- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Compensatory social behavior in nonhuman animals following maternal loss has been documented, but understanding of how orphans allocate bonding to reconstruct their social networks
is limited. Successful social integration may be critical to survival and reproduction for highly social species and, therefore, may be tied to population persistence. We examined the social
partners involved in affiliative interactions of female orphans and non-orphans in an elephant population in Samburu, northern Kenya that experienced heightened adult mortality driven by
drought and intense ivory poaching. We contrasted partners across different competitive contexts to gain insight to the influence of resource availability on social interactions. Though the
number of partners did not differ between orphans and non-orphans, their types of social partners did. Orphans interacted with sisters and matriarchs less while feeding than did non-orphans,
but otherwise their affiliates were similar. While resting under spatially concentrated shade, orphans had markedly less access to mature adults but affiliated instead with sisters, bulls,
and age mates. Orphan propensity to strengthen bonds with non-dominant animals appears to offer routes to social integration following maternal loss, but lack of interaction with adult
females suggests orphans may experience decreased resource access and associated fitness costs in this matriarchal society. SIMILAR CONTENT BEING VIEWED BY OTHERS COOPERATIVE PARTNER CHOICE
IN MULTI-LEVEL MALE DOLPHIN ALLIANCES Article Open access 25 March 2021 SOCIAL SUPPORT CORRELATES WITH GLUCOCORTICOID CONCENTRATIONS IN WILD AFRICAN ELEPHANT ORPHANS Article Open access 14
July 2022 SHARING AND CARING: TESTOSTERONE, FATHERING, AND GENEROSITY AMONG BAYAKA FORAGERS OF THE CONGO BASIN Article Open access 22 September 2020 INTRODUCTION Strong mother-offspring
bonds are widespread in philopatric species1. The importance of these bonds is demonstrated by the adverse consequences documented for orphans across species, including lower life
expectancy2,3, decreased physical condition4,5, and stunted vocal behavior6. Social processes may be especially predictive of the consequences of orphaning. For example, offspring survival
in baboons7 and horses8 is tied to the social relationships of mothers. The absence of those social relationships may therefore be expected to have detrimental effects on orphans, especially
for species highly dependent on social bonds2,9,10. Remaining socially integrated presents a challenge for orphans given the loss of their mothers’ social influence. However, compensatory
behavior has been documented in some species11,12,13,14, and access to important social partners is known to increase survival15,16. Orphans may therefore strive to mitigate the impacts of
maternal loss through compensatory social behavior. Though compensatory bonding is not well studied in nonhuman animals, it may be an important avenue by which sociality influences fitness.
Social behavioral comparisons between orphans and non-orphans provide a powerful framework to explore compensatory bonding. Because the number of strong relationships individuals can
maintain is limited, animals should bond with those that offer the greatest social benefit. In female baboons, preferences follow a hierarchy of availability of relatives: in the absence of
mothers, baboons bond strongly with maternal kin, and in the absence of maternal kin they bond with paternal kin or nonrelatives12. Baboon society is highly nepotistic17, and the prevalence
of these patterns across taxa with different social structures is unknown. In species disproportionately reliant on older individuals18,19, bonding with older conspecifics following maternal
loss may be expected to take precedence over bonding with relatives. Female African savannah elephants (_Loxodonta africana_) form matriarchal societies in which daughters remain with their
mothers and other female relatives in multi-generational groups for life20,21. The disproportionate poaching of older elephants for their larger ivory22,23 removes critical social partners
that offer ecological knowledge and resource access19,24,25. Aberrant behavior has been recorded in male elephant orphans that experienced impoverished social environments10. Recovery of
elephant populations may in part depend on the ability of orphans to remain socially integrated following maternal loss. Because of their decades-long reliance on their mothers and the
relationship of maternal loss to the current ivory poaching crisis26,27, elephants provide a highly relevant wild system in which to investigate orphan bonding patterns. Elephants’ social
patterns are structured by relatedness, as in other species1,21,28, but studies of disrupted populations suggest bonds with nonrelatives may replace those with relatives29,30,31,32. The weak
influence of nepotism in elephants33,34 also indicates a reduced reliance on kin relative to other species17. In this study, we extend previous work on partner preference12 to a different
social system to broaden understanding of the process of orphan social integration. We followed the social behavior of orphan and non-orphan female elephants in the Samburu population in
northern Kenya following drought and during a persistent period of poaching22,26, testing the prediction that orphans shift allocation of bonding effort to maternal relatives. Because
resource access structures social interaction, we separated behaviors during foraging of generally widely distributed resources and resting in spatially concentrated shade. We expected
orphans to interact less with dominant individuals while resting when competition is greater. We discuss the implications of our results for long term orphan social integration and elephant
population recovery. RESULTS Number of affiliative partners per time followed did not significantly differ between orphans and non-orphans at the α = 0.05 level (Kruskal-Wallis Rank Sum
Test: Feeding: χ2 = 0.017, p = 0.897; Resting: χ2 = 0.004, p = 0.949). However, we found a significantly lower age difference between orphans and their adult/older female social partners
(excluding bulls) while resting (Kruskal-Wallis Rank Sum Test: χ2 = 6.259, p = 0.012) but not while feeding (Kruskal-Wallis Rank Sum Test: χ2 = 1.855, p = 0.173) relative to that of
non-orphans. Related to the generally stronger interactions with bulls by orphans relative to non-orphans, we did not find significant differences in the age differences between the two
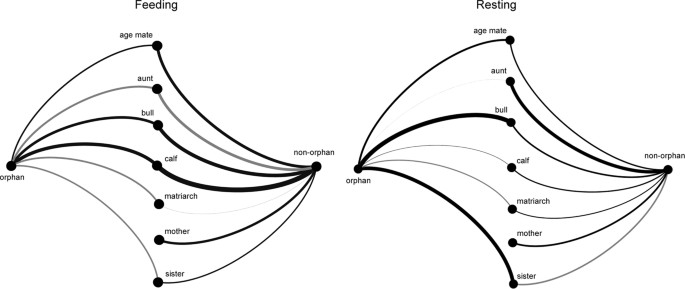
across all social partners (Feeding: χ2 = 1.603, p = 0.206; Resting: χ2 = 0.483, p = 0.487). While feeding, both orphans and non-orphans preferentially interacted with calves, age mates, and
bulls (Tables 1 and 2; Fig. 1). Non-orphans were more likely to interact with sisters relative to orphans, and demonstrated preference for their mothers. Aunts were unlikely feeding
affiliation partners for both orphans and non-orphans, and orphans were unlikely to affiliate with matriarchs when feeding. Younger orphans demonstrated more feeding affiliation, but orphans
that lost their mothers at an older age had more affiliative interactions while feeding (Table 1). Orphans were more likely to interact with sisters and bulls while resting than were
non-orphans (Tables 1 and 2). Resting non-orphans tended to affiliate more with calves than did orphans, but orphans tended to affiliate more with age mates than did non-orphans. Resting
orphans tended not to affiliate with matriarchs, and resting non-orphans tended to affiliate with their mothers (Fig. 2). In contrast to feeding, resting orphans that lost their mothers at
an earlier age affiliated more (Table 1). DISCUSSION The ability of orphans to adjust to their new circumstances presents a considerable challenge in socially dependent animals. Field
studies of wild populations have demonstrated compensatory behavior of orphans in different taxa11,13, but the process of social integration following maternal loss is not well understood.
Access to more experienced animals is thought to be one of the primary benefits of tight knit elephant social structure19,25, and whether or not orphans are able to compensate for lost
relationships with adults may be consequential for their survival and reproduction. Our comparisons of social behavior in elephant orphans and non-orphans in this wild population offer
insight into the drivers of elephant sociality and the challenges of orphaning in this society. Our models indicated strong differences between orphan and non-orphan interaction partners,
particularly while resting. Interactions while resting suggested that non-orphans affiliate more with young calves and their mothers, while orphans were primarily with younger individuals in
the aggregation, notably sisters, age mates, and bulls. While feeding (behavior focused on widely distributed resources), both orphans and non-orphans exhibited more diverse social
partners. Differences were apparent, however, most notably in avoidance of matriarchs by orphans and the lack of their mother as a social partner. The loss of their mother and apparently
related changes in their social interactions indicate that orphans lack direct access to mature female elephants. By assessing social contexts across activities with different resource
competition, we gained insight to the implications of the observed loss of social access for orphans5. Reducing potential conflicts may be important to orphans when interacting in
potentially competitive circumstances. We did not expect to find support for this during feeding when resources are more widespread, but orphans nonetheless exhibited lower than average
interaction rates with matriarchs while feeding. Despite the widespread nature of foraging resources in this system, elephants exhibit clear preferences among resource patches35. The more
limited social interaction between orphans and matriarchs while foraging indicates that access to high quality resource patches may vary depending on family history. Relatedly, the best
positions during resting are typically secured by older, more dominant animals, when competition for limited shade is common24,33. Anecdotally, non-orphans tended to cluster at the core of
family groups, whereas orphans were observed to occupy peripheral positions external to the group (Fig. 3), often in proximity to lower social status bulls, age mates, or sisters. These
observations may also explain why non-orphans were more likely to interact with young calves while resting, which tend to be at the core of resting groups. Our results are consistent with a
study of reindeer that found greater distances of orphans to adult females than non-orphans to adult females, especially in the context of spatially concentrated resources5. Lost
opportunities to access needed resources may be an important factor for orphan fitness across taxa. The differences apparent between orphans and non-orphans in access to older elephants
suggest disadvantages to being orphaned. Similar to baboons11,12, elephant orphans increase affiliation with sisters in response to maternal loss (at least while resting), which may buffer
them from the costs of orphaning. However, their compensatory bonding largely did not include adults (Fig. 2). Older female elephants confer known fitness advantages to their affiliates via
ecological knowledge36, dominance24, social knowledge19, and calf survival15,16. Loss of access to mature animals, therefore, may lower the fitness of orphans via several mechanisms in this
matriarchal society. Previous work has documented altered association patterns among female elephants in response to mortality, such that core social groups (defined by association patterns)
are sometimes comprised of nonrelatives13,32,37. While this previous work demonstrates their behavioral flexibility in times of disruption, our current study provides finer scale detail on
the social environments they experience. The behavioral flexibility and social processes of orphans are likely critical to population recovery in the face of the recent continental poaching
crisis. Future work should investigate how decreased access to knowledge repositories of adult elephants and compensatory social behavior alter fitness trajectories for this keystone
species. METHODS DATA COLLECTION We conducted near daily transects in Samburu and Buffalo Springs National Reserves in northern Kenya between May 2012 and April 2015 and between August and
September 2017. Aggregations of elephants were encountered randomly along transects. For each aggregation, we recorded the individuals present and whether all breeding females were
observed38. Association indices between pairs of elephants derived from high quality observations39 were used to define core social group membership, the strongest level of bonding in female
elephant society13,38. While female elephants are usually found with their core social group, aggregations of elephants on any given day may consist of any combination of core groups and
independent bulls, such that social context changes frequently38. Co-occurrence in aggregations was used to control for the availability of two elephants to interact in analyses (see below).
To determine the fine scale social patterns of elephants, we conducted focal follows (up to 30 minutes) of non-parous female elephants between six and 17 years old encountered along
transects (Feeding: Norphans = 28, Nmedian (IQR) minutes/orphan = 387.38 (96.75–540.19), Nmedian (IQR) follows/orphan = 20 (4.75–30), Nnon-orphans = 19, Nmedian (IQR) minutes/non-orphan =
310.5 (119–386.13), Nmedian (IQR) follows/non-orphan = 19 (6–23.5); Resting: Norphans = 30, Nmedian (IQR) minutes/orphan = 57 (30–86.88), Nmedian (IQR) follows/orphan = 3 (2–5), Nnon-orphans
= 18, Nmedian (IQR) minutes/non-orphan = 57.5 (34.13–86.19), Nmedian (IQR) follows/non-orphan = 2.5 (2–4.75); total sampling hours = 246.12 feeding and 49.14 resting; Supplementary Material
Fig. 1). To control for behavioral autocorrelation, no more than one focal follow in a particular activity was completed on a given animal in a given sampling day, and follows were
terminated before reaching 30 minutes if the animal went out of sight or switched activity. Competition for resources is expected to differ between feeding and resting because shade is a
limited point resource whereas forage is more diffusely distributed. We therefore distinguished between focal follows collected during the two activities (so that a maximum of 60 minutes was
collected on a focal animal in one day: 30 minutes resting and 30 minutes feeding). Sampling spanned both wet and dry seasons, and focal follows were only initiated if elephants appeared
unperturbed by the research vehicle. During focal follows we recorded any interactions with other elephants33,40,41,42. For this study on social bonding, we restricted analysis to
affiliative interactions indicative of bonding including bodily contact, trunk touching, greeting, allomothering, play behavior, and reaching a trunk to another elephant’s mouth40. We
conducted research with permission from the Kenya Wildlife Service, Samburu and Isiolo governments, and Colorado State University (IACUC 12–3414 A), and all methods adhere to relevant
guidelines and regulations. Data used in this study are included as Supplementary Material. DATA ANALYSIS In order to determine general differences between orphans and non-orphans, we
conducted Kruskal-Wallis rank sum tests to assess the number of social partners per time followed and the age differences between social partners and focal animals. We conducted an
additional Kruskal-Wallis rank sum test on age differences including only female partners older than focal animals to more directly assess affiliation with older individuals in female
society. To understand how orphans and non-orphans differ in the categories of their social partners, we constructed four hierarchical Bayesian negative binomial regression models with
uninformative priors predicting affiliative interactions with social partners during different activities (orphans feeding, non-orphans feeding, orphans resting, non-orphans resting). We
used the following process model: $$\mathrm{ln}({\lambda }_{i,j})={\alpha }_{j}+{\boldsymbol{\beta }}{{\boldsymbol{x}}}_{i,j}+\,\mathrm{ln}({\gamma }_{i,j}),$$ (1) where _λ_ _i,j_ is the
expected interaction count for elephant _j_ with social partner _i_ (across all focal follows of elephant _j_ during which social partner _i_ was observed in the same aggregation), _α_ _j_
is the random intercept for elephant _j_, _Β_ represents the vector of fixed effects coefficients associated with covariates _X_, and ln(_γ_ _i,j_ ) is an offset controlling for sampling
effort (the total number of focal minutes for elephant _j_ during which social partner _i_ was observed in the aggregation and therefore available to interact). The conditional probability
was defined as: $$({y}_{i,j}|{\boldsymbol{\beta }},{\alpha }_{j},{\mu }_{\alpha },{\tau }_{\alpha },r)\sim negbinom({p}_{i,j},r)$$ (2) $${\boldsymbol{\beta }}\, \sim normal(0,0.1)$$ (3)
$${\alpha }_{j}\, \sim normal({\mu }_{\alpha },{\tau }_{\alpha })$$ (4) $${\mu }_{\alpha }\, \sim normal(0,0.1)$$ (5) $${\tau }_{\alpha } \sim uniform(0.001,100)$$ (6) $$r\, \sim
uniform(0,100)$$ (7) where _µ_ _α_ and _τ_ _α_ are the mean and precision of _α_ _j_ , respectively, _r_ is the dispersion parameter, and _p_ _i,j_ is the probability that an interaction
occurs. Predictor variables included social partner categories and control variables that might affect interactions using information available from the long-term records of the population
(Table 3). No covariates included in a model were correlated above r = |0.7|. We fit models using JAGS43 and the _rjags_ package in R44,45 with Markov-Chain Monte Carlo by running three
parallel chains of 100,000 iterations each, and discarded the first 10% of iterations as burn-in after assessing convergence indicated by Gelman-Rubin diagnostic values < 1.146.
REFERENCES * Silk, J. B. The adaptive value of sociality in mammalian groups. _Philos. Trans. R. Soc. Lond. B. Biol. Sci._ 362, 539–559 (2007). Article PubMed PubMed Central Google
Scholar * Tung, J., Archie, E. A., Altmann, J. & Alberts, S. C. Cumulative early life adversity predicts longevity in wild baboons. _Nat. Commun._ 7, 11181 (2016). Article CAS PubMed
PubMed Central ADS Google Scholar * Lahdenperä, M., Mar, K. U. & Lummaa, V. Short-term and delayed effects of mother death on calf mortality in Asian elephants. _Behav. Ecol._ 27,
166–174 (2016). Article PubMed Google Scholar * Andres, D. _et al_. Sex differences in the consequences of maternal loss in a long-lived mammal, the red deer (_Cervus elaphus_). _Behav.
Ecol. Sociobiol._ 67, 1249–1258 (2013). Article Google Scholar * Holand, Ø. _et al_. Induced orphaning reveals post-weaning maternal care in reindeer. _Eur. J. Wildl. Res._ 58, 589–596
(2012). Article Google Scholar * Gultekin, Y. B. & Hage, S. R. Limiting parental feedback disrupts vocal development in marmoset monkeys. _Nat. Commun._ 8, 14046 (2017). Article CAS
PubMed PubMed Central ADS Google Scholar * Silk, J. B., Alberts, S. C. & Altmann, J. Social bonds of female baboons enhance infant survival. _Science_ 302, 1231–1234 (2003). Article
CAS PubMed ADS Google Scholar * Cameron, E. Z., Setsaas, T. H. & Linklater, W. L. Social bonds between unrelated females increase reproductive success in feral horses. _Proc. Natl.
Acad. Sci._ 106, 13850–13853 (2009). Article CAS PubMed PubMed Central ADS Google Scholar * Holt-Lunstad, J., Smith, T. B. & Layton, J. B. Social relationships and mortality risk:
A meta-analytic review. _PLoS Med_. 7 (2010). * Slotow, R., van Dyk, G., Poole, J., Page, B. & Klocke, A. Older bull elephants control young males. _Nature_ 408, 425–426 (2000). CAS
PubMed Google Scholar * Engh, A. L. _et al_. Behavioural and hormonal responses to predation in female chacma baboons (_Papio hamadryas ursinus_). _Proc. R. Soc. B_ 273, 707–712 (2006).
Article CAS PubMed Google Scholar * Silk, J. B., Altmann, J. & Alberts, S. C. Social relationships among adult female baboons (_Papio cynocephalus_) I. Variation in the strength of
social bonds. _Behav. Ecol. Sociobiol._ 61, 183–195 (2006). Article Google Scholar * Goldenberg, S. Z., Douglas-Hamilton, I. & Wittemyer, G. Vertical transmission of social roles
drives resilience to poaching in elephant networks. _Curr. Biol._ 26, 75–79 (2016). Article CAS PubMed Google Scholar * Carter, G. G., Farine, D. R. & Wilkinson, G. S. Social
bet-hedging in vampire bats. _Biol. Lett._ 13, 10–13 (2017). Article Google Scholar * Lahdenperä, M., Mar, K. U. & Lummaa, V. Nearby grandmother enhances calf survival and reproduction
in Asian elephants. _Sci. Rep._ 6, 27213 (2016). Article PubMed PubMed Central ADS CAS Google Scholar * Lee, P. C., Fishlock, V., Webber, C. E. & Moss, C. J. The reproductive
advantages of a long life: longevity and senescence in wild female African elephants. _Behav. Ecol. Sociobiol._ 70, 337–345 (2016). Article PubMed PubMed Central Google Scholar * Silk,
J. B., Alberts, S. C. & Altmann, J. Patterns of coalition formation by adult female baboons in Amboseli, Kenya. _Anim. Behav._ 67, 573–582 (2004). Article Google Scholar * Mueller, T.,
O’Hara, R. B., Converse, S. J., Urbanek, R. P. & Fagan, W. F. Social learning of migratory performance. _Science_ 341, 999–1002 (2013). Article CAS PubMed ADS Google Scholar *
McComb, K., Moss, C., Durant, S. M., Baker, L. & Sayialel, S. Matriarchs as repositories of social knowledge in African elephants. _Science._ 292, 491–494 (2001). Article CAS ADS
Google Scholar * Douglas-Hamilton, I. On the ecology and behaviour of the African elephant. (University of Oxford, 1972). * Archie, E. A., Moss, C. J. & Alberts, S. C. The ties that
bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. _Proc. R. Soc. B_ 273, 513–522 (2006). Article CAS PubMed Google Scholar *
Wittemyer, G., Daballen, D. & Douglas-Hamilton, I. Comparative demography of an at-risk African elephant population. _PLoS One_ 8, e53726 (2013). Article CAS PubMed PubMed Central
ADS Google Scholar * Chiyo, P. I., Obanda, V. & Korir, D. K. Illegal tusk harvest and the decline of tusk size in the African elephant. _Ecol. Evol._ 5, 5216–5229 (2015). Article
Google Scholar * Wittemyer, G., Getz, W. M., Vollrath, F. & Douglas-Hamilton, I. Social dominance, seasonal movements, and spatial segregation in African elephants: a contribution to
conservation behavior. _Behav. Ecol. Sociobiol._ 61, 1919–1931 (2007). Article Google Scholar * Foley, C., Pettorelli, N. & Foley, L. Severe drought and calf survival in elephants.
_Biol. Lett._ 4, 541–544 (2008). Article PubMed PubMed Central Google Scholar * Wittemyer, G. _et al_. Illegal killing for ivory drives global decline in African elephants. _Proc. Natl.
Acad. Sci._ 111, 13117–13121 (2014). Article CAS PubMed PubMed Central ADS Google Scholar * Turkalo, A. K., Wrege, P. H. & Wittemyer, G. Slow intrinsic growth rate in forest
elephants indicates recovery from poaching will require decades. _J. Appl. Ecol_ (2016). * Arnaud, C. M., Dobson, F. S. & Murie, J. O. Philopatry and within-colony movements in Columbian
ground squirrels. _Mol. Ecol._ 21, 493–504 (2012). Article PubMed Google Scholar * Charif, R. _et al_. Spatial relationships and matrilineal kinship in African savanna elephant
(_Loxodonta africana_) clans. _Behav. Ecol. Sociobiol._ 57, 327–338 (2005). Article Google Scholar * Wittemyer, G. _et al_. Where sociality and relatedness diverge: the genetic basis for
hierarchical social organization in African elephants. _Proc. Biol. Sci._ 276, 3513–21 (2009). Article PubMed PubMed Central Google Scholar * Gobush, K., Kerr, B. & Wasser, S.
Genetic relatedness and disrupted social structure in a poached population of African elephants. _Mol. Ecol._ 18, 722–734 (2009). Article CAS PubMed Google Scholar * Vidya, T. N. C.,
Varma, S., Dang, N. X., Van Thanh, T. & Sukumar, R. Minimum population size, genetic diversity, and social structure of the Asian elephant in Cat Tien National Park and its adjoining
areas, Vietnam, based on molecular genetic analyses. _Conserv. Genet._ 8, 1471–1478 (2007). Article Google Scholar * Archie, E. A., Morrison, T. A., Foley, C. A. H., Moss, C. J. &
Alberts, S. C. Dominance rank relationships among wild female African elephants. _Loxodonta africana. Anim. Behav._ 71, 117–127 (2006). Article Google Scholar * Wittemyer, G. & Getz,
W. M. Hierarchical dominance structure and social organization in African elephants, Loxodonta africana. _Anim. Behav._ 73, 671–681 (2007). Article Google Scholar * Owen-Smith, R. N.
_Megaherbivores: The Influence of Very Large Body Size on Ecology_. (Cambridge University Press, 1988). * McComb, K. _et al_. Leadership in elephants: the adaptive value of age. _Proc. R.
Soc. B_ 278, 3270–3276 (2011). Article PubMed PubMed Central Google Scholar * Wittemyer, G. _et al_. Where sociality and relatedness diverge: the genetic basis for hierarchical social
organization in African elephants. _Proc. R. Soc. B_ 276, 3513–3521 (2009). Article PubMed PubMed Central Google Scholar * Wittemyer, G., Douglas-Hamilton, I. & Getz, W. The
socioecology of elephants: analysis of the processes creating multitiered social structures. _Anim. Behav._ 69, 1357–1371 (2005). Article Google Scholar * Ginsberg, J. & Young, T.
Measuring association between individuals or groups in behavioural studies. _Anim. Behav._ 44, 377–379 (1992). Article Google Scholar * Poole, J. H. & Granli, P. In _The Amboseli
Elephants: A Long-Term Perspective on a_ _Long-Lived Mammal_ (eds. Moss, C. J., Croze, H. & Lee, P. C.) 109–124 (University of Chicago Press, 2011). * Lee, P. Allomothering among African
elephants. _Anim. Behav._ 35, 278–291 (1987). Article Google Scholar * Goldenberg, S. Z. Ivory poaching, sociality, and the role of behavior in conservation. (Colorado State University,
2016). * Plummer, M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling (2003). * Plummer, M. rjags: Bayesian graphical models using MCMC. _R package version_
3–13 19, http://cran.r-project.org/package=rjags (2016). * R Development Core Team. R: A language and environment for statistical computing. (2010). * Gelman, A. & Rubin, D. B. Inference
from iterative simulation using multiple sequences. _Stat. Sci._ 7, 457–511 (1992). Article Google Scholar Download references ACKNOWLEDGEMENTS The Kenyan Office of the President, the
Kenya Wildlife Service, and the Samburu and Isiolo governments gave us permission to work in the national reserves. We thank David Daballen, Jerenimo Lepirei and Iain Douglas-Hamilton for
facilitating data collection. Elizabeth Archie, Kevin Crooks, and Dhruba Naug offered constructive feedback on an earlier version of this manuscript, and Kristin Broms assisted in model
design. This work was funded by National Science Foundation Graduate Research Fellowship DGE-1321845 (S.Z.G.), Save the Elephants, Warner College of Natural Resources and Graduate Degree
Program in Ecology at Colorado State University, and through the generous support of Singer Rankin and WorldWomenWork. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Fish,
Wildlife, and Conservation Biology, Fort Collins, CO, 80523, USA Shifra Z. Goldenberg & George Wittemyer * Save the Elephants, Nairobi, 00200, Kenya Shifra Z. Goldenberg & George
Wittemyer Authors * Shifra Z. Goldenberg View author publications You can also search for this author inPubMed Google Scholar * George Wittemyer View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS S.Z.G. and G.W. designed the study, S.Z.G. collected and analyzed the data, and S.Z.G. and G.W. wrote the manuscript. CORRESPONDING
AUTHOR Correspondence to Shifra Z. Goldenberg. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S
NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY MATERIAL DATASET
FEEDING DATASET RESTING RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Goldenberg, S.Z., Wittemyer, G. Orphaned female elephant social bonds reflect lack of access to mature adults. _Sci Rep_ 7, 14408 (2017).
https://doi.org/10.1038/s41598-017-14712-2 Download citation * Received: 07 June 2017 * Accepted: 13 October 2017 * Published: 31 October 2017 * DOI:
https://doi.org/10.1038/s41598-017-14712-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative





