deficiency in _Jun_, _Jnk2/3_, and _Dlk_ did not prevent excitotoxicity-induced RGC death. It is unclear why the results presented here differ with previous studies showing protection
from excitotoxic injury after inhibiting the JNK/JUN signaling pathway20,21,22. Two previous studies used pharmaceutical approaches to inhibit JNK signaling20,22. For these studies it is
possible that there was inhibition of other kinases that contributed to the protection55. Munemasa and colleagues specifically tested the importance of JUN activation in NMDA induced RGC
death by intravitreally injecting _Jun_ anti-sense oligodeoxynucleotides21. It is possible that this experiment also resulted in off target effects. Furthermore, all of these experiments
counted total ganglion cell layer neurons and not just RGCs. The ganglion cell layer in the mouse consists of approximately equal numbers of RGCs and amacrine cells56 and both cell types die
after excitotoxic insult28. Thus, it is possible that there is protection of amacrine cells that explains the differences between the studies. It is also possible that the genetic deletion
of JNK signaling molecules during development alters the response of RGCs to excitotoxic insult. The lack of protection observed in this study by components of the JNK/JUN signaling pathway
is an intriguing result given the known importance of JNK/JUN signaling in RGCs after other insults, particularly after axonal injury38,57,58,59. Thus, these results suggest that in RGCs
different molecular pathways are activated depending on the insult. Furthermore, given the potential importance of excitotoxicity in diabetes and ischemic optic neuropathy and axonal injury
in glaucoma it suggests that RGCs die by different mechanisms in common human diseases. It is important to note that the experiments described here have not ruled out the potential
importance of a JNK1 contribution, particularly a JUN-independent JNK1 function, in mediating RGC death after an excitotoxic insult. It will be important in the future to test the role of
JNK1 and even the simultaneous inhibition of all three JNKs to fully define the importance of JNK signaling in excitotoxicity induced RGC death. After an excitotoxic insult, ER stress
components are upregulated and active in RGCs, including _Ddit3_25. Furthermore, _Ddit3_ deficiency has been shown to protect about 10 cells/mm in the ganglion cell layer more than in
C57BL/6 J after an excitotoxic injury23. _Ddit3_ deficiency did not provide protection to RGCs 7 days after insult, suggesting it is not required for RGC death after and excitotoxic insult.
Both JNK/JUN and ER stress signaling contribute to RGC death after axonal injury27,38. Recently, our group showed that these pathways appeared to be independently regulated in RGCs after
axonal injury44. In fact, absence of _Jun_ and _Ddit3_ provided robust, long-term protection to RGCs after an axonal insult44. Thus, it is possible that both of these pathways could
contribute to RGC death after an excitotoxic insult and that each is sufficient to kill RGCs. To critically test this hypothesis, RGCs were counted from mice deficient in both _Jun_ and
_Ddit3_ after an excitotoxic insult. Deficiency of both _Jun_ and _Ddit3_ failed to provide protection to RGCs after intravitreal NMDA injection 7 days after insult. Furthermore, RGC loss
after intravitreal injection of kainic acid was not lessened by _Jun/Ddit3_ deficiency. Together these data show that JUN and DDIT3 dependent pathways are not required to prevent RGC death
after an excitotoxic injury. This result was surprising given that both _Jun_ and _Ddit3_ are known RGC pro-death molecules and are expressed in RGCs after an excitotoxic injury. Studies
have reported that manipulating various pathways provide protection of RGCs7,21,22,23,60. However, most of these studies have shown only a minor or short-term protection, especially those
targeting parts of the pathway downstream of glutamate receptor activation. Furthermore, to date, no one method of intervention has shown complete protection from excitotoxic RGC death.
These studies coupled with the results described here indicate that multiple, independent pro-death pathways are active after an excitotoxic insult. This potentially makes preventing
excitotoxic RGC death very difficult and designing therapeutic targets challenging in chronic diseases where excitotoxicity has been implicated. Simply inhibiting NMDA and KA channels for
the duration of the chronic diseases are likely be detrimental to the system or become ineffective. Additionally, NMDA receptor antagonists failed to show efficacy in multiple clinical
trials for stroke and TBI, relegating development of potential therapeutics to downstream pathogenic signaling pathways61. Thus, multiple pro-death pathways may need to be targeted to
prevent vision loss in diseases were excitotoxicity contributes to RGC death. In the future, it will be important to define all of the pro-death pathways active in RGCs after excitotoxic
insult in order to identify common upstream regulators of these pathways. It is important to note that this study used a typical procedure of giving a single intravitreal injection of a
fairly large amount of NMDA or KA. It is unlikely that this type of injury is what occurs during disease. To understand the signaling pathways that control excitotoxic RGC death in human
diseases it will be important to model the precise nature of the excitotoxic insult in terms of both concentration and length of insult. Therefore, to properly model disease relevant
exposures to excitotoxins, experiments should be performed where excitotoxins are chronically delivered to the retina at low or varying levels. MATERIALS AND METHODS MICE Mice carrying null
alleles of _Tnf (Tnf__tm1Gkl_), _Bid_ (_Bid__tm1Sjk_), _Jnk2_ (_Mapk9__tm1Flv_) and _Jnk3_ (_Mapk10__tm1Flv_), or _Ddit3_ (_Ddit3__tm2.1Dron_) were obtained from the Jackson Laboratory.
Additionally, floxed alleles of _Dlk_ (_Dlk__fl_)62; and _Jun_ (_Jun__fl_)40; were used. Floxed alleles were recombined using a retinal expressed cre, Tg(Six3-cre)69Frty mice (referred to as
Six3-cre)41 Note a “?” for an allele denotes that for controls some animals carried a WT allele while others carried either a null or floxed allele as appropriate (e.g.+/? means both +/+
and +/− genotypes were used). All colonies were maintained by intercrossing. All alleles were on a C57BL/6 J genetic background except for the _Jnk2_ and _Jnk3_ cross which was on a mixed
C57BL/6 J and DBA/2 J genetic background. Mice were housed in a 12-hour light dark cycle and were fed chow and water ad libitum. All mice used were adults (2–5 months of age). All
experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology’s statement on the use of animals in ophthalmic research and were approved by the
University of Rochester’s University Committee on Animal Resources. INTRAOCULAR INJECTION Mice were anaesthetized with an intraperitoneal injection of 0.05 ml/10 g solution containing
ketamine (20 mg/mL) and xylazine (2 mg/mL). A small incision was made with a 30-gauge needle behind the limbus at the superiotemporal quadrant through the conjunctiva at a 45° angle. 2 mM,
20 mM, and 80 mM concentrations of N-Methyl-D-aspartate (NMDA; Sigma) and 10 mM, 12 mM, 15 mM, and 20 mM KA were made in sterile 0.1 M PBS. 2 μl of either NMDA or KA concentrations were
injected into the vitreous through the sclera just behind the limbus, using a 5 μL Hamilton syringe equipped with a 33-gauge removable needle. The contralateral eye was injected with 2 μL of
vehicle (0.1 M PBS) to serve as a control. To reduce an increase in intraocular pressure, the intravitreal injections were conducted over 2 minutes. The condition of the injected eyes was
monitored to ensure no problems arose due to the injection (e.g. cataract). IMMUNOHISTOCHEMISTRY AND CELL COUNTS RGC survival was assessed using the RGC marker TUJ1, whose expression is
sustained following injury63,64,65. Eyes were processed as previously described38,64. Following fixation in 4% paraformaldehyde (PFA), the anterior segment of each eye was removed and the
posterior eyecup was processed for whole mount immunostaining or cryosectioning. For whole mount immunostaining, retinas were blocked in 0.4% Triton X-100 in PBS containing 10% horse serum
for 3–4 hours. Retinas were then incubated in primary antibody against mouse anti-βIII tubulin (mouse anti-TUJ1, BioLegend, 1:1000) diluted in 0.3% Triton X-100 in PBS for 3 days at 4 °C.
Following washes in PBS, the retinas were incubated with Alexa Fluor conjugated secondary antibodies (Invitrogen, 1:1000) diluted in PBS for 1 day at 4 °C and then mounted ganglion cell
layer up onto slides. RGC density varies greatly with respect to retinal location. For each retina, images were obtained from eight 40× fields around the peripheral retina (two from each
quadrant), each field approximately 220 μm from the peripheral edge of the retina (one half of a 40× field in from the peripheral margin). The numbers of neurons immunolabeled with TUJ1 in
each image were quantified using the cell-counter tool in ImageJ (NIH). Counts from each field were summed. For immunohistochemistry on retinal sections, cryosections were blocked by
incubating in 10% horse serum in 0.1% Triton X-100 in PBS (PBST) for 2–3 h at room temperature. Sections were incubated with primary antibodies (mouse anti-TUJ1, BioLegend, 1:1000; rabbit
anti-pJUN, Cell Signaling, 1:250) diluted in PBST overnight at 4 °C. The following day the sections were washed and incubated with Alexa Fluor-conjugated secondary antibodies (Invitrogen,
1:1000) diluted in PBST for a minimum of 2 hours. Sections were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Fisher Scientific). For terminal deoxynucleotidyl
transferase-mediated dUTP end labeling (TUNEL), mice were euthanized and cryosections were prepared as described above. TUNEL assays were performed according to the manufacturer’s
instructions (ApoTag, EMD Millipore). STATISTICAL ANALYSIS During quantification of results, the experimenter was masked to genotype and/or experimental group. Experiments with two groups
were analyzed for differences using a one-way ANOVA and three or more groups, either multiple time points or genotypes, underwent statistical analyses using a two-way ANOVA with significance
determined at _P_ values < 0.05, followed by the Tukey’s or Sidak’s multiple comparisons test for group comparisons. REFERENCES * Olney, J. W. Brain lesions, obesity, and other
disturbances in mice treated with monosodium glutamate. _Science_ 164, 719–721 (1969). Article ADS CAS PubMed Google Scholar * Araszkiewicz, A. & Zozulinska-Ziolkiewicz, D. Retinal
neurodegeneration in the course of diabetes - pathogenesis and clinical perspective. _Curr. Neuropharmacol_. (2016). * Barber, A. J. A new view of diabetic retinopathy: a neurodegenerative
disease of the eye. _Prog. Neuropsychopharmacol. Biol. Psychiatry_ 27, 283–290 (2003). Article CAS PubMed Google Scholar * Ishikawa, M. Abnormalities in Glutamate Metabolism and
Excitotoxicity in the Retinal Diseases. _Scientifica_ 2013, e528940 (2013). Article Google Scholar * Fischer, A. J., Pickett Seltner, R. L., Poon, J. & Stell, W. K. Immunocytochemical
characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. _J. Comp. Neurol._ 393, 1–15 (1998). Article CAS PubMed Google Scholar *
Lam, T. T., Abler, A. S., Kwong, J. M. K. & Tso, M. O. M. N-Methyl-D-Aspartate (NMDA)–Induced Apoptosis in Rat Retina. _Invest. Ophthalmol. Vis. Sci._ 40, 2391–2397 (1999). CAS PubMed
Google Scholar * Lebrun-Julien, F. _et al_. Excitotoxic death of retinal neurons _in vivo_ occurs via a non-cell-autonomous mechanism. _J. Neurosci. Off. J. Soc. Neurosci._ 29, 5536–5545
(2009). Article CAS Google Scholar * Libby, R. T. _et al_. Susceptibility to Neurodegeneration in a Glaucoma Is Modified by Bax Gene Dosage. _PLoS Genet_. 1 (2005). * Sucher, N. J.,
Lipton, S. A. & Dreyer, E. B. Molecular basis of glutamate toxicity in retinal ganglion cells. _Vision Res._ 37, 3483–3493 (1997). Article CAS PubMed Google Scholar * Tobaben, S. _et
al_. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. _Cell Death Differ._
18, 282–292 (2011). Article CAS PubMed Google Scholar * Uchibayashi, R., Tsuruma, K., Inokuchi, Y., Shimazawa, M. & Hara, H. Involvement of Bid and caspase-2 in endoplasmic reticulum
stress- and oxidative stress-induced retinal ganglion cell death. _J. Neurosci. Res._ 89, 1783–1794 (2011). Article CAS PubMed Google Scholar * Li, Y., Schlamp, C. L., Poulsen, K. P.
& Nickells, R. W. Bax -dependent and Independent Pathways of Retinal Ganglion Cell Death Induced by Different Damaging Stimuli. _Exp. Eye Res._ 71, 209–213 (2000). Article CAS PubMed
Google Scholar * Hibi, M., Lin, A., Smeal, T., Minden, A. & Karin, M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation
domain. _Genes Dev._ 7, 2135–2148 (1993). Article CAS PubMed Google Scholar * Isenmann, S. & Bähr, M. Expression of c-Jun Protein in Degenerating Retinal Ganglion Cells after Optic
Nerve Lesion in the Rat. _Exp. Neurol._ 147, 28–36 (1997). Article CAS PubMed Google Scholar * Kim, B.-J. _et al_. _In vitro_ and _in vivo_ neuroprotective effects of cJun N-terminal
kinase inhibitors on retinal ganglion cells. _Mol. Neurodegener._ 11, 30 (2016). Article PubMed PubMed Central Google Scholar * Kwong, J. M. K. & Caprioli, J. Expression of
phosphorylated c-Jun N-terminal protein kinase (JNK) in experimental glaucoma in rats. _Exp. Eye Res._ 82, 576–582 (2006). Article CAS PubMed Google Scholar * Levkovitch-Verbin, H. _et
al_. The transcription factor c-jun is activated in retinal ganglion cells in experimental rat glaucoma. _Exp. Eye Res._ 80, 663–670 (2005). Article CAS PubMed Google Scholar * Munemasa,
Y. _et al_. P-JNK expression of the rat retina in NMDA-induced neurotoxicity. _ARVO Meet. Abstr._ 45, 724 (2004). Google Scholar * Roth, S. _et al_. Mitogen-Activated Protein Kinases and
Retinal Ischemia. _Invest. Ophthalmol. Vis. Sci._ 44, 5383–5395 (2003). Article PubMed Google Scholar * Munemasa, Y. _et al_. Contribution of mitogen-activated protein kinases to
NMDA-induced neurotoxicity in the rat retina. _Brain Res._ 1044, 227–240 (2005). Article CAS PubMed Google Scholar * Munemasa, Y. _et al_. Pro-apoptotic role of c-Jun in NMDA-induced
neurotoxicity in the rat retina. _J. Neurosci. Res._ 83, 907–918 (2006). Article CAS PubMed Google Scholar * Bessero, A.-C., Chiodini, F., Rungger-Brändle, E., Bonny, C. & Clarke, P.
G. H. Role of the c-Jun N-terminal kinase pathway in retinal excitotoxicity, and neuroprotection by its inhibition. _J. Neurochem._ 113, 1307–1318 (2010). CAS PubMed Google Scholar *
Awai, M. _et al_. NMDA-induced retinal injury is mediated by an endoplasmic reticulum stress-related protein, CHOP/GADD153. _J. Neurochem._ 96, 43–52 (2006). Article CAS PubMed Google
Scholar * Concannon, C. G. _et al_. NMDA receptor-mediated excitotoxic neuronal apoptosis _in vitro_ and _in vivo_ occurs in an ER stress and PUMA independent manner. _J. Neurochem._ 105,
891–903 (2008). Article CAS PubMed Google Scholar * Shimazawa, M. _et al_. Involvement of ER stress in retinal cell death. _Mol. Vis._ 13, 578–587 (2007). CAS PubMed PubMed Central
Google Scholar * Sokka, A.-L. _et al_. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. _J. Neurosci. Off. J. Soc. Neurosci._ 27,
901–908 (2007). Article CAS Google Scholar * Hu, Y. _et al_. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. _Neuron_
73, 445–452 (2012). Article CAS PubMed PubMed Central Google Scholar * Luo, X., Baba, A., Matsuda, T. & Romano, C. Susceptibilities to and Mechanisms of Excitotoxic Cell Death of
Adult Mouse Inner Retinal Neurons in Dissociated Culture. _Invest. Ophthalmol. Vis. Sci._ 45, 4576–4582 (2004). Article PubMed Google Scholar * Bai, N. _et al_. NMDA receptor subunits
have different roles in NMDA-induced neurotoxicity in the retina. _Mol. Brain_ 6, 34 (2013). Article CAS PubMed PubMed Central Google Scholar * De Groef, L. _et al_. Decreased TNF
Levels and Improved Retinal Ganglion Cell Survival in MMP-2 Null Mice Suggest a Role for MMP-2 as TNF Sheddase. _Mediators Inflamm._ 2015, e108617 (2015). Article Google Scholar * Zhao, J.
_et al_. (+)-Pentazocine Reduces NMDA-Induced Murine Retinal Ganglion Cell Death Through a σR1-Dependent Mechanism. _Investig. Opthalmology Vis. Sci._ 57, 453 (2016). Article CAS Google
Scholar * Danial, N. N. & Korsmeyer, S. J. Cell Death. _Cell_ 116, 205–219 (2004). Article CAS PubMed Google Scholar * Pozniak, C. D. _et al_. Dual leucine zipper kinase is required
for excitotoxicity-induced neuronal degeneration. _J. Exp. Med._ 210, 2553–2567 (2013). Article CAS PubMed PubMed Central Google Scholar * Fernandes, K. A., Harder, J. M., Kim, J.
& Libby, R. T. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. _Exp. Eye Res._ 112, 106–117 (2013). Article CAS PubMed PubMed Central Google
Scholar * Fernandes, K. A., Harder, J. M., John, S. W., Shrager, P. & Libby, R. T. DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells
following axonal injury. _Neurobiol. Dis._ 69, 108–116 (2014). Article CAS PubMed PubMed Central Google Scholar * Liu, J. & Lin, A. Role of JNK activation in apoptosis: A
double-edged sword. _Cell Res._ 15, 36–42 (2005). Article ADS PubMed Google Scholar * Yang, D. D. _et al_. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking
the Jnk3 gene. _Nature_ 389, 865–70 (1997). Article ADS CAS PubMed Google Scholar * Fernandes, K. A. _et al_. JNK2 and JNK3 are major regulators of axonal injury-induced retinal
ganglion cell death. _Neurobiol. Dis._ 46, 393–401 (2012). Article CAS PubMed PubMed Central Google Scholar * Lingor, P., Koeberle, P., Kügler, S. & Bähr, M. Down-regulation of
apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death _in vivo_. _Brain_ 128, 550–558 (2005). Article PubMed Google Scholar * Behrens, A. _et al_. Impaired
postnatal hepatocyte proliferation and liver regeneration in mice lacking c‐jun in the liver. _EMBO J._ 21, 1782–1790 (2002). Article CAS PubMed PubMed Central Google Scholar * Furuta,
Y., Lagutin, O., Hogan, B. L. & Oliver, G. C. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. _Genes. N. Y. N 2000_ 26, 130–132 (2000). CAS Google
Scholar * Hilberg, F., Aguzzi, A., Howells, N. & Wagner, E. F. c-Jun is essential for normal mouse development and hepatogenesis. _Nature_ 365, 179–181 (1993). Article ADS CAS PubMed
Google Scholar * Johnson, R. S., van Lingen, B., Papaioannou, V. E. & Spiegelman, B. M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in
culture. _Genes Dev._ 7, 1309–1317 (1993). Article CAS PubMed Google Scholar * Syc-Mazurek, S. B., Fernandes, K. A., Wilson, M. P., Shrager, P. & Libby, R. T. Together JUN and DDIT3
(CHOP) control retinal ganglion cell death after axonal injury. _Mol. Neurodegener._ 12, 71 (2017). Article PubMed PubMed Central Google Scholar * Jarrard, L. E. Use of excitotoxins to
lesion the hippocampus: Update. _Hippocampus_ 12, 405–414 (2002). Article PubMed Google Scholar * Schwarcz, R. & Coyle, J. T. Kainic acid: neurotoxic effects after intraocular
injection. _Invest. Ophthalmol. Vis. Sci._ 16, 141–148 (1977). CAS PubMed Google Scholar * Zheng, X.-Y., Zhang, H.-L., Luo, Q. & Zhu, J. Kainic Acid-Induced Neurodegenerative Model:
Potentials and Limitations. _BioMed Res. Int._ 2011, e457079 (2010). Google Scholar * Cazevieille, C. & Osborne, N. N. Retinal neurones containing kainate receptors are influenced by
exogenous kainate and ischaemia while neurones lacking these receptors are not–melatonin counteracts the effects of ischaemia and kainate. _Brain Res._ 755, 91–100 (1997). Article CAS
PubMed Google Scholar * Johnson, T. V. & Tomarev, S. I. Rodent models of glaucoma. _Brain Res. Bull._ 81, 349–358 (2010). Article PubMed Google Scholar * Mali, R. S., Cheng, M.
& Chintala, S. K. Plasminogen Activators Promote Excitotoxicity-Induced Retinal Damage. _FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol._ 19, 1280 (2005). CAS Google Scholar * Spigolon,
G., Veronesi, C., Bonny, C. & Vercelli, A. c-Jun N-terminal kinase signaling pathway in excitotoxic cell death following kainic acid-induced status epilepticus. _Eur. J. Neurosci._ 31,
1261–1272 (2010). Article PubMed Google Scholar * Ali, S. A. M., Hosaka, Y. Z. & Uehara, M. Expression of Small Leucine-Rich Proteoglycans in the Developing Retina and Kainic
Acid-Induced Retinopathy in ICR Mice. _J. Vet. Med. Sci._ 73, 439–445 (2011). Article CAS PubMed Google Scholar * Watkins, T. A. _et al_. DLK initiates a transcriptional program that
couples apoptotic and regenerative responses to axonal injury. _Proc. Natl. Acad. Sci. USA_ 110, 4039–4044 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Welsbie, D. S.
_et al_. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. _Proc. Natl. Acad. Sci. USA_ 110, 4045–4050 (2013). Article
ADS CAS PubMed PubMed Central Google Scholar * Koch, P., Gehringer, M. & Laufer, S. A. Inhibitors of c-Jun N-terminal kinases: an update. _J. Med. Chem._ 58, 72–95 (2015). Article
CAS PubMed Google Scholar * Schlamp, C. L. _et al_. Evaluation of the percentage of ganglion cells in the ganglion cell layer of the rodent retina. _Mol. Vis._ 19, 1387–1396 (2013). CAS
PubMed PubMed Central Google Scholar * Crocker, S. J. _et al_. c-Jun mediates axotomy-induced dopamine neuron death _in vivo_. _Proc. Natl. Acad. Sci. USA_ 98, 13385–13390 (2001). Article
ADS CAS PubMed PubMed Central Google Scholar * Pirianov, G. _et al_. Deletion of the c-Jun N-terminal kinase 3 gene protects neonatal mice against cerebral hypoxic-ischaemic injury.
_J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab._ 27, 1022–1032 (2007). Article CAS Google Scholar * Ries, V. _et al_. JNK2 and JNK3 combined are essential for
apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. _J. Neurochem._ 107, 1578–1588 (2008). Article CAS PubMed PubMed Central Google Scholar
* Behrens, A., Sibilia, M. & Wagner, E. F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. _Nat. Genet._ 21, 326–329 (1999).
Article CAS PubMed Google Scholar * Ikonomidou, C. & Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? _Lancet Neurol._ 1,
383–386 (2002). Article CAS PubMed Google Scholar * Miller, B. R. _et al_. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. _Nat. Neurosci._
12, 387–389 (2009). Article CAS PubMed PubMed Central Google Scholar * Cui, Q., Yip, H. K., Zhao, R. C. H., So, K.-F. & Harvey, A. R. Intraocular elevation of cyclic AMP
potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. _Mol. Cell. Neurosci._ 22, 49–61 (2003). Article CAS PubMed Google Scholar *
Harder, J. M. & Libby, R. T. BBC3 (PUMA) regulates developmental apoptosis but not axonal injury induced death in the retina. _Mol. Neurodegener._ 6, 50 (2011). Article PubMed PubMed
Central Google Scholar * Park, K. K. _et al_. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. _Science_ 322, 963–966 (2008). Article ADS CAS PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The authors would like to thank Drs. DiAntonio (Dlkfl), Furuta (Six3-cre) and Wagner (Junfl) for generously providing
mice. This work was supported by EY018606 (RTL) and Research to Prevent Blindness, an unrestricted grant to the Department of Ophthalmology at the University of Rochester Medical Center. The
funding agencies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Ophthalmology, University of Rochester Medical Center, Rochester, NY, 14642, USA Berkeley K. Fahrenthold, Kimberly A. Fernandes & Richard T. Libby * Neuroscience Graduate
Program, University of Rochester Medical Center, Rochester, NY, 14642, USA Berkeley K. Fahrenthold * Department of Biomedical Genetics, University of Rochester Medical Center, Rochester, NY,
14642, USA Richard T. Libby * The Center for Visual Sciences, University of Rochester Medical Center, Rochester, NY, 14642, USA Richard T. Libby Authors * Berkeley K. Fahrenthold View
author publications You can also search for this author inPubMed Google Scholar * Kimberly A. Fernandes View author publications You can also search for this author inPubMed Google Scholar *
Richard T. Libby View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.K.F., K.A.F. and R.T.L. designed research; B.K.F. and K.A.F. performed
research; B.K.F., K.A.F. and R.T.L. analyzed data; B.K.F. and R.T.L. wrote the paper. All authors have reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Richard T. Libby.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fahrenthold, B.K., Fernandes, K.A. & Libby, R.T. Assessment of intrinsic and
extrinsic signaling pathway in excitotoxic retinal ganglion cell death. _Sci Rep_ 8, 4641 (2018). https://doi.org/10.1038/s41598-018-22848-y Download citation * Received: 20 December 2017 *
Accepted: 01 March 2018 * Published: 15 March 2018 * DOI: https://doi.org/10.1038/s41598-018-22848-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative