- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Studies confirm physical long-range cell-cell communication, most evidently based on electromagnetic fields. Effects concern induction or inhibition of cell growth. Their natural
function is unclear. With the protozoan _Paramecium caudatum_ I tested whether the signals regulate cell density and are electromagnetic. Up to 300 cells/mL, cell growth in clones of this
study is decreasingly pronounced. Using cuvettes as chemical barriers enabling physical communication I placed 5 indicator cells/mL, the inducer populations, into smaller cuvettes that
stand in bigger and contained 50, 100, 200 or 300 cells/mL. Under conditions of _total darkness_ such pairs were mutually exposed for 48 hours. The hypothesis was that indicator cells, too,
grow less the more neighbor cells there are. The bigger inducer populations were in the beginning the less they grew. The indicator populations grew accordingly; the more cells they were
surrounded by the less they grew. The suppressing neighbors-effect disappeared when inner cuvettes were shielded by graphite known to shield electromagnetic radiation from GHz to PHz, i.e.
to absorb energy from microwaves to light. These are the first results demonstrating non-contact physical _quorum sensing_ for cell population density regulation. I assume rules intrinsic to
electromagnetic fields interacting with matter and life. SIMILAR CONTENT BEING VIEWED BY OTHERS COMBINED RESPONSE OF POLAR MAGNETOTAXIS TO OXYGEN AND PH: INSIGHTS FROM HANGING DROP ASSAYS
AND MICROCOSM EXPERIMENTS Article Open access 09 November 2024 A REVIEW OF QUORUM-SENSING AND ITS ROLE IN MEDIATING INTERKINGDOM INTERACTIONS IN THE OCEAN Article Open access 05 February
2025 CHANGES IN INTERACTIONS OVER ECOLOGICAL TIME SCALES INFLUENCE SINGLE-CELL GROWTH DYNAMICS IN A METABOLICALLY COUPLED MARINE MICROBIAL COMMUNITY Article Open access 07 January 2023
INTRODUCTION How does a population of cells in a multicellular organism maintain its cell density? Basic understanding is coming from studies with unicellular organisms where cells release
chemical signals in dependence of cell density leading to a corresponding regulation of the cell cycle1,2,3. Beside this molecule-based, i.e. chemical _quorum sensing_, there is also
(contact-based) physical _quorum sensing_ reported in the context of dispersal rates4. Further, effects among cells on cell cycle occur also through glass or quartz barriers where chemical
signaling is prevented5,6,7. These effects seem to be governed by physical factors, too, most evidently electromagnetic (EM) signals generated by the cells themselves8,9, enabling – due to
their physical nature – to cross glass or quartz barriers10. In the present study, this seemingly non-chemical cell-to-cell communication was further investigated as its possible biological
role was analyzed. The study organism was the freshwater protozoan _Paramecium caudatum_, as already used in previous studies by the present author showing _intraspecific_ 11 as well as
_interspecific_ 12 effects from one population on the other across chemical barriers. One possible function of this non-chemical cell communication in _Paramecium_ caudatum is assumed to be
the regulation of population density. I distinct here the main-hypothesis of density regulation due to a physical signal that can trespass chemical barriers and the sub-hypothesis that these
physical signals are of electromagnetic nature. _Paramecium caudatum_ used in this study are maintained in microcosms where they reach an average density of about 300 cells/mL. Even though
they receive food (bacteria) _ad libidum_ and each cell occupies only about 0.1% of the average volume available for each cell one never finds the cells overshooting that particular density.
In the present study I wanted to find out whether physical signals are used to regulate the cell density in _Paramecium caudatum_. For this, I placed cells at four different densities,
(i.e., 50, 100, 200 and 300 cells per mL) into cuvettes. It is expected that low-density populations would still grow during the course of the experiment whereas populations with higher
densities would have a slower growth, and those at carrying capacity would stop growing - or just stay in a balance between cell mortality and division. Yet, what if we place smaller
cuvettes containing 5 cells (per mL) into those above-mentioned populations? Would these 5 tester cells act as indicators in that they would grow in dependence on the density of the
population they are surrounded by? The hypothesis of a physical (presumably electromagnetic) cell density regulation is based on the conclusion that the bigger the outer population is at the
onset of the experiment the lower would not only be their own growth but also the lower would be the growth of those 5 original (indicator) cells placed inside. This was assumed to be so
because the presumed endogenous (electromagnetic) signal with which the inducer population regulates its own density trespasses to the inner cuvette, where the tester population adopts its
growth decision accordingly, hence indicating that the big inducer population can regulate its density via non-chemical cell-to-cell communication. The results of this study deliver strong
evidence for a physical regulation of population density: across chemical barriers the five inner cells grew less, the more neighbors they had. This indicates that the inducer populations
released a signal that regulates cell density. When shielding against presumed electromagnetic signals, the growth decreasing effect from neighboring populations disappeared, supporting the
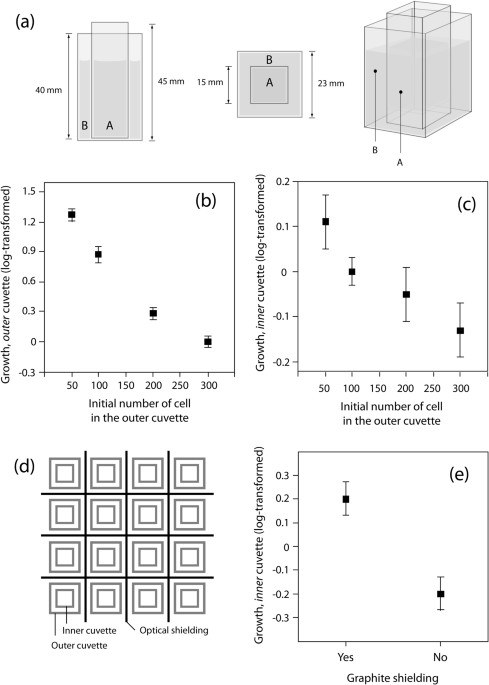
assumption that an endogenous electromagnetic signal was transmitted from the outer inducer population to the inner tester population. RESULTS EXPERIMENT 1: DENSITY-EFFECT The inducer
populations showed a highly significant negative relation between initial population size and cell division rate (ANOVA (linear fit): DF = 1; SS = 16.257; F-ratio = 610.6; p > F =
0.0001****) with the biggest populations (300 cells at origin) performing no more growth (Fig. 1(b)). Cell division rates of tester cells and controls had similar mean values (ANOVA (linear
fit): DF = 1; SS = 0.007; F-ratio = 0.148; p > F = 0.702 ns). In either of the above tests there was a highly significant effect from repeating the experiment (Tab 1), but no material
effects due to different cuvette material (statistics not shown) which latter allowed to merge the glass and quartz treatment groups which leading then to n = 16 per treatment group.
Regarding the working hypothesis, there was a strong significant negative effect between initial densities of inducer populations and cell division rates of the tester populations (Table 1,
Fig. 1(c)). Cases of mortality in the inducer populations were omitted in the above analysis because this adds the variable mortality known for tremendous release of endogenously generated
photons13. Mortality was assessed in 10 inducer populations with originally 300 cells/mL (8 cases) and 200 cells/mL (2 cases). While tester populations were positively correlated with
inducer populations when the latter were growing (ANOVA (linear fit): DF = 1; SS = 0.870; F-ratio = 8.014; p > F = 0.0066**), note that they were negatively correlated when inducer
populations displayed mortality i.e., tester populations grew significantly better the higher the mortality rate in the inducer populations (ANOVA (linear fit): DF = 1; SS = 0.490; F-ratio =
9.007; p > F = 0.017*). EXPERIMENT 2: GRAPHITE-SHIELDING Tester populations that were shielded with graphite from inducer populations grew significantly better (ANOVA (linear fit): DF =
1; SS = 0.774; F-ratio = 14.437; p > F = 0.0014**) than those not shielded (Fig. 1(e)). There was no difference in growth of inducer populations due to exposure to the graphite-layer on
the outside of the inner cuvette (statistics not shown). DISCUSSION The results of the present study provide strong evidence for a physical signal organizing the regulation of population
density in the unicellular organism _Paramecium caudatum_. This physical signal is most probably of electromagnetic nature since the effect disappeared when shielding tester populations
against electromagnetic signals assumed to be emitted by inducer populations. Further, under the shielding conditions, presumed volatiles (i.e., volatile chemical compounds mediating
long-range cell-to-cell signaling) could still have induced an effect but, such an effect was absent. The absence of volatiles in the experimental system is extensively discussed
elsewhere11. Since the results indicate that cells can sense the _quorum_, i.e., the number of cells the population consists in, I suggest here to interpret this study as evidence for a
non-contact _physical_ (most probably electromagnetic) _quorum sensing_. Despite the confirmation of the initial hypothesis, the study delivered two unexpected results: (i) The absence of
effects from different material types (normal glass or quartz glass) of separating cuvettes was opposed to a previous study with the same cell system11. It suggests that during this
experiment the inducing signals were in the wavelength-range that could be transmitted through both, glass and quartz cuvettes. Such wavelengths refer to the UV-A range and below. (ii) The
effects coming from inducer populations that began to decrease in cell number during the experiment and were inducing a growth increase response in tester populations were an interesting
side observation of the experiment and demands an intense investigation on effects of mortality on cell division rates and regulation, respectively. The major effects described in this study
deliver indirect evidence that cells use signals that offshoot from the cellular generation of electromagnetic fields8,14,15. Surely, direct evidence is desirable, but as we are not
technically able to (i) catch, (ii) store and (iii) release at will electromagnetic waves released by one subpopulation and emitted to another, the here-mentioned method for testing
non-chemical cell-to-cell communication is in principal (still) the best; for a detailed discussion about this aspect please refer to Fels10. To get more information about the true nature of
the signals, the graphite-shielding method is useful but delivers only indirect evidence. For direct evidence, therefore, the use of multimode optical fibers with a transmission window
allowing optical communication is planned. The significance of this study is the support of the assumption that the non-avoidable endogenous generation of electromagnetic fields plays an
active role in cell dynamics. This supports the basic hypothesis that the endogenous fields of the cell feedback on cell components some of which having generated these fields16. The work
is, in addition, mutually supportive to giving credit to works on electrostatic or electromagnetic induction on cell processes being exogenous17,18,19,20 or endogenous21,22,23,24. The
results demand to give more attention to the physics of the cell. This may also include to look at effects from physics-based non-invasive therapies and basic research leading to such forms
of therapy, respectively25. This, however, means that we cannot exclude the possibility that life’s inner organization is also driven by rules intrinsic to electromagnetic fields interacting
with matter and life. MATERIALS AND METHODS THE STUDY ORGANISM _Paramecium caudatum_ (Phylum: Ciliata) is a freshwater protozoan of the Eurasian plate and a common research organism in
different biological fields such as ecology and cell behaviour26,27 or host-parasite interactions28. They are large cells with a length of about 250 µm and width of about 50 µm. In this
study, I work with _Paramecia_ that are maintained in an incubator with a 24 hrs cycle of 15 hrs artificial light at 23 °C and 9 hrs darkness at 18 °C. Once per week the densities of the
cells were assessed with the use of a binocular microscope, glass wells and a hand counter. The cells were thereafter fed with a medium that contains the prokaryote _Serratia sp_. (on which
_Paramecia_ feed) and dried Lettuce (_Lactuca sativa var_. _capitata_) leaves (on which the prokaryotes feed). Under such conditions, different clones maintain clone specific densities. In
this study, the clone K8 was used with an average maximum cell density, i.e., carrying capacity of 300 cells/mL (for more information refer29). THE GLASS-BARRIER METHOD The use of
glass-barriers (i.e., cuvettes) goes back to the original works of Alexander Gurwitsch5 who employed it to prevent transmission of chemical – but not electromagnetic – signals from one
population to another, and was used in many variations ever since30,31,32. In the present study two populations were separated from each other by using two sizes of cuvettes (vials). Big
cuvettes with a base of 23 mm × 23 mm and a height of 40 mm, and small cuvettes with a base of 15 mm × 15 mm and a height of 45 mm the thickness of the walls being 1.5 mm (see Fig. 1(a)).
The cuvettes consist either of normal glass (which is a filter for electromagnetic radiation in the optical ultra-violet (UV) spectrum, i.e., UV-B [280–315 nm] and UV-C [100–280 nm]) or
quartz glass allowing transmission of the whole UV range; for glass and quartz transmission spectra see elsewhere11. THE SEPARATION OF TWO CELL POPULATIONS In order to separate two cell
populations of _Paramecium caudatum_, one is placed in a small cuvette and the other in a bigger cuvette (for a detailed description see10). The smaller cuvette is then placed into the big
cuvette, leading to a chemical separation of these two populations (Fig. 1(a)). Under the conditions of the experiment each cuvette contains 1 mL of medium (as described above) leading to a
height of about 6 mm of medium in the inner and of about 4 mm in the outer cuvette (when containing a small inner cuvette). Such pairs of cuvettes (referred to as “units”) are then randomly
placed in a grid (Fig. 1(d)); note that units were separated by black carbon paper disabling light transfer from one unit to the other. The grid itself stands in a lightproof box leading to
_total-darkness_ conditions during the experiment(s). EXPERIMENT 1: DENSITY-EFFECT This experiment tested for a differing effect on tester populations due to differing cell densities in
neighbouring inducer populations. At the beginning of an experiment the inducer populations contained either 50, 100, 200 or 300 cells/mL. These densities were obtained by diluting the
well-grown clones – reaching population sizes of 300 cells/mL – with medium. The inducer populations were in the outer cuvettes while the tester populations were in the inner cuvettes. The
tester populations consisted at the beginning of the experiment always of 5 (individually and randomly picked) cells. A control was added with no inducer population but 1 mL medium in the
outer cuvette. The inner and outer cuvettes consisted either both of _normal glass_ or _both of quartz_ glass. An experimental block contained a random design (Fig. 1(d)) of two replicates
in a total of ten treatment groups and was kept for 48 hrs under conditions of total darkness in an incubator at (constant) 26 °C before assessing the growth of the cells. Note, _Paramecia_
grow well at room temperature below 30 °C (26). The experiment itself was repeated four times leading to a sample size of n = 8 per treatment group (as there were no effects from separating
material glass or quartz found [see results], data could be merged leading to n = 16). EXPERIMENT 2: GRAPHITE-SHIELDING Shielding is a commonly used method when looking for electromagnetic
effects between organisms or cells30,33,34. If the signal in the _Paramecium caudatum_ system is electromagnetic, then a thin layer of colloidal graphite around the inner cuvette should
prevent electromagnetic signals35 coming from the outer inducer population that could induce an effect on the inner tester population. Using purest colloidal graphite in solution (CRAMOLIN®
GRAPHIT) a graphite-layer was twice sprayed onto the bottom and up to a height of 15 mm on the outer side of the small cuvettes. Graphite has the capability of strongly decreasing the
transmission of electromagnetic signals; it is known to shield electromagnetic field efficiently in the range of radiofrequency/microwave region up to wavelengths in the light spectrum, i.e.
from GHz to PHz35,36,37,38. The experiment consisted of five cells in the inner (small) cuvettes surrounded by 100 neighbouring cells and separated from each other either with or without an
additional graphite-shield. Only quartz cuvettes were in use. An experimental block consisted of five replicates of each treatment group arranged in a random design. Each block was kept for
48 hrs under conditions of total darkness in an incubator at constantly 26 °C before assessing the growth of the cells. The experiment was repeated two times leading to a sample size of n =
10 per treatment group. ANALYSIS All data were log-transformed and analysed with JMP statistics39. REFERENCES * Falciatore, A. & Bowler, C. Revealing the molecular secrets of marine
diatoms. _Annual Review of Plant Biology_ 53, 109–30 (2002). Article CAS PubMed Google Scholar * Waters, C. & Bassler, L. B. Quorum sensing: Cell-to-Cell Communication in Bacteria.
_The Annual Review of Cell and Developmental Biology_ 21, 319–346 (2005). Article CAS PubMed Google Scholar * Tannières, M., Lang, J., Barnier, C., Shykoff, J. A. & Faure, D.
Quorum-quenching limits quorum-sensing exploitation by signal- negative invaders. _Scientific Reports_ 7, 40126, https://doi.org/10.1038/srep40126 (2017). Article ADS PubMed PubMed
Central Google Scholar * Fellous, S., Duncan, A. & Coulon, A. & Kaltz, O. Quorum Sensing and Density-Dependent Dispersal in an Aquatic Model System. _PLoS ONE_ 7(11), e48436,
https://doi.org/10.1371/journal.pone.0048436 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Gurwitsch, A. & Gurwitsch, L. Das Problem der Zellteilung physiologisch
betrachtet in _Monographien aus dem Gesamtgebiet der Physiologie der_ Pflanzen _und der Tiere_ 11 (eds Gildmeister, M., Goldschmid, R., Neuberg, C., Parnas, J. & Ruhland, W.) 1–221
(Springer, 1926). * Musumeci, F., Scordino, A., Triglia, A., Blandino, G. & Milazzo, I. Intercellular communication during yeast cell growth. _Europhysics Letters_ 47, 736–742 (1999).
Article ADS CAS Google Scholar * Scholkmann, F., Fels, D. & Cifra, M. Non-chemical and non-contact cell-to-cell communication: a short review. _Am J Transl Res_ 5(6), 586–593 (2013).
PubMed PubMed Central Google Scholar * Cifra, M., Fields, J. Z. & Farhadi, A. Electromagnetic cellular interactions. _Progress in Biophysics and Molecular Biology_ 105, 223–246
(2011). Article CAS PubMed Google Scholar * Laager, F. Light based cellular interactions: hypothesis and perspectives. _Front. Phys._ 3, 55, https://doi.org/10.3389/fphy.2015.00055
(2015). Article Google Scholar * Fels D Electromagnetic cell communication and the barrier method. _Fields of theCell_, eds Fels D, Cifra M, Scholkmann F (Research Signpost, Trivandrum,
India) pp 149–162 (2015). * Fels, D. Cellular Communication through Light. _PLoS ONE._ 4(4), e5086 (2009). Article ADS PubMed PubMed Central Google Scholar * Fels, D. Physical
Non-Contact Communication between Microscopic Aquatic Species: Novel Experimental Evidences for an Interspecies Information Exchange. _Journal of Biophysics_ 2016, 7406356,
https://doi.org/10.1155/2016/7406356 (2016). Article PubMed PubMed Central Google Scholar * Slawinski, J. Necrotic photon emission in stress and lethal interactions. _Current topics in
Biophysics_ 19, 8–27 (1990). Google Scholar * Reguera, G. When microbial conversations get physical. _Trends in Microbiology_ 19, 105–113 (2011). Article CAS PubMed PubMed Central
Google Scholar * Fleming, A.H.J. A range of fields over the spectrum in a cell colony may control the timing of its cell cycle. _Proceedings of PIERS at St Petersburg_. Russia. May 22–25
(2017). * Fels, D., Cifra, M. & Scholkmann, F. Introduction in _Fields of the_ Cell (eds Fels, D., Cifra, M. & Scholkmann, F.) ii–vii (Research Signpost, 2015). * Panel, H. _et al_.
Influence on Cell Proliferation of Background Radiation or Exposure to Very Low, Chronic Gamma Radiation. _Health Physics_ 52(5), 571–8 (1987). Article Google Scholar * Sun, Y., Wang, C.
& Dai, J. Biophotons as neural communication signals demonstrated by _in situ_ biophoton autography. _Photochemical and Photobiological Sciences_ 9, 315–322 (2010). Article CAS PubMed
Google Scholar * Cammaerts, M.-C. & Debeir, O. Cammaerts, R. _Changes in Paramecium caudatum (Protozoa) near a switched-on GSM telephone. Electromagnetic Biology and Medicine_ 30,
57–66 (2011). Google Scholar * Scholkmann, F. _et al_. The circadecadal rhythm of oscillation of umbilical cord blood parameters correlates with geomagnetic activity – An analysis of
long-term measurements (1999–2011). _Chronobiology International_ 33(9), 1136–1147 (2016). Article PubMed Google Scholar * Beloussov, L.V. Ultraweak photon emission as a tool for
analysing collective processes in cells and developing embryos in _Biophotonics and Coherent_ Systems _in Biology_ (eds Beloussov, L.V., Voeikov, V.L. & Martynyuk, V.S.) 139–157
(Springer, 2007). * Levin, M. Bioelectric mechanisms in regeneration: unique aspects and future perspectives. _Semin Cell Dev Biol_ 20, 543–556 (2009). Article PubMed PubMed Central
Google Scholar * Levin, M. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation _in vivo_. _Molecular Biology of the Cell_
25(24), 3835–3850 (2014). Article PubMed PubMed Central Google Scholar * Durant, F. _et al_. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric
gradients. _Biophysics Journal_ 112, 2231–2243 (2017). Article ADS CAS Google Scholar * Markov, M. S. _Electromagnetic Fields in Biology and Medicine_, 452 (Taylor & Francis, 2015).
* Wichtermann, R. _The Biology of Paramecium_, 599 (2nd ed, New York, Plenum, (1986). * Görtz, H.-D. _Parameciu_m, 444 (Springer, (1988). * Fels, D., Vignon, M. & Kaltz, O. Ecological
and genetic determinants of multiple infection and aggregation in a microbial host-parasite system. _Parasitology_ 135, 1373–1383 (2008). Article CAS PubMed Google Scholar * Fels, D.
& Kaltz, O. Temperature-dependent transmission and latency of Holospora undulata, a micronucleus-specific parasite of the ciliate _Paramecium caudatum_. _Proceedings of the Royal
Society, B._ 273, 1031–1038 (2006). Article Google Scholar * Jaffe, L. F. Marine plants may polarize remote Fucus eggs via luminescence. _Luminescence_ 20, 414–418 (2005). Article PubMed
Google Scholar * Farhadi, A. _et al_. Evidence, for non-chemical, non-electrical intercellular signalling in intestinal epithelial cells. _Bioelectrochemistry_ 71, 142–148 (2007). Article
CAS PubMed Google Scholar * Rossi, C., Foletti, A., Magnani, A. & Lamponi, S. New perspectives in cell communication: Bioelectromagnetic interactions. _Seminars in Cancer Biology_
21, 207–214 (2011). Article CAS PubMed Google Scholar * Albrecht-Buehler, G. Rudimentary form of cellular “vision”. _Proceedings of the Natural Academy of Sciences_ 89, 8299–8292 (1992).
Article Google Scholar * Shen, X., Mei, W. & Xu, X. Activation of neutrophils by a chemically separated but optically coupled neutrophil population undergoing respiratory burst.
_Experientia (Basel)_ 50, (963–968 (1994). Google Scholar * Chung, D. D. L. Electromagnetic interference shielding effectiveness of carbon materials. _Carbon_ 39, 279–285 (2001). Article
CAS Google Scholar * Cao, J. & Chung, D. D. L. Colloidal graphite as an admixture in cement and as a coating on cement for electromagnetic interference shielding. _Cement and Concrete
Research_ 33, 1737–1740 (2003). Article CAS Google Scholar * Papoular, R. J. & Papoular, R. Some optical properties of graphite from IR to millimetric wavelengths. _MNRAS_ 443,
2974–2982 (2014). Article ADS CAS Google Scholar * Bond, T. C. & Bergstrom, R. W. Light Absorption by Carbonaceous Particles: An Investigative Review. _Aerosol Science and
Technology_ 40(1), 27–67 (2006). Article ADS CAS Google Scholar * SAS 2003. _JMP Statistics and Graphics Guide_ (Vs. 5.0.1.2). CARY, N.C., SAS Institute. Download references
ACKNOWLEDGEMENTS I am greatly thankful to Thomas Boller for mental support and early comments on the manuscript, to Felix Scholkmann proofreading and commenting the last version of the
manuscript and to the FAG (Freiwillige Akademische Gesellschaft, Basel, Switzerland) for financially supporting the publication. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * University of
Basel, Dept. of Environmental Sciences, Hebelstrasse 1, 4056, Basel, Switzerland Daniel Fels Authors * Daniel Fels View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS Daniel Fels wrote the main manuscript text and prepared Fig. 1. CORRESPONDING AUTHOR Correspondence to Daniel Fels. ETHICS DECLARATIONS COMPETING INTERESTS The
author declares that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Fels, D. Endogenous physical regulation of population density in the freshwater protozoan _Paramecium caudatum_ . _Sci Rep_ 7, 13800 (2017).
https://doi.org/10.1038/s41598-017-14231-0 Download citation * Received: 04 July 2017 * Accepted: 06 October 2017 * Published: 23 October 2017 * DOI:
https://doi.org/10.1038/s41598-017-14231-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






