- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The highly energetic electrons in non-thermal plasma generated by gas phase pulsed corona discharge (PCD) produce hydroxyl (OH) radicals via collision reactions with water
molecules. Previous work has established that OH radicals are formed at the plasma-liquid interface, making it an important location for the oxidation of aqueous pollutants. Here, by
contacting water as aerosol with PCD plasma, it is shown that OH radicals are produced on the gas side of the interface, and not in the liquid phase. It is also demonstrated that the
gas-liquid interfacial boundary poses a barrier for the OH radicals, one they need to cross for reactive affinity with dissolved components, and that this process requires a gaseous atomic H
scavenger. For gaseous oxidation, a scavenger, oxygen in common cases, is an advantage but not a requirement. OH radical efficiency in liquid phase reactions is strongly temperature
dependent as radical termination reaction rates increase with temperature. SIMILAR CONTENT BEING VIEWED BY OTHERS RESONANT DUAL-PULSE LASER IGNITION TECHNIQUE BASED ON OXYGEN REMPI
PRE-IONIZATION Article Open access 16 November 2020 A WATER COOLED, HIGH POWER, DIELECTRIC BARRIER DISCHARGE REACTOR FOR CO2 PLASMA DISSOCIATION AND VALORIZATION STUDIES Article Open access
06 May 2023 OBSERVATION AND RATIONALIZATION OF NITROGEN OXIDATION ENABLED ONLY BY COUPLED PLASMA AND CATALYST Article Open access 20 January 2022 INTRODUCTION Atmospheric pressure
non-thermal plasma technologies have been an on-going focus of research in water treatment over the last two decades. The most common laboratory-scale studies have included investigation of
dielectric barrier discharge (DBD) and pulsed corona discharge (PCD), the latter forming the subject of discussion in this paper. Plasma treatment of water is chemically similar with
traditional ozonation, as several oxidants are formed in plasma reactions from oxygen and water, including ozone O3, hydroxyl radical OH, hydrogen peroxide H2O2, atomic oxygen O(3P) and
singlet oxygen O2(1Δg). Of these, O3 and OH are specially recognized as the major oxidants in plasma water treatment1,2,3,4,5. The latter is largely produced via inelastic electron
collisions with water as in Eq. (1)3: $${{\rm{H}}}_{2}{\rm{O}}+{e}^{-}\to {\rm{OH}}+{\rm{H}}+{e}^{-}$$ (1) For water treatment purposes, the gas-liquid interface where the OH radicals are
formed is a crucial environment and the plasma-liquid contact surface area thus makes an important variable4,5,6. In earlier work, a vertical PCD reactor in which the treated solution is
allowed to shower through a perforated plate positioned above the plasma zone was employed to generate a large plasma-liquid contact surface area6. Increasing this contact surface improves
the oxidation energy efficiency mainly due to the enhanced action of superficial OH radicals4,6. Although OH radical activity at the plasma-liquid interface is evident, it is extinguished in
complete absence of O2 4. So far this property, however, seems only observed with the present configuration; several other types of non-thermal plasmas applied in oxidation of aqueous
organic compounds seem to have effect also in the absence of molecular O2 7,8,9,10,11, suggesting that these processes include various mechanisms. The occurrence of such reports is
understandably limited as in most water treatment studies the gas phase contains air for practical reasons, and thus O2 at least as a partial constituent. Oxidation for example under Ar has
shown similar efficiency with air11, but papers describing oxidation under pure nitrogen plasma are scarce. More importantly, these reports tend to describe plasma discharge types that
substantially differ from our PCD configuration. It should be noted that here, the absence of O2 does not suggest that Eq. (1) would be invalid under these circumstances. Instead, it is
proposed in4 that the atomic hydrogen H (a product in Eq. (1)) recombines with the OH radical in the absence of O2, which is a strong H scavenger following Eq. (2)5.
$${\rm{H}}+{{\rm{O}}}_{2}+M\to {{\rm{HO}}}_{2}+M$$ (2) In other words, OH radicals are not able to react with the dissolved species if H is not scavenged. It is, however, interesting to
consider why OH radicals in this case are completely unreactive in the liquid phase without O2, while they can be effective even in anaerobic natural waters12. In the present study, we
address this problem by suggesting that OH radicals are formed only from water vapor and their transfer through the gas-liquid interface is the decisive process for successful oxidation of
dissolved components. We arrive at this conclusion by describing: (i) OH radical oxidation of gaseous nitrogen and acetone without ambient O2; (ii) zero oxidation of oxalic acid (OA) in the
liquid phase in the absence of O2; and (iii) demonstrating the temperature dependence of the possibility of the OH radical diffusing unreacted through the interface under air plasma. These
observations provide essential information for the conceptualization of new PCD-based OH radical processes by enabling mapping of the kind of redox reactions that are achievable. RESULTS AND
DISCUSSION Ambient plasma is known to result in oxidation of N2, producing dissolved NOx in water treatment applications. In this study, the experiments showed temperature and pulse
frequency as having no effect on the yield of NOx (per energy dose), measured as aqueous total nitrogen (TN). Since water vapor pressure increases with temperature, it would be reasonable to
assume that the density of gaseous OH radicals would correlate with it. The observed temperature independence of N2 oxidation is therefore very interesting, as it does not display any
correlation to water vapor pressure, which at 13…30 °C ranges from 1.51 to 4.25 kPa. In agreement with previous studies13,14, the indifference towards pulse frequency indicates that only
short-lived species present during and shortly after the pulses contributed to NOx formation (OH radical lifetime on water surface is ~2.7 µs15, 1–2 orders of magnitude longer above the
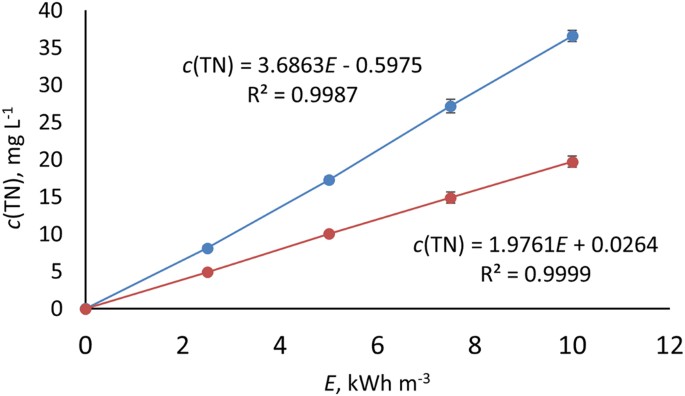
surface5). Under N2, however, the TN formation rate at 1.98 g kWh−1 was almost half that in air, 3.69 g kWh−1. It should be noted that the yield in air is over twice the TN 1.77 g kWh−1
calculated from NO3 − formation presented in14, which is probably due to the water being introduced in aerosol form, rather than the previously applied showering approach, substantially
increasing the gas-liquid contact area (see Experimental Methods). The dissolved TN evolution observed under N2, i.e. in the absence of O2, suggests OH radical induced oxidation taking place
in the gas phase because simultaneously there was zero oxidation in the liquid phase, as will be discussed below. The difference in formation rates under air and N2 indicates that nitrogen
oxidation is supported by oxygen, which acts as a strong atomic H scavenger and contributes to the formation of strong oxidants. The evolution of TN concentration under air and N2 is
presented in Fig. 1. Since variation in the process parameters (temperature and pulse frequency) had no effect on TN formation rate against delivered energy, the values presented are
averages of all experiments under a given atmosphere. In agreement with previous findings14, the dissolved TN in treatment under air was found to consist only of NO3 −. Without oxygen, also
NO2 − was identified, which may simply be explained by the absence of O3 since dissolved O3 readily oxidizes NO2 −, producing mainly NO3 − and O2(1Δg)16. The gas phase oxidation was
confirmed with concentrated acetone solutions (17%); acetone oxidation under N2 resulted in notable accumulation of dissolved oxidation products, acetic17 and formic acid18, during the
treatment. The IC detector response for acetate and formate showed an increase consistent with delivered energy dose. Some inaccuracy of the acetate and formate concentrations may occur due
to the partial overlapping of the chromatogram peaks (the chromatograms are presented in Supplementary Discussion 1). The evolution of the oxidation products, however, confirm the gas phase
formation of OH radicals, whereas with the dissolved probe compound (OA), there was no indication of liquid phase OH radical activity under N2 atmosphere (discussed further below). It should
be noted that under these conditions, the presence of lower oxidation state NOx may enhance the OH induced oxidation process via secondary reactions19, the extent of which may provide
excellent topics for further research. The ion chromatograms of the acetone oxidation products with NO2 − and NO3 − are presented in Fig. 2. Temperature and pulse frequency displayed
consistent correlation with OA oxidation energy efficiency in the experiments conducted under air, the reaction rates exhibiting kinetic profile change from zero to first order along the
decrease in both parameters from the highest to the lowest applied values, as can be seen in Fig. 3. The oxidation efficiency at 300 and 500 pps is at similar range while distinctly lower at
833 pps. The difference stems mainly from the role of O3 6. At equal energy doses, the amount of pulses and thus the amount of OH from Eq. (1) can be considered constant: the role of O3 can
then be considered from the difference in treatment times at different frequencies. The OA oxidation yield at 833 pps is almost halved from 13 to 30 °C, while its improvement when changing
to 500 pps, attributable to longer treatment time, varies very little at any temperature, which emphasizes the temperature dependence of OH radical in the oxidation of the dissolved species
(calculations in Supplementary Discussion 2). By taking the reaction rates from the 0…5 kWh m−3, wherein the degradation is practically linear in all cases (see Fig. 3), the frequency
dependent decelerations of the OA oxidation rates caused by temperature increase can be directly visualized (Fig. 4). Given the very low reactivity of O3 and OA at low pH, the magnitude of
the temperature effect seen in Fig. 4 - strong even at high pulse frequency - is largely attributed to OH radicals. For reference, at similar concentrations and pH, aqueous OA reduction by
O3 alone was reported in20,21 as a maximum of ~10% for 30…60 min treatment. The applied O3 concentration ranged from similar20 to an order of magnitude higher21 compared to our process7.
Since formation of OH radicals is induced by electrons with ~3 orders of magnitude higher temperature than that of ions and molecules22, the rate of it cannot practically be dependent on the
gas temperature range in the current experiments. It can be therefore concluded that high temperature increases OH radical activity instead, which, however, results in decreasing the
oxidation rates of dissolved components due to promotion of premature radical termination reactions. Several possibilities for these reactions are described in23,24. Under N2, TOC remained
unchanged i.e. zero oxidation for OA was observed at any combination of pulse frequency and applied temperature. The result is in agreement with earlier research, where even phenol remained
unoxidized in PCD treatment without molecular oxygen4. The liquid phase OH radical formation testing by using KMnO4 as an atomic H scavenger also yielded zero oxidation of OA, further
demonstrating that no OH radicals are formed in the liquid phase, and that the radicals must therefore come from the gas side of the interface. During the treatment, the permanganate was
gradually reduced to Mn(IV)O2, which is an inevitable development due to the NOx formation. This development is presented in Supplementary Discussion 3. These findings extend and are
supported by the report of Kanazawa _et al_.5, where gas-phase OH radical formation and its dissolution into liquid phase are discussed and OH lifetime on water surface found to be
substantially shorter than in humid air. In summary, via simple experiments with a PCD plasma water treatment system under air and N2, we have showed that although OH radicals are active
also in the liquid phase, in the present kind of plasma-water interaction they are formed in the gas phase only (i.e. from water vapor), and that the gas-liquid interface is a major barrier
for the efficient utilization of the radicals in oxidation of aqueous compounds. The absence of atomic H scavengers in the gas phase promotes OH recombination with H, which is too fast for
OH radicals to cross the gas-liquid interface boundary unreacted. Liquid-phase H scavenging does not enable OH induced oxidation, suggesting that no radicals are produced on the liquid side.
In the absence of gaseous H scavengers, oxidation by OH still occurs in the gas phase because the radicals do not need to cross an interfacial boundary. Temperature increases OH radical
reactivity, which hinders oxidation energy efficiency of dissolved compounds by promoting premature reactions, i.e. reactions occurring prior to successful transfer through the interface.
METHODS The PCD reactor used is a vertical wire-plate configuration with a pulse generator adjustable to deliver identical pulses (22 kV and 180 A peak amplitude) at 50 to 833 pulses per
second (pps) at corresponding nominal power of 6 to 100 W (0.12 J per pulse). Water was sprayed from above into direct contact with the plasma, using five axial-flow full cone atomizer
nozzles, at volumetric flow rate of 1.8 L min−1. Below the reactor is a tank holding the treated solution, a jacketed vessel coupled with a thermostat for adjusting the operating
temperature. For experiments under N2, oxygen absence was confirmed by measuring gas composition in the water tank headspace, using an oxygen analyzer (based on paramagnetic susceptibility
measurements). The practical concept is illustrated in Fig. 5. A more detailed technical description of the PCD system (excluding the atomizer setup) and a pulse oscillogram can be found in
our previous publication6. Oxalic acid (OA) concentrations were measured by analyzing the total organic carbon (TOC) concentrations (applying catalytic combustion at 680 °C) coupled with a
total nitrogen (TN) unit for simultaneous TN analysis. Since OA has no organic oxidation products, i.e. is directly oxidized to CO2, TOC corresponds directly to OA concentration. Similarly,
in the experiments under air, TN value can be used to determine NO3 concentrations since PCD yields no other dissolved NOx species under air, as mentioned above. Acetone oxidation products,
formate and acetate, were observed using an ion chromatograph (IC) with an anion column. In the experiments under N2 atmosphere, IC was also used to identify any presence of NO2 −. For the
OH reactions with dissolved components, OA at 60 mg L−1 (pH ~3.4) was used as the organic probe compound for favoring OH radical over O3 reactions, following the low reactivity of OA with
ozone (_k_ ≤ 4 ∙ 10−2 M−1 s−1 25 at pH 5–6; for the reaction with OH _k_ = 5.3 ∙ 106 M−1 s−1 26), emphasized under acidic conditions. The experiments were conducted in 10 L batches and
sampling was done at 2.5 kWh m−3 intervals of discharge energy per treated water volume until 10 kWh m−3. The experiments were carried out at three pulse frequencies, 300, 500 and 833 pps:
due to the identic pulses, increasing frequency at fixed energy doses results in reduced treatment time, lower frequency therefore giving the longer living species, like O3, more time to
react with the probe compound. The experiments were conducted at temperatures of 13, 20 and 30 °C. For the experiments under N2, the system was flushed with N2 until all oxygen was replaced.
For these experiments, the treated solution was also degassed before introduction into the tank and kept running through the system under N2 atmosphere for 40 minutes before starting the
experiment to ensure negligible dissolved oxygen. N2 inflow was kept at 7 L min−1 throughout the experiments to ensure zero oxygen intake from ambient air. To ascertain whether OH radical
formation in the liquid phase occurs, potassium permanganate KMn(VII)O4 (0.1 mM) was used in a separate experiment as an atomic H scavenger in the dissolved phase. For any reaction following
Eq. (1), the permanganate would not be oxidized by OH but instead serve as an oxidant for atomic H. Under N2, thus scavenged atomic H in the liquid phase would enable OH radical reactions
with OA. To exclude oxalate oxidation by permanganate, sodium oxalate was in this case used at neutral pH and at the lowest applied temperature of 13 °C, under which conditions permanganate
is not reactive with oxalate. Gas phase oxidation under N2 was studied with a 17% acetone solution, exploiting the high volatility of the substance. Run under N2 atmosphere, acetone was used
to demonstrate organic species oxidation in the gas phase by OH radicals from water vapor, in the absence of O2. The experimenting was conducted at 833 pps and 20 °C and acetone solution
volume was reduced to 7 L for convenience, and the process overall duration was extended to 18.03 kWh m−3 of delivered energy to highlight the observable effects. DATA AVAILABILITY The
datasets generated during the current study are available from the corresponding author on reasonable request. REFERENCES * Dobrin, D., Bradu, C., Magureanu, M., Mandache, N. B. &
Parvulescu, V. I. Degradation of diclofenac in water using a pulsed corona discharge. _Chem. Eng. J._ 234, 389–396 (2013). Article CAS Google Scholar * Grabowski, L. R., van Veldhuizen,
E. M., Pemen, A. J. M. & Rutgers, W. R. Corona above water reactor for systematic study of aqueous phenol degradation. _Plasma Chem. Plasma Process._ 26, 3–17 (2006). Article CAS
Google Scholar * Ono, R. & Oda, T. Dynamics of ozone and OH radicals generated by pulsed corona discharge in humid-air flow reactor measured by laser spectroscopy. _J. Appl. Phys._ 93,
5876–5882 (2003). Article CAS ADS Google Scholar * Preis, S., Panorel, I., Kornev, I., Hatakka, H. & Kallas, J. Pulsed corona discharge: the role of ozone and hydroxyl radical in
aqueous pollutants oxidation. _Water Sci. Technol._ 68, 1536–1542 (2013). Article CAS PubMed Google Scholar * Kanazawa, S. _et al_. Observation of OH radicals produced by pulsed
discharges on the surface of a liquid. _Plasma Sources Sci. Technol._ 20, 034010 (2011). Article ADS Google Scholar * Ajo, P., Kornev, I. & Preis, S. Pulsed corona discharge in water
treatment: the effect of hydrodynamic conditions on oxidation energy efficiency. _Ind. Eng. Chem. Res._ 54, 7452–7458 (2015). Article CAS Google Scholar * Shen, Y., Lei, L., Zhang, X.,
Zhou, M. & Zhang, Y. Effect of various gases and chemical catalysts on phenol degradation pathways by pulsed electrical discharges. _J. Haz. Mat._ 150, 713–722 (2008). Article CAS
Google Scholar * Zhang, Y., Zhou, M., Hao, X. & Lei, L. Degradation mechanisms of 4-chlorophenol in a novel gas–liquid hybrid discharge reactor by pulsed high voltage system with oxygen
or nitrogen bubbling. _Chemosphere_ 67, 702–711 (2007). Article CAS PubMed ADS Google Scholar * Chandana, L., Reddy P, M. K. & Subrahmanyam, C. Atmospheric pressure non-thermal
plasma jet for the degradation of methylene blue in aqueous medium. _Chem. Eng. J._ 282, 116–122 (2015). Article CAS Google Scholar * Hsieh, K. C., Wandell, R. J., Bresch, S. & Locke,
B. R. Analysis of hydroxyl radical formation in a gas-liquid electrical discharge plasma reactor utilizing liquid and gaseous radical scavengers. _Plasma Process. Polym._ 14, 1600171
(2017). Article Google Scholar * Hayashi, D. _et al_. Influence of gaseous atmosphere on corona-induced degradation of aqueous phenol. _J. Phys. D Appl. Phys._ 33, 2769 (2000). Article
CAS ADS Google Scholar * Vaughan, P. P. & Blough, N. V. Photochemical formation of hydroxyl radical by constituents of natural waters. _Environ. Sci. Technol._ 32, 2947–2953 (1998).
Article ADS Google Scholar * Preis, S., Panorel, I., Llauger Coll, S. & Kornev, I. Formation of nitrates in aqueous solutions treated with pulsed corona discharge: the impact of
organic pollutants. _Ozone: Sci. Eng._ 36, 94–99 (2014). Article CAS Google Scholar * Kornev, I., Osokin, G., Galanov, A., Yavorovskiy, N. & Preis, S. Formation of nitrite- and
nitrate-ions in aqueous solutions treated with pulsed electric discharges. _Ozone: Sci. Eng._ 35, 22–30 (2103). Article Google Scholar * Attri, P. _et al_. Generation mechanism of hydroxyl
radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. _Sci. Rep._ 5, 9332 (2015). Article CAS PubMed PubMed Central Google Scholar *
Naumov, S., Mark, G., Jarocki, A. & von Sonntag, C. The reactions of nitrite ion with ozone in aqueous solution – new experimental data and quantum-chemical considerations. _Ozone: Sci.
Eng._ 32, 430–434 (2010). Article CAS Google Scholar * Schaefer, T., Schindelka, J., Hoffmann, D. & Herrmann, H. Laboratory kinetic and mechanistic studies on the OH-initiated
oxidation of acetone in aqueous solution. _J Phys Chem A._ 116, 6317–6326 (2012). Article CAS PubMed Google Scholar * Xu, W., Raftery, D. & Francisco, J. S. Effect of irradiation
sources and oxygen concentration on the photocatalytic oxidation of 2-propanol and acetone studied by _in situ_ FTIR. _J Phys Chem B._ 107, 4537–4544 (2003). Article CAS Google Scholar *
Richards-Henderson, N., Goldstein, A. H. & Wilson, K. R. Large enhancement in the heterogeneous oxidation rate of organic aerosols by hydroxyl radicals in the presence of nitric oxide.
_J Phys Chem Lett._ 6, 4451–4455 (2015). Article CAS PubMed Google Scholar * Huang, Y. _et al_. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage
sludge-derived activated carbon as catalysts. _Sci. Total Environ._ 575, 50–57 (2017). Article CAS PubMed ADS Google Scholar * Jeirani, Z. & Soltan, J. Improved formulation of
Fe-MCM-41 for catalytic ozonation of aqueous oxalic acid. _Chem. Eng. J._ 307, 756–765 (2017). Article CAS Google Scholar * Müller, S. & Zahn, R. Air pollution control by non-thermal
plasma. _Contrib. Plasma Phys._ 47, 520–529 (2007). Article ADS Google Scholar * Ono, R. & Oda, T. Measurement of gas temperature and OH density in the afterglow of pulsed positive
corona discharge. _J. Phys. D._ 41, 035204 (2008). Article ADS Google Scholar * Joshi, A. A., Locke, B. R., Arce, P. & Finney, W. C. Formation of hydroxyl radicals, hydrogen peroxide
and aqueous electrons by pulsed streamer corona discharge in aqueous solution. _J. Haz. Mat._ 41, 3–30 (1995). Article CAS Google Scholar * Hoigné, J. & Bader, H. Rate constants of
reactions of ozone with organic and inorganic compounds in water—II: dissociating organic compounds. _Water Res._ 17, 185–194 (1983). Article Google Scholar * Getoff, N., Schwörer, F.,
Markovic, V. M., Sehested, K. & Nielsen, S. O. Pulse radiolysis of oxalic acid and oxalates. _J. Phys. Chem._ 75, 749–755 (1971). Article Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * School of Engineering Science, Lappeenranta University of Technology, P.O. Box 20, 53851, Lappeenranta, Finland Petri Ajo * School of Advanced
Manufacturing Technologies, Tomsk Polytechnic University, 2A Lenina Ave., 634028, Tomsk, Russia Iakov Kornev & Sergei Preis Authors * Petri Ajo View author publications You can also
search for this author inPubMed Google Scholar * Iakov Kornev View author publications You can also search for this author inPubMed Google Scholar * Sergei Preis View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.A. conducted the experimental part and wrote the body of the manuscript. I.K. and S.P. participated in the results
analysis and experimental design, and writing the final version of the paper. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Petri Ajo. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ajo, P., Kornev, I. & Preis, S. Pulsed Corona Discharge Induced
Hydroxyl Radical Transfer Through the Gas-Liquid Interface. _Sci Rep_ 7, 16152 (2017). https://doi.org/10.1038/s41598-017-16333-1 Download citation * Received: 01 September 2017 * Accepted:
09 November 2017 * Published: 23 November 2017 * DOI: https://doi.org/10.1038/s41598-017-16333-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative









