- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Mouse papillomavirus has shown broad tissue tropism in nude mice. Previous studies have tested _cutaneous_ infections in different immunocompromised and immunocompetent mouse
strains. In the current study, we examined _mucosal_ infection in several immunocompetent and immunocompromised mouse strains. Viral DNA was monitored periodically by Q-PCR of lavage
samples. Immunohistochemistry and _in situ_ hybridization were used to determine viral capsid protein and viral DNA respectively. All athymic nude mouse strains showed active infections at
both cutaneous and mucosal sites. Interestingly, NOD/SCID mice, which have a deficiency in T, B, and NK cells, showed minimal disease at cutaneous sites but developed persistent infection at
the mucosal sites including those of the anogenital region and the oral cavity. Three strains of _immunocompetent_ mice supported mucosal infections. Infections of the lower genital tract
in heterozygous (immunocompetent) mice of the NU/J strain progressed to high grade dysplasia and to carcinoma _in situ_. Anti-MmuPV1 neutralizing antibodies were detected in the sera of all
immunocompetent animals. Our findings demonstrate that the mucosae may be the preferred sites for this virus in mice. The mouse model is expected to be a valuable model for the study of
mucosal papillomavirus disease, progression, and host immune control. SIMILAR CONTENT BEING VIEWED BY OTHERS HUMAN PAPILLOMAVIRUSES: DIVERSITY, INFECTION AND HOST INTERACTIONS Article 14
September 2021 ROLES OF HUMAN PAPILLOMAVIRUS IN CANCERS: ONCOGENIC MECHANISMS AND CLINICAL USE Article Open access 24 January 2025 VIRAL INFECTION AND ANTIVIRAL IMMUNITY IN THE ORAL CAVITY
Article 12 November 2024 INTRODUCTION Human papillomaviruses (HPVs) are obligate factors for the development of cervical cancer, which is responsible for the deaths of 250,000 women
worldwide each year1. In addition, these viruses are increasingly implicated in head and neck cancers, anal cancers, and some skin cancers2,3. The three existing vaccines against HPVs are
all prophylactic in nature and, while effective in preventing new infections of important subsets of papillomaviruses, offer little help to the many people already infected with the virus4.
In addition, the uptake of the vaccines has been disappointingly low meaning that those people unable to clear the disease will continue to be at risk for the development of cancer over
time5. Papillomaviruses are species-specific and therefore it is not possible to study a HPV in an animal model. For many years, the cottontail rabbit papillomavirus (CRPV) model was the
system of choice for several laboratories including our own, in part because CRPV lesions progress to cancer over time6,7,8,9,10. Much about the immunology, molecular biology and malignant
potential of papillomaviruses has been learned using this system and it is anticipated that the model will continue to be a valuable resource in the years to come11. However, there are
limitations to the model. For one thing, most HPV-associated cancers are of mucosal origin and the CRPV lesions are cutaneous12. In addition, reagents for the rabbit are quite limited
relative to those for the most common laboratory animal, the mouse. Unfortunately, until 2011 when Ingle _et al_. reported finding Mouse papillomavirus 1(MmuPV1) in a colony of nude mice in
India13, no mouse virus had been identified that could infect a common laboratory strain14,15. The discovery of MmuPV1 intrigued the papillomavirus research community although enthusiasm was
tempered by the early report that the virus was strictly cutaneous in nature13. A number of laboratories began to study the virus with most work assuming cutaneous tropism16,17,18,19,20.
Sundberg _et al_. looked at strain and site differences and noted the formation of trichoblastomas on the dorsal skin21. Handisurya _et al_. also looked at strain differences and T cell
involvement in clearance17. They showed that T cell depletion via anti-CD3 antibody rendered immunocompetent animals permissive for cutaneous infections. Wang _et al_. studied immunologic
control of the virus and noted cutaneous viral persistence in one strain of immunocompetent mice, the hairless SKH-118. This work was followed up by that of Jiang _et al_. in a paper in
which the utility of this animal was demonstrated for the study of clearance of PV disease19. Uberoi _et al_. reported that systemic immunosuppression induced by a high dose of UVB promoted
cancer development in MmuPV1 infected ear skin of FVB/NJ immunocompetent mice20. In our laboratory, we found and reported on the first _mucosal_ infections with the MmuPV1 virus and have
definitively shown that oral, vaginal, anal and penile tissues are all highly susceptible to the virus, putting to rest the idea that the virus is restricted to cutaneous sites22,23,24,25.
These observations were further confirmed by studies in another group21,26. In addition to the active anogenital infections and dysplasia in these animals, we have also observed that the
single circumvallate papilla of the mouse tongue is uniquely susceptible to infection by the virus. This site is comparable to back of the tongue sites so commonly found in oral
papillomavirus-associated cancers in humans, for which an increasing incidence is reported in younger male Caucasians27. We anticipate that this new mouse model will be of use in studying
progression of oral papillomavirus disease. Active infections can be readily established in immunocompromised animals at mucosal sites22,23,24. To study viral-host interactions, an
immunocompetent mouse strain with intact immune response is desirable. Different immunocompetent mouse strains including C57BL/6, hairless SKH-1, and FVB/NJ have revealed differences in
cutaneous site susceptibilities in previous studies17,18,19,20,21,28. We were interested in following up on these observations and in determining whether we could identify a mucosally
susceptible immunocompetent strain as well. The experiments reported in this manuscript were designed to expand on our observations of mucosal MmuPV1 infections by investigating several
different mouse strains. Among the animals selected, we first tested immunocompetent C57BL/6 mice and SKH-1 hairless elite mice. They showed strong immune responses to MmuPV1 infection and
cleared the infection quickly. We then decided to investigate the heterozygous siblings of the homozygous immunocompromised NU/J, Hsd: NU and B6 animals, which we had previously shown to be
permissive for viral infections17,21. These heterozygotes are immunocompetent. To follow the infections longitudinally, we monitor viral DNA copy numbers via QPCR analysis of DNA isolated
from lavage samples, which we collect regularly over time23. This allows us to use a small number of animals to obtain robust data. It obviates the need for large numbers of animals to be
sacrificed over time while still allowing for extensive data collection. The lavages have proven to be a powerful tool and have been used to study vaginal, penile, anal and oral
infections23. We found that the NU/J, Hsd: NU and C57BL/6 _heterozygotes_ were permissive for infection at the anogenital tissues. Furthermore all NU/J mice proceeded to develop carcinoma
_in situ_ in the vaginal infected tissues by 7.5 months post infection. Interestingly, the cutaneous tissues of these same mice showed only subclinical infections and were not permissive for
papillomavirus lesion development. These important findings provide opportunities for the study of mucosal papillomavirus infections and malignancies under the influence of an intact immune
system and in a biologically relevant site, the vaginal canal. They further help to cement the utility of this new MmuPV1 model for the study of papilloma diseases, progression,
immunological response and viral-host interaction. RESULTS IMMUNOCOMPETENT SKH1-HRHR AND C57BL/6J MICE WERE SUSCEPTIBLE TO MMUPV1 INFECTION AT MUCOSAL SITES BUT CLEARED THE INFECTION QUICKLY
Previous studies have demonstrated that adaptive immunity is sufficient to eliminate MmuPV1 infection in outbred hairless euthymic SKH1-Elite (Crl: SKH1-Hrhr) and C57BL/6J immunocompetent
mice at cutaneous sites19,20. Whether or not mucosal sites, including the lower genital tract, were susceptible to MmuPV1 infection was not tested. Four SKH1-Elite and eight inbred C57BL/6J
mice were infected vaginally with MmuPV1 (Table 1). Infection was followed by the detection of viral DNA in vaginal lavage, a tool that has proven to be very robust in our hands23. Viral DNA
was detected in the lavages at week two post-infection in both strains but was undetectable at week four post-infection (Supplementary Fig. 1A and B). We detected anti-viral antibodies in
serum samples from these infected animals (Supplementary Fig. 1C and D) indicating that transient mucosal infections probably occurred26. Serum conversion was also reported in cutaneously-
infected immunocompetent mice19. These findings suggest that both immunocompetent mouse strains are susceptible to MmuPV1 infection at vaginal mucosae, and that the infections rapidly clear.
Eight C57LB/6J mice were used to test the ability of these immunocompetent mice to sustain oral infection. Viral DNA was detected in the DNA from oral lavages by QPCR at week three
post-infection in six of the mice and became undetectable after week four (Supplementary Fig. 2A). Two mice with the highest viral DNA copy numbers were sacrificed for histological analysis.
In neither of these animals was viral DNA detected by _in situ_ hybridization. Serum samples from all animals were harvested for antibody detection at week seven post-infection. All eight
orally-infected B6 mice generated detectable antibodies against the mouse papillomavirus (Supplementary Fig. 2B). No viral DNA was detected at the infected sites by _in situ_ hybridization
analysis in any of the animals at week seven post infection, supporting the rapid clearance of disease. In our previous studies of immunocompromised mice, we have found anal sites to be
somewhat less permissive to MmuPV1 infection than vaginal and oral sites23. Previous studies have shown that CD4 and CD8 T cells are crucial to the elimination of MmuPV1 in immunocompetent
mice at cutaneous sites17,18. We, therefore, decided to deplete CD4 and CD8 cells in three C57BL/6J mice by using anti-mouse CD4 (Clone GK1.5) and anti-mouse CD8 (clone 2.43 against CD8a)
for seven weeks following anal viral infections. The CD4 (Supplementary Fig. 3A) and CD8 (Supplementary Fig. 3B) levels were evaluated after the termination of the experiment by one-color
flow cytometry analysis29. Although significantly lower levels of CD8 were found in the depleted animals (Supplementary Fig. 3C, P < 0.05, unpaired Student T-test), the CD4 T cell
population was not significantly different between the two groups (Supplementary Fig. 3C, P > 0.05, unpaired Student T-test). Anal infections were detected at week five post-infection
(Supplementary Fig. 3D and E, P < 0.05, unpaired Student T-test) but no viral DNA was detected after week six post-infection. We concluded that the observation that MmuPV1 infection could
not be sustained in the anal mucosae of immunocompetent C57BL/6J mice, even under antibody depletion conditions, may have been due to insufficient CD4 T cell depletion. NOD/SCID MICE WERE
SUSCEPTIBLE TO MUCOSAL INFECTIONS BUT RESISTANT TO CUTANEOUS INFECTIONS A previous study reported that NOD/SCID mice were resistant to cutaneous MmuPV1 infections (19). In the current study,
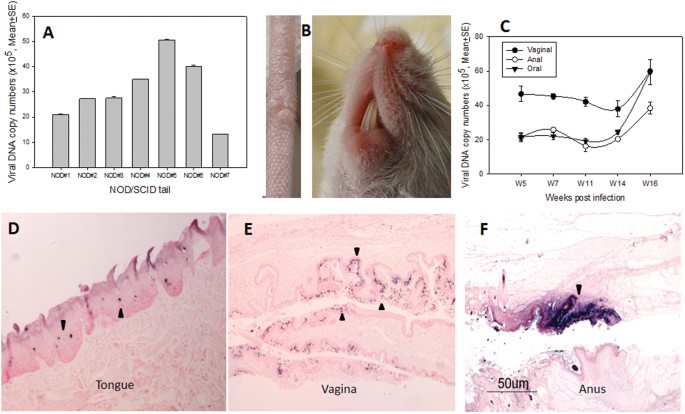
we tested eight NOD/SCID mice for both cutaneous and mucosal infection. Although no visible cutaneous lesions were detected in seven of the eight infected mice, we were able to detect viral
DNA at ten weeks post-infection in the infected tail tissues of all animals by Q-PCR (Fig. 1A). One small tail lesion on one of the infected mice was observed; no muzzle lesions were
detected (Fig. 1B). On the other hand, high levels of viral DNA were detected in vaginal, anal, and oral samples of all animals by Q-PCR (Fig. 1C). Selected oral (Fig. 1D), anal (Fig. 1E),
and vaginal (Fig. 1F) sites were subjected to _in situ_ hybridization following sacrifice and all were positive (arrows). These observations have confirmed that the mucosal tissues of
another immunocompromised mouse are permissive for MmuPV1 infection but that the cutaneous sites are refractive for productive viral infection. PERSISTENT INFECTION WAS ESTABLISHED IN
HETEROZYGOTES AT MUCOSAL SITES A previous study reported that inbred nude mice of the NU/J strain were resistant to viral infection at back sites (19). We tested both the immunocompromised
homozygous NU/J (nude) and immunocompetent heterozygous NU/J (hairy) mice of this inbred strain in the current study. We also tested the HSD: NU outbred (homozygous) nudes, which have been
used in our previous studies. Animals were infected at both mucosal and cutaneous sites. Lesions were detected at the tail and the muzzle sites of the inbred NU/J nude mice although the
lesions were significantly smaller when compared with those in the outbred nude mice (HSD: NU) (Fig. 2). On the other hand, comparable _mucosal_ infections were detected in both strains of
nude mice. The viral DNA copy number in oral, anal and vaginal lavages was monitored through week 20 post viral infection in all three strains. Infection was detected in all although
significantly lower levels of viral DNA were seen in the heterozygous mice when compared with the corresponding homozygous mice (Fig. 3A and B, P < 0.001 after week seven post infection,
unpaired Student T-test). The viral infection in all animals persisted over time. Unlike homozygous mice with visible lesions at both muzzle and tail sites (Fig. 4A), no muzzle lesions were
detected in the heterozygous animals and only, a single lesion was detected on one of the tails of these animals; this lesion regressed within two weeks (Fig. 4B). ADVANCED DISEASE WAS FOUND
IN MMUPV1-INFECTED NU/J HETEROZYGOTES Four NU/J heterozygous (He#1-He#4) NU/J mice were sacrificed to examine histological changes at the lower genital tract at 7.5 months post-infection.
All of these animals were positive for viral DNA by _in situ_ hybridization (ISH, Fig. 5A–D, 10×, and arrows) and for viral capsid protein by immunohistochemistry (IHC, Fig. 5E–H). Detailed
pathological analysis revealed carcinoma _in situ_ in the vaginal tracts of all four animals (Fig. 5I–K, and arrows). Ambiguous stromal microinvasion by basal cells was seen in one of the
four genital tracts (H&E, Fig. 5I). Cell invasion was detected at multiple lesions (H&E, Fig. 5J–K, 20×, and arrows). No significant signals were detected in the oral cavity and the
anal canal of these mice. MMUPV1 DISEASE PERSISTED IN MUCOSAL TISSUES IN HOMOZYGOUS NU/J MICE One of the four infected homozygous NU/J mice died before the termination of the experiment. The
remaining three animals (Ho#1-#3) were positive for viral capsid protein by immunohistochemistry of the lower genital tract (Fig. 6A–C, 10×, and arrows). There were scattered foci of
ambiguous microinvasion in these infected tissues (Fig. 6D–F, 20×, and arrows). The stratified squamous cornifying epithelium of the anal canal displayed diffuse mild hyperplasia and
hyperkeratosis (Fig. 6G, 10×, and arrows). There was minimal atypia or obvious viral cytopathic effect (CPE) in the anal canal and low to moderate numbers of ISH-positive cells (Fig. 6H,
10×, and arrows), mostly located near the recto-anal junction. A small plaque was found on the ventral surface of the tongue just rostral to the duct of the sublingual salivary gland and was
positive for viral DNA (Fig. 6I–J, 10×, and arrows). HETEROZYGOTES OF HSD: NU AND C57BL/6 (B6) NUDE MICE ALSO SUPPORTED MUCOSAL INFECTIONS We had shown that immunocompetent NU/J
heterozygotes not only supported mucosal MmuPV1 infections but that these infections progressed to cancer (Fig. 5). To determine if other immunocompetent strains of mice might also be
permissive five Hsd: NU heterozygotes and four C57BL/6 nude heterozygotes (Foxn1nu/+) were infected at oral, anal and vaginal sites. Weekly or biweekly lavages demonstrated that infection
was maintained in the Hsd: NU heterozygotes up to at least week 23 (Fig. 7A). The B6 Nude heterozygotes were permissive (Fig. 7B) but did not show positivity after week 23. These experiments
are on-going and final results will be reported at a later date. INFILTRATION OF NEUTROPHILS IN THE LOWER GENITAL TRACT WAS DETECTED IN INFECTED ANIMALS The infected vaginal tissues from
all heterozygous and homozygous NU/J mice were positive for viral DNA by _in situ_ hybridization (Figs 5, 6). To explore whether innate immune cells such as neutrophils infiltrated into the
infected sites, we used a neutrophil-specific monoclonal antibody (LY6.B2) to detect these cells in the lower genital tract of both heterozygous (Fig. 8A) and homozygous (Fig. 8B) mice.
Significantly more neutrophils were identified at the infected vaginal tissues of heterozygous mice when compared to those of homozygous mice (Fig. 8A,B). NEUTRALIZING ANTIBODIES WERE
GENERATED IN THE INFECTED ANIMALS The sera of all heterozygous mice and two of the four homozygous mice were positive for anti-MmuPV1 antibody (Supplementary Fig. 4). These sera were able to
neutralize the MmuPV1 infection of a mouse keratinocyte cell line. REGRESSION OF MUCOSAL MMUPV1 INFECTION IS DELAYED IN IFNΑ/ΒR KNOCKOUT MICE To begin to look at the role of Type I
interferons in innate immune control of MmuPV1 infection, IFNα/βR knockout mice (two females 2–7 L, R and one male 1–7 L) were infected mucosally and disease was tracked by quantitation of
viral DNA in lavage samples30. Relative to annually-infected B6 mice under transient CD4 and CD8 T cell depletion (Supplementary Fig. 3) and vaginally infected wild type B6 (Supplementary
Fig. 4A), disease persisted at the anal and vaginal canals for a longer time in Type I interferons receptor knockout animals (Supplementary Fig. 5A and B respectively). DISCUSSION MmuPV1 was
discovered in a nude mouse colony in 201113. Later, a variant was reported in the house mouse from another laboratory31. Most studies to date have been based on the MmuPV1 isolate from nude
mice17,18,20,21,25. Our laboratory has done pioneering work with this virus and has demonstrated secondary mucosal infection in mice that were originally infected at cutaneous sites25,30.
This finding demonstrates a broad tropism of this virus in athymic mice22,23,24,32. Mucosal infection was later reported in two other studies thus confirming our findings21,26. Several
laboratories have attempted to establish cutaneous infections in immunocompetent animals17,18,19,20,21. Handisurya, _et al_. found that Cyclosporin A treatment is required for induction and
maintenance of MmuPV1-induced papillomas in immunocompetent mice17. Uberoi _et al_. found that several mouse strains including FVB/NJ, C57BL/6, and BALB/c mice were susceptible to MmuPV1
infection after exposure to high doses of UVB20. UVB radiation is associated with systemic immune suppression as measured by inhibition of DTH responses in these mice20. In tissues
supporting MmuPV1 infection in FVB/NJ mice, persistent papillomas developed at focal regions consistent with squamous cell carcinoma, including invasion extending into follicular structures
deep within the dermis20. Jiang _et al_., in a recent study, also observed that 20% of hairless SKH-1 mice established persistent infections19. These studies suggest that specific strains of
immunocompetent animals are susceptible to viral infection at cutaneous sites. Most are able to eliminate the disease due to strong host immune responses but persistence and even cancers
are possible17,18. _Mucosal_ infections in immunocompetent animals have not been reported. Our current study was designed to examine whether such infections could be established and
maintained. This was of special interest to us because of the association between papillomavirus disease and cervical, penile, and head and neck cancers. In our current study, we tracked
mucosal infections in several immunocompetent mouse strains by monitoring viral DNA copy numbers in lavage samples as well as by antibody generation19. Hairless SKH-1 and B6 mice showed
transient infections at anogenital mucosae and the oral cavity (Supplementary Fig. 1). Anti-papillomavirus antibody was detected in these animals. These observations indicate that the virus
is presented to the immune system even in the absence of overt disease. The antibody detected in these immunocompetent animals may be generated either from the original virus exposure or be
a consequence of a latent infection that was detected following immunosuppression19,20. Transient infection parallels most HPV infections in the human population. Almost all people will
contract HPV infections in their lifetimes, but most will clear the infections unless their immune responses are compromised33. CD4 and CD8 T cells are the important components of adaptive
immunity for the control of viral infections34. Previous studies demonstrated that depletion of either CD4 or CD8 T cells was not sufficient to enable MmuPV1 to produce lesions at cutaneous
sites such as the tail of B6 mice17,18,19. Interestingly, CD4 depleted animals showed higher viral transcripts at the infected sites when compared with CD8 depleted animals indicating CD4 T
cells might have helped to eradicate the infection in these animals by producing neutralizing antibodies to block the viral spread. On the basis of these observations, we simultaneously
depleted both CD4 and CD8 T cells in the anally infected B6 mice and tracked viral DNA in the anal lavage. We chose to do this procedure in experiments with anal infections because it has
been our observation, in immunocompromised mice, that anal infections are not as robust as oral and genital infections and we wanted to optimize opportunities for infection23. When we
examined the CD4 and CD8 T cell populations in these mice, the depletion of CD8 T cells was more successful than that of CD4 T cells. The normal CD4/CD8 cell ratio is 2:1. We used the same
amount of monoclonal antibody for each depletion. One out of the three mice failed to show an effective CD4 depletion which made the statistical analysis not significant (Supplementary Fig.
3A–C). Viral DNA peaked at week five post-infection and disappeared around week six post-infection (Supplementary Fig. 3D,E). In agreement with previous studies17,18,19, we speculate that
the residual CD4 T cells may have helped to eradicate the infection in these animals by producing neutralizing antibodies to block the viral spread. E6-specific MmuPV1 CD8+ T cells can
eliminate MmuPV1-induced papillomas in athymic mice by adoptive transfer18,19. Intriguingly, we observed that NOD/SCID mice, a mouse strain that is deficient in T, B and NK cells, still
managed to control cutaneous infections at subclinical levels but showed persistent infection at the three tested mucosal sites (Fig. 1). We hypothesize that host defense factors independent
of and in addition to CD4 and CD8 T cells may play a key role in local viral clearance in these animals. Innate immunity has been reported to play a role in the control of viral infection
at mucosal sites35,36. In a previous study, IFNα/βR- mice were found to be free of MmuPV1-induced cutaneous lesions 18implying that this receptor may not be important for viral control.
Whether latent infections were established at those sites were unclear. When we tested MmuPV1 mucosal infection in IFNα/βR- mice, a prolonged time to regression was found at mucosal sites of
these mice30; Infections were detected up to three months post infection (Supplementary Fig. 5). These findings suggest that type I interferon might play a role in disease outcome. Innate
immune cells including NK cells and neutrophils are important in host defense against viral infections37. In the current study, we detected more neutrophils in the tissues of heterozygous
NU/J mice with a milder disease than in those of homozygous mice with more severe disease indicating that neutrophils may have contributed to the disease outcome (Fig. 8). The role of these
and other immune cells in MmuPV1 infection and persistence needs to be further investigated. Interestingly, two immunocompetent mouse strains, the heterozygous NU/J mouse and the
heterozygous Hsd: NU mouse, were able to control cutaneous infection at muzzle and tail sites but failed to clear infection at mucosal sites such as the anogenital tract and the oral cavity.
This result demonstrates that immune responses are not equally effective at all anatomical sites. We hypothesize that factors in the local microenvironment may play a role in this
differential disease outcome as we observed in NOD/SCID mice, a mouse strain with T, B and NK cell deficiencies. These mice showed a pattern of mucosal infection similar to that in outbred
nude mice but, unlike the outbred nude mice, were more resistant to cutaneous infections. Further studies will investigate the role of local host defenses including innate immunity in the
control of viral infection at cutaneous sites and the role of innate immune cells in disease clearance. The finding that MmuPV1 can establish persistent infection in the anogenital tracts of
at least two strains of immunocompetent mice and that those infections in the NU/J heterozygotes progress to carcinoma _in situ_ over time is important. Lesion classification was based on
the degree and extent of dysplasia and the presence or absence of progressive differentiation luminal to the basement membrane. To detect progression of disease in the living animals, we
collected vaginal lavages periodically for cytological analysis. This technique is the basis of the Pap smear test used in clinical practice to identify hyperplastic changes in human
samples. The most common human cancer associated with papillomavirus is carcinoma of the cervix and most often it is associated with HPV16. The MmuPV1 infections did reach the cervix and we
hypothesize that had the animals been maintained longer, these sites, too, would have progressed to carcinoma. We should note that papillomavirus-associated cancers of the human vagina while
less common than those of the cervix, do occur38. We have not detected hyperplastic changes in the oral epithelium of the NU/J mice in the current study. We hypothesize that the difference
in the disease outcome is due to the genetic background of mouse strains. In our published studies, we identified dysplasia in the oral infections of Hsd Nude mice22. In that study, we found
hyperplasia and dysplasia in lesions of the circumvallate papillae of the tongues. These back-of-tongue sites are anatomically related to the sites of human papillomavirus-associated oral
cancers. Ever since investigators have been aware of the association between papillomaviruses and human cancers, a suitable mouse model to study the disease and its progression has been
sought12. Not until 2011 was a papillomavirus that could infect a common laboratory mouse discovered13. This virus was identified in a colony of immunocompromised mice and was reported to be
strictly cutaneous but our work and that of others has shown both cutaneous and mucosal tropism30. The work reported here clearly shows the potential for genital infections with this virus
to progress to cancer and to do so in at least one immunocompetent strain of laboratory mouse. This observation paves the way for the establishment of a robust model in which to study
disease progression at a site relevant to human infections and in an animal with a competent immune system. MATERIALS AND METHODS VIRAL STOCK Infectious virus was isolated from lesions on
the tails of mice from our previous study25. In brief, the lesions were scraped from the tail with a scalpel blade and homogenized in phosphate-buffered saline (1 × PBS) using a Polytron
homogenizer (Brinkman PT10–35) at highest speed for three minutes while chilling in an ice bath. The homogenate was spun at 10,000 rpm and the supernatant was decanted into Eppendorf tubes
for storage at −20 °C. For these experiments, the virus was diluted 1:5 in 1 × PBS and 200 μl was passed through a 0.2μm cellulose acetate sterile syringe filter. This was chased by the
addition of 200 μl 1 × PBS. The PBS filtrate was added to the filtered virus to give a total of 250 μl sterile virus solution when taking into account loss in the filter. Viral DNA was
quantitated by extraction of the DNA from 5 μl of this stock. 1 μl of the DNA extract contains 1.4 × 107 viral genome equivalents23. About 1 × 108 viral DNA genome equivalents were used for
each infection. ANIMALS AND VIRAL INFECTIONS All mouse work was approved by the Institutional Animal Care and Use Committee of Pennsylvania State University’s College of Medicine (COM) and
all methods were performed in accordance with guidelines and regulations. Hsd: NU outbred homozygotes (Foxn1nu/nu) and heterozygotes (Foxn1nu/+) mice (6–8 weeks) were obtained from Harlan
Laboratories (ENVIGO), outbred hairless euthymic SKH1-Elite (Crl: SKH1-Hrhr), inbred C57BL/6 J mice [wild type, homozygotes(Foxn1nu/nu), and heterozygotes(Foxn1nu/+)], NU/J [outbred
homozygotes (Foxn1nu/nu) and heterozygotes (Foxn1nu/+)] and NOD.CB17-Pkdcscid/SzJ (NOD/SCID) were obtained from the Jackson Laboratory (Table 1). All animals were housed (2–3 mice/cage) in
sterile cages within sterile filter hoods and were fed sterilized food and water in the COM BL2 animal core facility. Mice were sedated i.p. with 0.1 ml/10 g body weight with
ketamine/xylazine mixture (100 mg/10 mg in 10 mls ddH2O). For vaginal infection, mice were inoculated subcutaneously with 3 mg Depo-Provera (Pfizer) in 100 µl PBS three days before the viral
infection as described previously23. Depo was not administered for anal and oral infections. The vaginal and anal tracts were wounded with Doctors’ Brush Picks coated with Conceptrol (Ortho
Options, over the counter). Twenty-four hours after wounding, the mice were again anesthetized and challenged with 25 μl (3.5 × 108) and 10 μl (1.4 × 108) of the sterilized viral suspension
at the vaginal and anal tracts respectively24. For tongue infection, tongues were withdrawn using a sterile forceps and microneedles were used to wound the ventral surface of the tongues22.
Care was taken to minimize the bleeding. The following day, each animal was again anesthetized. The ventral surface of tongues was again gently abraded and 10 µl of sterile virus (1.4 ×
108) was applied to the freshly abraded surfaces. Animals were placed on their backs during recovery to minimize loss of virus from the infection sites. Monitoring was conducted weekly and a
photographic log was created for each animal23. VAGINAL, ANAL, AND ORAL LAVAGE FOR DNA EXTRACTION The vaginal, anal and oral lavages were conducted using 25 μl of sterile 0.9% NaCl
introduced into the vaginal and anal canals with a disposable filter tip. The rinse was gently pipetted in and out of the vaginal canal and stored at −20 °C before being processed. For oral
lavage, a swab (Puritan purflock Ultra, puritan diagnostics LLC) soaked in 25 μl of sterile 0.9% NaCl was used. For DNA extraction, the DNeasy kit (QIAGEN) was used according to the
instructions of the manufacturer. All DNA samples were eluted into 50 µl EB buffer23. MOUSE CD4/CD8 DEPLETION Mouse CD4 and CD8 depletions were conducted according to the literature with
some modification17. Mice were injected with 300 µg of anti-mouse CD4 (Clone GK1.5) and 300 µg of anti-mouse CD8 (clone 2.43 against CD8a) I.P. in 200 µl 1 × PBS at day 1, 2, 3 and
challenged with 10 µl (1 × 107) MmuPV1 on day seven. All of the mice were treated with anti-CD4 and anti-CD8 twice weekly until week seven and once weekly after week seven. Two control mice
without any treatment were used as positive controls. At week ten after viral infection, blood samples were collected from these animals and examined for the efficiency of T-cell depletion
(BD Biosciences, Anti-CD4–APC (clone RM 4–5; BD Pharmingen) and Anti-CD8a-PE and Anti-CD8b-FITC (clone53-5.8; BD Pharmingen) with flow cytometry. Viral DNA was examined from the anal lavage
at weeks three and six post-infection. Blood samples were also collected from these animals for anti-MPV antibody detection by standard ELISA. VIRAL DNA COPY NUMBER ANALYSIS Linearized
MmuPV1 genome DNA was used for standard curve determination by SYBR Green Q-PCR analysis (FastStart Universal SYBR Green Master (Rox), Roche). The primer pairs (5′GCCCGA AGACA ACACCG CCACG3′
and 5′CCTCCGCCTC GTCCCCA AATGG 3′) that amplify E2 were used. Viral copy numbers in 1 µl of 50 µl DNA extract from a lavage sample were converted into equivalent DNA load using a formula
(1ng viral DNA = 1.2 × 108 copy number, http://cels.uri.edu/gsc/cndna.html). Viral copy number per sample initially provides a simple positive or negative answer and is well adapted to a
clinical setting23. The Q- PCR reactions were run in a Stratagene Mx Pro-Mx3000 P (Stratagene). Each reaction consisted of 7.5 µl of ultrapure water, 5pmol of each primer, 7.5 µl of SYBR
Green-PCR Master Mix (Roche) and 1 μl of DNA template. PCR conditions were: initial denaturation at 95 °C for 10 min, then 40 cycles at 95 °C for 15 s and at 60 °C for 1 min. All samples
were tested in at least duplicates. Viral titers were calculated according to the standard curve. In some cases we also calculated the difference in cycle time (Ct) between the 18sRNA gene
and viral DNA (ΔCt). Fold change (2ΔCt) demonstrates the relative viral DNA load in each sample as described previously23,39. ANTIBODY DETECTION BY ELISA Mouse sera were collected at the
termination of the experiment. Mouse papillomavirus virus-like particles (VLPs) or HPV16 VLPs were used as the antigen for ELISA. Anti-MmuPV1 monoclonal antibody (MPV.B9) and Anti-HPV16
monoclonal antibody (H16.V5) were used as the positive and negative control for the corresponding antigens respectively23. The ELISA was conducted as reported previously40. _IN VITRO_
NEUTRALIZATION ASSAY A mouse keratinocyte cell line (K38, a generous gift from Dr. Julia Reichelt41, University of Newcastle, UK) was seeded at 1.5 × 105 cells per well in DMEM/Ham’s F-12,
with 4.5 g/l D-Glucose, 50uM CaCl2, with L-Glutamine and Na-Pyruvate (Cedarlane), in 10% FBS with calcium depleted at 32 °C. 1 µl of viral extract from tail papillomas was incubated with
various dilutions of mouse sera (1:50–1:100 dilution) in media for 1hr at 37 °C and added onto K38 cells incubated in 12-well plates at 32 °C for 72 hours. The cells were harvested with
TRIzol reagent (Life Technologies). Total RNA was extracted from the infected cells, and infectivity was assessed by measuring viral E1^E4 transcripts with QRT-PCR (E1^E4-forward,
5′-CATTCGAGTC ACTGCTTCTGC-3′; E1^E4-reverse, 5′-GATGCAGGTTTGTCGTTCTCC-3′; E1^E4-probe, 5′-6-carboxyfluorescein (FAM)-TGGAAAACGATAAAGCTCCTCCTC AGCC-6-carboxytetramethylrhodamine (TAMRA)-3′ as
previously described with a few modifications 17as follows: The Brilliant II Master mix kit (Agilent) was used for the QRT-PCR reactions. The following cycling conditions were applied: 50
°C for 30 minutes (the reverse transcription), 95 °C for 10 minutes, and 40 cycles of 94 °C for 15 seconds and 60 °C for 1 minute. At the end of each amplification cycle, three fluorescence
readings were detected. Analysis of the amplification efficiencies was performed using the REST software42. IMMUNOHISTOCHEMISTRY AND _IN SITU_ HYBRIDIZATION ANALYSES OF INFECTED TISSUES
After termination of the experiment, the animals were euthanized and tissues of interest were fixed in 10% buffered formalin as described previously. Hematoxylin and eosin (H &E)
analysis, _in situ_ hybridization (ISH) and immunohistochemistry (IHC) were conducted as described in previous studies22,24. For IHC, a goat group specific antibody (GSA) to a conserved
region of L1 (ViroStat #5001), and an in-house anti-MmuPV1 L1 monoclonal antibody (MPV.B9) were used on FFPE sections. Rat anti-mouse LY6.B2 (MCA771GT, Bio-Rad) was used for neutrophil
detection. STATISTICAL ANALYSIS The data were statistically analyzed with one-way ANOVA analysis for multiple groups in Sigmaplot 12 software. Unpaired student T-test was also used to
compare two different groups of animals after viral infections in the studies. Differences were considered to be significant at P < 0.05. REFERENCES * Bigby, S. M., Eva, L. J., Fong, K.
L. & Jones, R. W. The Natural History of Vulvar Intraepithelial Neoplasia, Differentiated Type: Evidence for Progression and Diagnostic Challenges. _Int J Gynecol Pathol_ 35, 574–584,
https://doi.org/10.1097/PGP.0000000000000280 (2016). Article CAS PubMed Google Scholar * Benevolo, M., Dona, M. G., Ravenda, P. S. & Chiocca, S. Anal human papillomavirus infection:
prevalence, diagnosis and treatment of related lesions. _Expert Rev Anti Infect Ther_ 14, 465–477, https://doi.org/10.1586/14787210.2016.1174065 (2016). Article CAS PubMed Google Scholar
* Shigeishi, H. & Sugiyama, M. Risk Factors for Oral Human Papillomavirus Infection in Healthy Individuals: A Systematic Review and Meta-Analysis. _J Clin Med Res_ 8, 721–729,
https://doi.org/10.14740/jocmr2545w (2016). Article PubMed PubMed Central Google Scholar * Iversen, O. E. _et al_. Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in
Girls and Boys vs a 3-Dose Regimen in Women. _JAMA_ 316, 2411–2421, https://doi.org/10.1001/jama.2016.17615 (2016). Article CAS PubMed Google Scholar * Dochez, C., Bogers, J. J.,
Verhelst, R. & Rees, H. HPV vaccines to prevent cervical cancer and genital warts: an update. _Vaccine_ 32, 1595–1601, https://doi.org/10.1016/j.vaccine.2013.10.081 (2014). Article CAS
PubMed Google Scholar * Brandsma, J. L. The cottontail rabbit papillomavirus model of high-risk HPV-induced disease. _Methods Mol. Med_ 119, 217–235 (2005). CAS PubMed Google Scholar
* Christensen, N. D. Cottontail rabbit papillomavirus (CRPV) model system to test antiviral and immunotherapeutic strategies. _Antivir. Chem. Chemother_ 16, 355–362 (2005). Article CAS
PubMed Google Scholar * Peh, W. L. _et al_. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. _J. Virol_ 76, 10401–10416 (2002). Article CAS PubMed
PubMed Central Google Scholar * Breitburd, F., Salmon, J. & Orth, G. The rabbit viral skin papillomas and carcinomas: a model for the immunogenetics of HPV-associated carcinogenesis.
_Clin. Dermatol_ 15, 237–247 (1997). Article CAS PubMed Google Scholar * Huber, E., Vlasny, D., Jeckel, S., Stubenrauch, F. & Iftner, T. Gene profiling of cottontail rabbit
papillomavirus-induced carcinomas identifies upregulated genes directly Involved in stroma invasion as shown by small interfering RNA-mediated gene silencing. _J. Virol_ 78, 7478–7489
(2004). Article CAS PubMed PubMed Central Google Scholar * Campo, M. S. Animal models of papillomavirus pathogenesis. _Virus Res_ 89, 249–261 (2002). Article CAS PubMed Google
Scholar * Christensen, N. D., Budgeon, L. R., Cladel, N. M. & Hu, J. Recent advances in preclinical model systems for papillomaviruses. _Virus Res_ 231, 108–118,
https://doi.org/10.1016/j.virusres.2016.12.004 (2017). Article CAS PubMed Google Scholar * Ingle, A. _et al_. Novel laboratory mouse papillomavirus (MusPV) infection. _Vet Pathol_ 48,
500–505, https://doi.org/10.1177/0300985810377186 (2011). Article CAS PubMed Google Scholar * Van Doorslaer, K. _et al_. Complete genomic characterization of a murine papillomavirus
isolated from papillomatous lesions of a European harvest mouse (Micromys minutus). _J Gen Virol_ 88, 1484–1488, https://doi.org/10.1099/vir.0.82615-0 (2007). Article PubMed Google Scholar
* Muller, H. & Gissmann, L. Mastomys natalensis papilloma virus (MnPV), the causative agent of epithelial proliferation: Characterization of the virus particle. _J. Gen. Virol_ 41,
315–323 (1978). Article CAS PubMed Google Scholar * Handisurya, A. _et al_. Characterization of Mus musculus papillomavirus 1 infection _in situ_ reveals an unusual pattern of late gene
expression and capsid protein localization. _J. Virol_ 87, 13214–13225, https://doi.org/10.1128/JVI.02162-13 (2013). * Handisurya, A. _et al_. Strain-Specific Properties and T Cells Regulate
the Susceptibility to Papilloma Induction by Mus musculus Papillomavirus 1. _PLoS. Pathog_ 10, e1004314, https://doi.org/10.1371/journal.ppat.1004314 (2014). Article PubMed PubMed Central
Google Scholar * Wang, J. W. _et al_. Immunologic Control of Mus musculus Papillomavirus Type 1. _PLoS Pathog_ 11, e1005243, https://doi.org/10.1371/journal.ppat.1005243 (2015). Article
PubMed PubMed Central Google Scholar * Jiang, R. T. _et al_. Spontaneous and vaccine-induced clearance of Mus musculus Papillomavirus type 1 (MmuPV1/MusPV1) infection. _J Virol_.
https://doi.org/10.1128/JVI.00699-17 (2017). Google Scholar * Uberoi, A., Yoshida, S., Frazer, I. H., Pitot, H. C. & Lambert, P. F. Role of Ultraviolet Radiation in
Papillomavirus-Induced Disease. _PLoS Pathog_ 12, e1005664, https://doi.org/10.1371/journal.ppat.1005664 (2016). Article PubMed PubMed Central Google Scholar * Sundberg, J. P. _et al_.
Immune status, strain background, and anatomic site of inoculation affect mouse papillomavirus (MmuPV1) induction of exophytic papillomas or endophytic trichoblastomas. _PLoS One_ 9,
e113582, https://doi.org/10.1371/journal.pone.0113582 (2014). Article ADS PubMed PubMed Central Google Scholar * Cladel, N. M. _et al_. Mouse papillomavirus MmuPV1 infects oral mucosa
and preferentially targets the base of the tongue. _Virology_ 488, 73–80, https://doi.org/10.1016/j.virol.2015.10.030 (2016). Article CAS PubMed Google Scholar * Hu, J. _et al_. Tracking
vaginal, anal and oral infection in a mouse papillomavirus infection model. _J Gen Virol_ 96, 3554–3565, https://doi.org/10.1099/jgv.0.000295 (2015). Article CAS PubMed PubMed Central
Google Scholar * Cladel, N. M. _et al_. A novel pre-clinical murine model to study the life cycle and progression of cervical and anal papillomavirus infections. _PLoS One_ 10, e0120128,
https://doi.org/10.1371/journal.pone.0120128 (2015). Article PubMed PubMed Central Google Scholar * Cladel, N. M. _et al_. Secondary infections, expanded tissue tropism, and evidence for
malignant potential in immunocompromised mice infected with Mus musculus papillomavirus 1 DNA and virus. _J. Virol_ 87, 9391–9395, https://doi.org/10.1128/JVI.00777–13 (2013). * Joh, J. _et
al_. MmuPV1 infection and tumor development of T cell-deficient mice is prevented by passively transferred hyperimmune sera from normal congenic mice immunized with MmuPV1 virus-like
particles (VLPs). _Exp Mol Pathol_ 100, 212–219, https://doi.org/10.1016/j.yexmp.2016.01.003 (2016). Article CAS PubMed Google Scholar * Marur, S. & Forastiere, A. A. Head and Neck
Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. _Mayo Clin Proc_ 91, 386–396, https://doi.org/10.1016/j.mayocp.2015.12.017 (2016). Article PubMed Google Scholar
* Meyers, J. M., Uberoi, A., Grace, M., Lambert, P. F. & Munger, K. Cutaneous HPV8 and MmuPV1 E6 Proteins Target the NOTCH and TGF-beta Tumor Suppressors to Inhibit Differentiation and
Sustain Keratinocyte Proliferation. _PLoS Pathog_ 13, e1006171, https://doi.org/10.1371/journal.ppat.1006171 (2017). Article PubMed PubMed Central Google Scholar * Hu, J. _et al_. An
HLA-A2.1-Transgenic Rabbit Model to Study Immunity to Papillomavirus Infection. _J. Immunol_ 177, 8037–8045 (2006). Article CAS PubMed Google Scholar * Hu, J., Cladel, N. M., Budgeon, L.
R., Balogh, K. K. & Christensen, N. D. The Mouse Papillomavirus Infection Model. _Viruses_ 9, https://doi.org/10.3390/v9090246 (2017). * Schulz, E. _et al_. Isolation of three novel rat
and mouse papillomaviruses and their genomic characterization. _PLoS One_ 7, e47164, https://doi.org/10.1371/journal.pone.0047164 (2012). Article ADS CAS PubMed PubMed Central Google
Scholar * Cladel, N. M. _et al_. Mouse papillomavirus infections spread to cutaneous sites with progression to malignancy. _J Gen Virol_, https://doi.org/10.1099/jgv.0.000926 (2017). *
Heard, I., Palefsky, J. M. & Kazatchkine, M. D. The impact of HIV antiviral therapy on human papillomavirus (HPV) infections and HPV-related diseases. _Antivir. Ther_ 9, 13–22 (2004).
CAS PubMed Google Scholar * Tewari, K. S. & Monk, B. J. New strategies in advanced cervical cancer: from angiogenesis blockade to immunotherapy. _Clin Cancer Res_ 20, 5349–5358,
https://doi.org/10.1158/1078-0432.CCR-14-1099 (2014). Article CAS PubMed Google Scholar * Gregorczyk, K. P. & Krzyzowska, M. Innate immunity to infection in the lower female genital
tract. _Postepy Hig. Med. Dosw. (Online_.) 67, 388–401, doi:1048816 [pii] (2013). * Moerman-Herzog, A. & Nakagawa, M. Early Defensive Mechanisms against Human Papillomavirus Infection.
_Clin Vaccine Immunol_ 22, 850–857, https://doi.org/10.1128/CVI.00223-15 (2015). Article CAS PubMed PubMed Central Google Scholar * Amador-Molina, A., Hernandez-Valencia, J. F., Lamoyi,
E., Contreras-Paredes, A. & Lizano, M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. _Viruses_ 5,
2624–2642, https://doi.org/10.3390/v5112624 (2013). Article PubMed PubMed Central Google Scholar * van Poelgeest, M. I. _et al_. Vaccination against Oncoproteins of HPV16 for Noninvasive
Vulvar/Vaginal Lesions: Lesion Clearance Is Related to the Strength of the T-Cell Response. _Clin Cancer Res_ 22, 2342–2350, https://doi.org/10.1158/1078-0432.CCR-15-2594 (2016). Article
PubMed Google Scholar * Moreau, F. _et al_. Detection and genotyping of human papillomavirus by real-time PCR assay. _J Clin Virol_ 56, 244–249, https://doi.org/10.1016/j.jcv.2012.11.003
(2013). Article PubMed Google Scholar * Hu, J. _et al_. Long-peptide therapeutic vaccination against CRPV-induced papillomas in HLA-A2.1 transgenic rabbits. _Trials Vaccinol_ 3, 134–142,
https://doi.org/10.1016/j.trivac.2014.06.002 (2014). Article PubMed PubMed Central Google Scholar * Reichelt, J. & Haase, I. Establishment of spontaneously immortalized keratinocyte
lines from wild-type and mutant mice. _Methods Mol Biol_ 585, 59–69, https://doi.org/10.1007/978-1-60761-380-0_5 (2010). Article CAS PubMed Google Scholar * Hu, J. _et al_. Detection of
L1, infectious virions and anti-L1 antibody in domestic rabbits infected with cottontail rabbit papillomavirus. _J. Gen. Virol_ 88, 3286–3293 (2007). Article CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health
under Award Number R21AI121822 (Christensen and Hu) and the Jake Gittlen Memorial Golf Tournament. AUTHOR INFORMATION Author notes * Timothy K. Cooper Present address: Charles River
Laboratories – Contractor Supporting: National Institute of Allergy and Infectious Diseases (NIAID) Integrated Research Facility, Division of Clinical Research, 8200 Research Plaza – Fort
Detrick, Frederick, MD, 21702, United States of America AUTHORS AND AFFILIATIONS * The Jake Gittlen Laboratories for Cancer Research; Pennsylvania State University College of Medicine,
Hershey, Pennsylvania, United States of America Nancy M. Cladel, Lynn R. Budgeon, Karla K. Balogh, Sarah A. Brendle, Neil D. Christensen & Jiafen Hu * Department of Pathology,
Pennsylvania State University College of Medicine, Hershey, Pennsylvania, United States of America Nancy M. Cladel, Lynn R. Budgeon, Karla K. Balogh, Sarah A. Brendle, Neil D. Christensen
& Jiafen Hu * Department of Comparative Medicine, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, United States of America Timothy K. Cooper * Department of
Microbiology and Immunology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, United States of America Neil D. Christensen & Todd D. Schell Authors * Nancy M.
Cladel View author publications You can also search for this author inPubMed Google Scholar * Lynn R. Budgeon View author publications You can also search for this author inPubMed Google
Scholar * Karla K. Balogh View author publications You can also search for this author inPubMed Google Scholar * Timothy K. Cooper View author publications You can also search for this
author inPubMed Google Scholar * Sarah A. Brendle View author publications You can also search for this author inPubMed Google Scholar * Neil D. Christensen View author publications You can
also search for this author inPubMed Google Scholar * Todd D. Schell View author publications You can also search for this author inPubMed Google Scholar * Jiafen Hu View author publications
You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization: N.M.C., N.D.C. and J.H. Data curation: N.M.C., L.R.B., K.K.B., S.A.B. and J.H. Formal analysis:
N.M.C., T.K.C. and J.H. Funding acquisition: N.D.C. and J.H. Investigation: N.M.C., L.R.B., K.K.B., S.A.B., T.K.C., T.D.S. and J.H. Methodology: N.M.C., L.R.B., K.K.B., S.A.B. and J.H.
Project administration: N.M.C., L.R.B., K.K.B., S.A.B. and J.H. Resources: N.M.C., L.R.B., K.K.B., S.A.B. and J.H. Supervision: N.M.C., N.D.C. and J.H. Validation: N.M.C., L.R.B., K.K.B.,
S.A.B. and J.H. Visualization: N.M.C., L.R.B., K.K.B., S.A.B., T.K.C. and J.H. Writing – original draft: N.M.C. and J.H. Writing – review & editing: N.M.C., L.R.B., K.K.B., S.A.B.,
T.D.S., T.K.C., N.D.C. and J.H. CORRESPONDING AUTHOR Correspondence to Jiafen Hu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY
MATERIAL DATASET 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Cladel, N.M., Budgeon, L.R., Balogh, K.K. _et al._ Mouse papillomavirus infection persists in mucosal tissues of an immunocompetent mouse strain and progresses to cancer.
_Sci Rep_ 7, 16932 (2017). https://doi.org/10.1038/s41598-017-17089-4 Download citation * Received: 21 August 2017 * Accepted: 20 November 2017 * Published: 05 December 2017 * DOI:
https://doi.org/10.1038/s41598-017-17089-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative