- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Hedgehog (Hh) signaling pathway and Cyclin E are key players in cell proliferation and organ development. Hyperactivation of _hh_ and _cyclin E_ has been linked to several types of
cancer. However, coordination of the expression of _hh_ and _cyclin E_ was not well understood. Here we show that an evolutionarily conserved transcription factor Apontic (Apt) directly
activates _hh_ and _cyclin E_ through its binding site in the promoter regions of _hh_ and _cyclin E_. This Apt-dependent proper expression of _hh_ and _cyclin E_ is required for cell
proliferation and development of the _Drosophila_ wing. Furthermore, Fibrinogen silencer-binding protein (FSBP), a mammalian homolog of Apt, also positively regulates _Sonic hh_ (_Shh_),
_Desert hh_ (_Dhh_), _Cyclin E1_ (_CCNE1_) and _Cyclin E_2 (_CCNE_2) in cultured human cells, suggesting evolutionary conservation of the mechanism. Apt-mediated expression of _hh_ and
_cyclin E_ can direct proliferation of Hh-expressing cells and simultaneous growth, patterning and differentiation of Hh-recipient cells. The discovery of the simultaneous expression of Hh
and principal cell-cycle regulator Cyclin E by Apt implicates insight into the mechanism by which deregulated _hh_ and _cyclin E_ promotes tumor formation. SIMILAR CONTENT BEING VIEWED BY
OTHERS ETV2 REGULATES ENHANCER CHROMATIN STATUS TO INITIATE SHH EXPRESSION IN THE LIMB BUD Article Open access 21 July 2022 TARGETING OF SET/I2PP2A ONCOPROTEIN INHIBITS GLI1 TRANSCRIPTION
REVEALING A NEW MODULATOR OF HEDGEHOG SIGNALING Article Open access 06 July 2021 SOX2 LEVELS REGULATE THE CHROMATIN OCCUPANCY OF WNT MEDIATORS IN EPIBLAST PROGENITORS RESPONSIBLE FOR
VERTEBRATE BODY FORMATION Article Open access 12 May 2022 INTRODUCTION Animal development requires the organ growth and patterning. How these two processes are coordinated remains poorly
understood. The _Drosophila_ wing is an excellent model to study the regulation of gene expression during the organ growth and patterning. The wing disc is a sac-like structure composed of
disc proper (DP) cells and peripodial epithelium (PE). During larval development, both DP and PE cells proliferate extensively and are patterned, finally give rise to the adult wing1,2,3.
The Hh signaling and Cyclin E can contribute to growth and patterning of the wing disc during development4,5. Hh pathway is one of the major conserved signaling pathways that control animal
development from _Drosophila_ to humans, which has been implicated in stem cell maintenance, cell migration, axon guidance and tissue regeneration6,7,8,9. Most vertebrate species have three
_hh_: _Shh_, _Indian hedgehog_ (_Ihh_) and _Dhh_, each with different expression patterns and functions. In the _Drosophila_ wing disc, morphogen Hh expresses in posterior (P) compartment
cells and spreads into approximately 12 cells-wide of anterior (A) cells along A/P boundary, where it regulates target gene expression in the A compartment to control entire wing patterning
through stabilizing full-length Cubitus interruptus (CiF)4,10,11,12. Therefore, the expression of _hh_ is vital during wing development. Engrailed (En) induces the expression of _hh_ in the
P compartment, at the same time, represses the Hh downstream component Ci11,13. Ci can exist in two forms: CiF and a repressor form (CiR). CiR represses the expression of _hh_ in anterior
cells12,14. However, regulatory factor that directly activates _hh_ transcription remained to be identified. Cyclin E belongs to the cyclin family, which is required for cell division15.
Dysregulation of _cyclin E_ correlates with various tumors, including breast cancer and lung cancer16,17. Besides, deregulated Cyclin E activity causes cell lineage-specific abnormalities,
such as impaired maturation due to unregulated cell proliferation18. In _Drosophila_, Cyclin E is essential for G1-to-S phase transition in the posterior cells of eye disc19. It has been
reported that _cyclin E_ is a potential target gene of Hh signaling in _Drosophila_. Hh pathway activates _cyclin E_ expression through its unique transcription factor Ci in the posterior
cells of eye disc20. In the wing disc, Hh pathway is turned on exclusively in the A cells near A/P boundary21. However, _cyclin E_ expresses throughout the wing disc5. This contradiction
suggests that other factors are involved in regulating the expression of _cyclin E_. Therefore, it is fruitful to investigate the regulation of _cyclin E_ in the wing disc and the
relationship between Cyclin E and Hh pathway. Apt has been identified as a transcription factor involved in development of tracheae, head, heart and nervous system22,23,24,25. Apt can
suppress metastasis26 and is required in the nervous system for normal sensitivity to ethanol sedation27. Moreover, Apt participates in JAK/STAT signaling pathway to limit border cells
migration28. The human homolog of Apt, FSBP, is a cancer-related factor that is expressed in many tissues29,30. However, the role of Apt in the organ growth and patterning is unknown. In
this study, we unveiled a fundamental role of Apt in growth and patterning of the wing disc through coordinated expression of morphogen _hh_ and cell cycle regulator _cyclin E_. Both loss of
function and overexpression of _apt_ resulted in defective wings. Further studies demonstrated that loss of _apt_ function attenuated the expression of _hh_ and _cyclin E_, while _apt_
overexpression upregulated _hh_ and _cyclin E_. Mutating the inherent Apt binding sites in the promoter region of _hh_ and _cyclin E_ compromised the expression of _hh_ and _cyclin E_.
Collectively, Apt directly activates the expression of _hh_ and _cyclin E_ to allow proper wing development. In addition, we found that Apt-dependent expression of _hh_ and _cyclin E_ is
evolutionarily conserved in human cells. RESULTS APT IS EXPRESSED IN THE WING DISC AND IS REQUIRED FOR WING DEVELOPMENT As the first attempt to investigate the function of _apt_ during wing
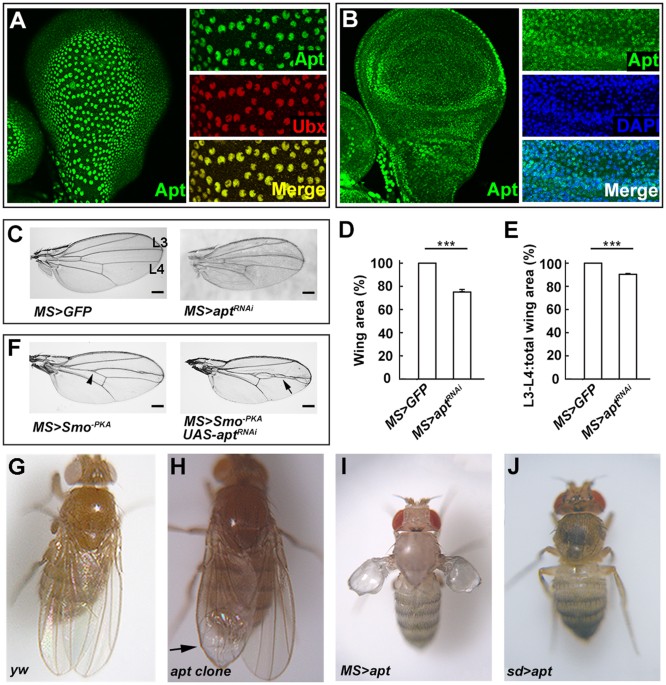
development, we analyzed _apt_ expression pattern in the wing disc by immunostaining using anti-Apt antibody. In the wing disc, Apt was detected in PE cells as revealed by co-localization
with a PE marker Ubx (Fig. 1A). Apt was also detected in DP cells (Fig. 1B). These data clearly demonstrate that Apt is expressed in both the PE and DP of the wing disc, suggesting its
possible role in wing development. To analyze the role of Apt during wing development, we would examine the developing wing of homozygous _apt_ null mutant. However, _apt_ null homozygotes
die as embryos22. Therefore, we firstly examined the phenotype of _apt_ knockdown using an _MS1096_-_GAL4_ driver. RNAi-mediated knockdown of _apt_ resulted in a small wing, and also reduced
the width between vein 3 and vein 4 (Fig. 1C–E). Overexpression of a dominant-negative form of Smoothened (Smo−PKA) caused a “fused wing” phenotype31,32,33. Knockdown of _apt_ enhanced the
“fused wing” phenotype (Fig. 1F, arrowhead indicates the “fused wing” phenotype and arrow indicates enhancement of the “fused wing” phenotype). Furthermore, we induced _apt_ loss of function
mutant clones in the wing disc using the _FLP/FRT_ system34. The formation of these clones resulted in a small wing with a blistered phenotype (Fig. 1H) compared with the control wing (Fig.
1G). To investigate the effect of _apt_ overexpression, we employed the _MS1096-Gal4_ driver. Abnormal wings were induced by overexpression of _apt_ (Fig. 1I). The wing was diminished and
blistered, and the pattern of veins was disrupted and extra abnormal bristles were induced in the wing margin. In addition, when _apt_ was overexpressed by a stronger driver (_sd-Gal4_),
both wings and halters were lost (Fig. 1J). Taken together, the loss-of-function and overexpression analyses indicate that Apt is indispensable for wing development. APT ACTIVATES THE
EXPRESSION OF _HH_ IN THE WING DISC Given that the space between vein 3 and vein 4 is a characteristic monitor of Hh activity21,35, the observed narrowing the space between vein 3 and vein 4
upon knockdown of _apt_ (Fig. 1C–E) implies that Apt can modulate expression of _hh_ in the wing disc. The enhanced dominant-negative phenotype of Smo−PKA by knockdown of _apt_ (Fig. 1F)
supports the notion. To examine the relationship between _apt_ and _hh_, we first compared the expression of _apt_ and _hh_, and found that Apt and _hh-lacZ_ were co-expressed in PE cells
(Fig. 2A–C) and P compartment cells of the DP (Fig. 2D–F) in the early third instar larval disc. Furthermore, _apt_ exhibited genetic interaction with _hh_. Ninety-seven percent of _hh_
_bar3_ mutant (n = 40) showed slightly reduced area between L3 and L4 (Fig. 2H) and the remaining three percent showed wing blistering phenotype (Supplementary Fig. S5B). While heterozygotes
of _apt_ null allele showed normal wings (Fig. 2G), the same heterozygotes under the _hh_ _bar3_ background exhibited more severe phenotypes of reduced L3-L4 area and smaller wing with
blister (Fig. 2I), which reproduced the _apt_ loss of function phenotype (Fig. 1H). Transheterozygotes of two sets of _hh_ alleles (_hh_ _bar3_/_hh_ 2 and _hh_ _Mir_/_hh_ 2) showed a smaller
wing with an extra crossvein (Supplementary Fig. S1A–E), demonstrating that it is a loss of function phenotype of _hh_. While wings of animal heterozygous for _hh_ 2 or _apt_ null mutant
were normal, trans-heterozygotes of the _apt_ null allele and _hh_ 2 showed the same wing phenotype (Supplementary Fig. S1F–I). These results suggest that Apt regulates the expression of
_hh_. To address the issue directly, we analyzed the expression of _hh_ under loss-of-function and overexpression of Apt. The expression of _hh-lacZ_ was significantly reduced in the _apt_
mutant clones in the PE (Fig. 3A–C) and the DP (Fig. 3D–F). In addition, the expression of _hh-lacZ_ also decreased in the _apt_-knocked down region (Fig. 3G–I). By contrast, overexpression
of Apt increased the expression of _hh-lacZ_ (Fig. 3J–L). Moreover, Apt regulates the mRNA levels of _hh_ and its target _dpp_ (Supplementary Fig. S2). These results demonstrate that Apt
activates the expression of _hh_. APT DIRECTLY CONTROLS _HH_ IN THE WING DISC To address how Apt activates the expression of _hh_, we focused on a 15–kb region of the _hh_ locus known to
reproduce the normal _hh_ expression pattern in the wing disc36. We identified one potential Apt binding sequence25 within the region (Fig. 4A). Chromatin immunoprecipitation (ChIP) assays
using early third instar wing discs detected Apt protein on the predicted wild-type Apt binding site but not in the regions upstream and downstream of the binding site (Fig. 4A and D). We
next assessed the function of the Apt-binding site in _hh_ using a CRISPR-Cas9 system37. Since the designed gRNA contained the Apt-binding site, four Apt-binding site deletion mutants and
two insertion mutants were generated (Fig. 4B and C; Supplementary Fig. S3A). The _hh_ _ΔaptDB1_ mutation abolished the occupancy of Apt on its binding site (Fig. 4D). Homozygotes of these
mutations showed reduced expression of _hh_ (Fig. 4E; Supplementary Fig. S3B) and exhibited the small wing and reduced vein 3–4 spacing phenotypes (Fig. 4F; Supplementary Fig. S3C and D).
Effect of _hh_ _ΔaptDB1_ mutation on the _hh_ function was also examined under the _hh_ 2 heterozygous background. While wings of animals heterozygous for _hh_ 2 or _hh_ _ΔaptDB1_ were
normal, transheterozygotes of _hh_ _ΔaptDB1_ and _hh_ 2 showed the same extra vein phenotype (Fig. 4G–I) as did transheterozygotes of _apt_-null allele and _hh_ 2 (Supplementary Fig. S1H).
Besides, we also examined the effect of _hh_ _ΔaptDB1_ mutation on the _hh_ function under expression of a dominant-negative form of Smoothened (Smo−PKA). While _MS_ > _Smo_ _−PKA_ alone
showed reduction of the intervein space between vein 3 and vein 4 (Fig. 4J and K, arrowhead), _hh_ _ΔaptDB 1_exhibited more severe defects and enhanced the “fused wing” phenotype of _MS_
> _Smo_ _−PKA_ (Fig. 4L). Taken together, these data demonstrate that Apt directly activates transcription of _hh_ in the wing disc for proper wing development. APT ACTIVATES THE _CYCLIN
E_ EXPRESSION IN THE WING DISC We have reported that Apt induces the _cyclin E_ expression in the eye disc38. Therefore, we examined whether Apt consistently regulates _cyclin E_ in the wing
disc. To do this, we performed a double-staining experiment using Apt antibody and Cyclin E antibody. In the wild-type wing disc, Apt and Cyclin E were co-expressed (Fig. 5A–C).
Furthermore, the expression of Cyclin E was significantly reduced in the _apt_ mutant clones (Fig. 5D–F). Compared with control disc (Fig. 5G), _apt_ knockdown decreased Cyclin E level (Fig.
5H), while _apt_ overexpression increased Cyclin E (Fig. 5I). In addition, the _cyclin E_ mRNA levels were decreased and increased upon RNAi-knockdown and overexpression of _apt_ in the
wing disc, respectively (Supplementary Fig. S4). These results indicate that Apt activates the expression of _cyclin E_ in the wing disc. APT DIRECTLY CONTROLS _CYCLIN E_ IN THE WING DISC
Since Apt directly activates the expression of _cyclin E_ in the eye disc, we anticipated a direct role of Apt in the expression of _cyclin E_ also in the wing disc. This expectation was
verified by transgenic reporter assays. The reporter gene38 carries the endogenous promoter and the _cyclin E_ regulatory element containing a wild-type Apt-binding site (_cycEPlacZ_) or a
mutated site (_cycEMPlacZ_). _cycEPlacZ_ with the wild type binding site recapitulated the _cyclin E_ expression in the wing disc (Fig. 5J–L). However, base substitutions in the Apt-binding
site in _cycEMPlacZ_ abolished the lacZ expression (Fig. 5M–O). These results indicate that Apt directly activates _cyclin E_ through its binding site in the regulatory region of _cyclin E_.
APT IS A GROWTH SENSOR TO CONTROL ORGAN GROWTH AND PATTERNING Because both Hh and Cyclin E are involved in cell death and cancer39,40,41, we asked whether the overexpression phenotypes are
caused by apoptosis. To test this, we investigated apoptosis in wing discs by staining with anti-Caspase-3 antibody. In the third instar wing disc, _apt_ mutant clones showed few apoptotic
cells (Fig. 6A). However, in the wing disc from an Apt-overexpressed larva, the number of apoptotic cells significantly increased compared with a control disc (Fig. 6B and C). This
presumably explains why wing size was reduced upon strong overexpression of Apt (Fig. 1K and L). Besides, we examined the growth of wing discs upon _apt_ knockdown in the dorsal region using
an _ap-GAL4_ driver. Compared with a control disc (Fig. 6D), _apt_ knockdown region exhibited growth disadvantage (Fig. 6E and H). Overexpression of Apt using the _ap-GAL4_ driver resulted
in severely reduced dorsal region (Fig. 6F). When we inhibited cell death by simultaneous overexpression of a Caspase inhibitor P35, we observed outgrowth of cell layers from the disc in the
_apt_ and _p35_ overexpressed region (Fig. 6G and H). Homozygotes of _hh_ mutations for the Apt-binding site exhibited the small wing but not the blistered phenotype. However, _hh_ and
_cyclin E_ double mutant recapitulates the smaller and blistered wing. While _CycE_ 2/+ flies showed normal wings, three percent of _hh_ _bar3_/_hh_ _bar3_ and eighteen percent of _CycE_
2/+; _hh_ _bar3_/_hh_ _bar3_ flies showed the smaller and blistered phenotypes (Supplementary Fig. S5A–C). We also observed genetic interaction between _hh_ and _cyclin E_ in the extra
crossvein phenotype. While _CycE_ _JP_/+ and _hh_ 2/+ flies showed normal wings, fifty-four percent of _CycE_ _JP_/+; _hh_ 2/+ flies (n = 102) exhibited wings with the extra crossvein
(Supplementary Fig. S5D–F). Collectively, these data suggest that Apt controls wing development by inducing appropriate amounts of Hh and Cyclin E. FSBP POSITIVELY REGULATES _SHH_ AND
_CYCLIN E_ IN HUMAN CELLS FSBP, the mammalian homologue of _Drosophila_ Apt, is a cancer related factor. To examine whether FSBP regulates _Shh_ and _cyclin E_, we used human 293T cells to
knockdown or overexpress FSBP and analysed the mRNA levels of _Shh_, its signaling pathway genes and _cyclin E_. After transfection of FSBP siRNA, the mRNA level of FSBP decreased nearly 60
percent compared with mock. Under the condition, we observed marked decrease in the mRNA levels of _Shh_ and _Shh_ signaling pathway genes such as _Ptch_, _Gli_ and _Hhip_ (Fig. 7A). The
levels of _cyclin E_ (_CCNE1_ and _CCNE2_) mRNA showed less prominent but statistically significant decrease. When we overexpressed FSBP, mRNA level of _FSBP_ increased nearly 7.5 folds, and
that of _Shh_ increased dramatically 9 folds. The mRNA levels of _Shh_ targets and _cyclin E_ were also increased upon overexpression of FSBP (Fig. 7B). Interestingly, FSBP also regulates
the expression of _Dhh_, but not _Ihh_. Taking together, these data suggest that the regulation of _hh_/_Shh_ and _cyclin E_ by Apt/FSBP is conserved from _Drosophila_ to humans. DISCUSSION
Morphogen Hh and cell cycle regulator Cyclin E control growth and patterning in vertebrate and invertebrate. Here, we unravel a fundamental role of transcription factor Apt/FSPB as a
conserved regulator of _hh/Shh_ and _cyclin E/CCNE_. During _Drosophila_ wing development, Apt directly activates the expression of _hh_ and _cyclin E_ to control wing growth and patterning.
Both loss-of-function and overexpression assays clearly demonstrated that Apt is vital for wing development. Further studies showed that loss of _apt_ function attenuates, while
overexpression of _apt_ activates the expression of _hh_ and _cyclin E_. Moreover, we found that the homolog of Apt, FSBP, can positively regulate _Shh_ and its pathway genes, and _CCNE_ in
human cells. Hyperactivation of Hh pathway and _cyclin E_ has been implicated in many tumors16,39. In contrast, during development, cell proliferation must be precisely regulated and
coordinated with the processes of cell patterning and differentiation, which are also regulated by Hh and Cyclin E41,42. This delicate balance is probably maintained by Apt-mediated proper
expression of Hh and Cyclin E. Indeed, overexpression of Apt in the presence of apoptosis inhibitor P35 generated tumor-like outgrowth of cell layers in the wing disc. Apt-dependent
expression of _hh_ and _cyclin E_ can direct proliferation of Hh-expressing cells and simultaneous growth, patterning and fate specification of Hh-recipient cells. Although mechanisms are
quite different, this provides similar effects as an asymmetric division of a stem cell. To assess the importance of the Apt-binding site in the promoter region of _hh_, we first tried a
transgenic reporter assay. However, the regulatory region of _hh_ encompassing the upstream region and the 1st intron (~15 kb)36 is too large to make a reporter construct for conventional
P-element mediated transgenesis. Therefore, we employed the CRISPR-Cas9 system37 to mutagenize the endogenous Apt-binding site in the _hh_ promoter. All 6 independent mutants exhibited the
same phenotypes (reduced expression of _hh_, reduced wing size and the space between L3 and L4), suggesting that the observed phenotypes are not due to off-target effect of Cas9.
Nevertheless, we inspected the possibility of off-target effect. Since our gRNA carries the binding sequence for Apt, a binding site of Apt in other than the _hh_ promoter could be the most
likely candidate for off-target. However, all the 6 mutants showed the wild type sequence around the Apt-binding site in the _cyclin E_ promoter (Supplementary Fig. S6). Furthermore, we
observed clear genetic interactions between _hh_ _ΔaptDB1_ and other _hh_ mutants. Taken together, these data strongly suggest that the observed phenotypes are not due to off-target effect.
Although the expression of Apt and Hh overlapped in the P compartment of wing disc, how Apt specifically induces _hh_ in the P compartment is still not clear. Since CiR has been known to
repress the expression of _hh_ in anterior cells14, CiR may interrupt the activation of _hh_ by Apt in anterior cells. While our data strongly support that Apt is a transcription factor of
_hh_, mutating the Apt-binding site on _hh_ promoter alone induced the weak phenotype. However, the binding site mutation showed strongly enhanced “fused wing” phenotype in the background of
overexpression of Smo domain-negative form (Smo−PKA). These observations suggest that besides Apt, other factor(s) might also regulate _hh_ transcription during development. Therefore, both
knockdown and overexpression of Apt only moderately affected the expression of _hh_. Hh, as an important morphogen, plays multifaceted roles in segmentation and wing patterning. Previous
findings paid more attention on the protein modification of Hh but the regulatory mechanism underlying _hh_ transcription was not well understood. Here we identified Apt as the first
regulatory factor that directly activates _hh_ transcription. MATERIALS AND METHODS FLY STOCKS All the adult phenotypes were obtained from females. Strains used were as follows. _apt_
_PΔ4_22, _apt_ _p2_25, _apt_ 167 (gift from M. Starz-Gaiano), _cycEPlacZ_ and _cycEMPlacZ_ 38, _UAS-apt_ (gift of D. Montell), _UAS-GFP_ (gift of Y. Hiromi), _MS_ > _Smo_ _−PKA_31,32.
_hh_ _Mir_ was obtained from _Drosophila_ Genetic Resource Center. _hh_ 2, _hh_ _Mrt_, _hh_ _bar3_ _, CycE_ 2 _, CycE_ _JP_ _, hh-LacZ_, _MS1096-GAL4_, _sd-GAL4_ (8609) and _ap-Gal4_ were
obtained from Bloomington _Drosophila_ Stock Center. _UAS-apt_ _RNAi_ lines were obtained from Tsinghua Fly Center and Vienna _Drosophila_ Resource Center. _CAS-0001, TBX-000_2_, TBX-0004,
TBX-0008, TBX-0010_ were obtained from NIG-FLY Stock Center. CLONAL ANALYSIS Homozygous _apt_ loss-of-function clones were generated by _hs-FLP/FRT_ recombination34. _FRT42D_ and _apt_ _PΔ4_
_/CyO_ were recombined to generate _FRT42D_, _apt_ _PΔ4_. Six pairs of _FRT42D_, _apt_ _PΔ4_ cross to _Gla/CyO_ were allowed to lay eggs in G418-containing medium, and then test each line
with _apt_ _P2_ _/CyO_. _hs-FLP; FRT42D_, _Ubi-GFP/CyO_ crossed with _FRT42D_, _apt_ _PΔ4_ _/CyO_ were performed at 25 °C. Heat shocks were performed 32-56 hours after egg-laying for 1.5
hours at 37 °C. GENERATION OF CRISPR CONSTRUCTS To induce mutations in the Apt-binding site in the _hh_ promoter region, we used a Cas9–gRNA system. We designed gRNA in the _hh_ promoter
region carrying the binding sequence of Apt (Fig. 3A). The corresponding sequence was introduced into the pBFv-U6.2 vector and the gRNA transgenic flies were generated as described37. gRNA
females were crossed to Cas9 males to obtain the founder animals. Male founders were crossed to female balancer. Offspring male flies were balanced and stocked. Genomic DNA was extracted
from each offspring male and used for molecular characterization. PCR primers were designed to construct gRNA expression vectors and to amplify the promoter region of _cyclin E_
(Supplementary Table S1). CHROMATIN IMMUNOPRECIPITATION (CHIP) ChIP assays of wing discs were performed as previously described43. Briefly, 100 early third instar wing discs were dissected
in PBS and fixed by 1% formaldehyde at room temperature for 20 minutes. Sonicated chromatin was immunoprecipitated using 10 μl anti-Apt antibody. Quantitative PCR using 4 μl of the purified
DNA. RT-QPCRANALYSIS Total RNAs were prepared from the dissected tissues using an RNAprep Pure Tissue kit TIANGEN #DP431). cDNAs were synthesized using a Prime ScriptTM II1st strand cDNA
synthesis kit (TaKaRa #6210A). The real-time qPCR was conducted with Bio-Rad CFX96 real-time system using a SuperRealPreMix Plus (SYBR Green) Kit (TIANGEN #FP205) in a 20 ul reaction
containing 2 pmol of relevant primers. The amount of mRNA was normalized to that of control tubulin mRNA. PCR primers used are shown in Supplementary Table (Supplementary Table S1).
ANTIBODIES AND IMMUNOHISTOCHEMISTRY Staining of larval tissues was performed as described previously38,44. Larvae were dissected in PBS, fixed with 4% formaldehyde for 40 minutes on ice and
then permeabilized for 15 minutes at room temperature in PBS containing 0.5% NP-40. The following primary antibodies were used in overnight incubations at 4 °C in blocking solution: rabbit
anti-Apt (1:1000), rabbit anti-β-galactosidase (1:2000, Cappel), rabbit Caspase3 (1:50, Cell Signaling Technology), mouse anti-β-galactosidase (1:500, Sigma), mouse anti-Ubx (1:10,
Developmental Studies Hybridoma Bank (DSHB)), goat anti-Cyclin E (1:200, Santa Cruz). The secondary antibodies used were as follows: Alexa 488 donkey anti-rabbit IgG conjugate (1:500,
Molecular Probes), Alexa 488 donkey anti-mouse IgG (1:500, Molecular Probes), Cy3-conjugated donkey anti-mouse IgG (1:500, Sigma), Cy3-conjugated goat anti-rabbit IgG (1:500, CWBIO), bovine
anti-goat IgG-CFL 555 (1:500, Santa Cruz). Mounting used VECTASHIELD Mounting Medium with DAPI (Vector Labs). The caspase-3 staining was did as described previously45. CELL CULTURE AND
TRANSFECTION 293 T cells were cultured in DMEM (Gibco) containing 10% fetal bovine serum and 100 U/ml of penicillin/streptomycin. For RNA interference experiment, FSBP siRNA was designed by
ourselves, the sequences were: siFSBP-F: 5′-GCCUGGUAAGAGACAGGAAdTdT-3′, siFSBP-R: 5′-UUCCUGUCUCUUACCAGGCdTdT-3′. After cells were cultured for 24 h in 12-well plate, the culture medium was
changed to serum-free medium. Mock is no siRNA treatment. siRNA duplexes were transfected at a final concentration of 20 nM using lipofectamine 2000 (Invitrogen) according to the
manufacturer’s instructions. Cells are harvested for real-time PCR after cultured for 48–96 h with serum containing medium. For overexpression experiment, transfection was carried out using
PEI (polyethylenimine) transfection method. 293 T cells were transfected in 60 mm plates with 5 µg plasmid pcDNA3.1-FSBP, which was constructed by our lab, and pcDNA3.1-HisA/V5 plasmid was
transfected as control. Forty-eight to ninety-six hours after transfection, cells are harvested for real-time PCR analyses with standard protocols. The primers were used as showing in the
Supplementary Table S1. MICROSCOPY AND IMAGE TREATMENT Images were acquired in Olympus FV1200 confocal microscope and Olympus cellSens, treated with Adobe Photoshop CS6 image programs. Wing
size and space between vein 3 and vein 4 were measured on each picture using the ImageJ computer program. STATISTICAL ANALYSIS Results are given as means SEM; each experiment included at
least three independent samples and was repeated at least three times. Group comparisons were made by two-tailed unpaired Student’s t-tests. *P < 0.05; **P < 0.01, and ***P < 0.001.
REFERENCES * McClure, K. D. & Schubiger, G. Developmental analysis and squamous morphogenesis of the peripodial epithelium in Drosophila imaginal discs. _Development_ 132, 5033–5042,
https://doi.org/10.1242/dev.02092 (2005). Article CAS PubMed Google Scholar * Cho, K. O., Chern, J., Izaddoost, S. & Choi, K. W. Novel signaling from the peripodial membrane is
essential for eye disc patterning in Drosophila. _Cell_ 103, 331–342 (2000). Article CAS PubMed Google Scholar * Milner, M., Bleasby, A. & Kelly, S. The role of the peripodial
membrane of leg and wing imaginal discs of Drosophila melanogaster during evagination and differentiation _in vitro_. _Wilhelm Roux’s Arch. Dev. Biol._ 193, 180–186 (1984). Article Google
Scholar * Tabata, T. & Kornberg, T. B. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. _Cell_ 76, 89–102 (1994). Article CAS PubMed Google
Scholar * Neufeld, T. P., de la Cruz, A. F., Johnston, L. A. & Edgar, B. A. Coordination of growth and cell division in the Drosophila wing. _Cell_ 93, 1183–1193 (1998). Article CAS
PubMed Google Scholar * Beachy, P. A., Karhadkar, S. S. & Berman, D. M. Tissue repair and stem cell renewal in carcinogenesis. _Nature_ 432, 324–331,
https://doi.org/10.1038/nature03100 (2004). Article ADS CAS PubMed Google Scholar * Hochman, E., Castiel, A., Jacob-Hirsch, J., Amariglio, N. & Izraeli, S. Molecular pathways
regulating pro-migratory effects of Hedgehog signaling. _J Biol Chem_ 281, 33860–33870, https://doi.org/10.1074/jbc.M605905200 (2006). Article CAS PubMed Google Scholar * Clement, V.,
Sanchez, P., de Tribolet, N. & Radovanovic, I. & Ruiz i Altaba, A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. _Curr
Biol_ 17, 165–172, https://doi.org/10.1016/j.cub.2006.11.033 (2007). Article CAS PubMed Google Scholar * Charron, F., Stein, E., Jeong, J., McMahon, A. P. & Tessier-Lavigne, M. The
morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. _Cell_ 113, 11–23 (2003). Article CAS PubMed Google Scholar * Basler, K.
& Struhl, G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. _Nature_ 368, 208–214, https://doi.org/10.1038/368208a0 (1994). Article ADS CAS
PubMed Google Scholar * Aza-Blanc, P. & Kornberg, T. B. Ci: a complex transducer of the hedgehog signal. _Trends Genet_ 15, 458–462 (1999). Article CAS PubMed Google Scholar *
Methot, N. & Basler, K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. _Cell_ 96,
819–831 (1999). Article CAS PubMed Google Scholar * Tabata, T., Eaton, S. & Kornberg, T. B. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and
is a target of engrailed regulation. _Genes Dev_ 6, 2635–2645 (1992). Article CAS PubMed Google Scholar * Aza-Blanc, P., Ramirez-Weber, F. A., Laget, M. P., Schwartz, C. & Kornberg,
T. B. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. _Cell_ 89, 1043–1053 (1997). Article CAS PubMed Google
Scholar * Knoblich, J. A. _et al_. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. _Cell_ 77,
107–120 (1994). Article CAS PubMed Google Scholar * Donnellan, R. & Chetty, R. Cyclin E in human cancers. _FASEB journal: official publication of the Federation of American
Societies for Experimental Biology_ 13, 773–780 (1999). CAS Google Scholar * Keyomarsi, K. _et al_. Cyclin E, a potential prognostic marker for breast cancer. _Cancer research_ 54, 380–385
(1994). CAS PubMed Google Scholar * Minella, A. C. _et al_. Cyclin E phosphorylation regulates cell proliferation in hematopoietic and epithelial lineages _in vivo_. _Genes Dev_ 22,
1677–1689, https://doi.org/10.1101/gad.1650208 (2008). Article CAS PubMed PubMed Central Google Scholar * Richardson, H., O’Keefe, L. V., Marty, T. & Saint, R. Ectopic cyclin E
expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. _Development_ 121, 3371–3379 (1995). CAS PubMed Google Scholar *
Duman-Scheel, M., Weng, L., Xin, S. & Du, W. Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. _Nature_ 417, 299–304, https://doi.org/10.1038/417299a
(2002). Article ADS CAS PubMed Google Scholar * Strigini, M. & Cohen, S. M. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. _Development_
124, 4697–4705 (1997). CAS PubMed Google Scholar * Eulenberg, K. G. & Schuh, R. The tracheae defective gene encodes a bZIP protein that controls tracheal cell movement during
Drosophila embryogenesis. _EMBO J_ 16, 7156–7165, https://doi.org/10.1093/emboj/16.23.7156 (1997). Article CAS PubMed PubMed Central Google Scholar * Gellon, G., Harding, K. W.,
McGinnis, N., Martin, M. M. & McGinnis, W. A genetic screen for modifiers of Deformed homeotic function identifies novel genes required for head development. _Development_ 124, 3321–3331
(1997). CAS PubMed Google Scholar * Su, M. T., Venkatesh, T. V., Wu, X., Golden, K. & Bodmer, R. The pioneer gene, apontic, is required for morphogenesis and function of the
Drosophila heart. _Mech Dev_ 80, 125–132 (1999). Article CAS PubMed Google Scholar * Liu, Q. X., Jindra, M., Ueda, H., Hiromi, Y. & Hirose, S. Drosophila MBF1 is a co-activator for
Tracheae Defective and contributes to the formation of tracheal and nervous systems. _Development_ 130, 719–728 (2003). Article CAS PubMed Google Scholar * Woodhouse, E. C. _et al_.
Drosophila screening model for metastasis: Semaphorin 5c is required for l(2)gl cancer phenotype. _Proc Natl Acad Sci USA_ 100, 11463–11468, https://doi.org/10.1073/pnas.2031202100 (2003).
Article ADS CAS PubMed PubMed Central Google Scholar * McClure, K. D. & Heberlein, U. A small group of neurosecretory cells expressing the transcriptional regulator apontic and the
neuropeptide corazonin mediate ethanol sedation in Drosophila. _J Neurosci_ 33, 4044–4054, https://doi.org/10.1523/JNEUROSCI.3413-12.2013 (2013). Article CAS PubMed PubMed Central
Google Scholar * Starz-Gaiano, M., Melani, M., Wang, X., Meinhardt, H. & Montell, D. J. Feedback inhibition of Jak/STAT signaling by apontic is required to limit an invasive cell
population. _Dev Cell_ 14, 726–738, https://doi.org/10.1016/j.devcel.2008.03.005 (2008). Article CAS PubMed Google Scholar * Lau, K. F., Perkinton, M. S., Rodriguez, L., McLoughlin, D.
M. & Miller, C. C. An X11alpha/FSBP complex represses transcription of the GSK3beta gene promoter. _Neuroreport_ 21, 761–766, https://doi.org/10.1097/WNR.0b013e32833bfca0 (2010). Article
CAS PubMed PubMed Central Google Scholar * Kim, K. Y., Ki, D. H., Jeung, H. C., Chung, H. C. & Rha, S. Y. Improving the prediction accuracy in classification using the combined
data sets by ranks of gene expressions. _BMC bioinformatics_ 9, 283, https://doi.org/10.1186/1471-2105-9-283 (2008). Article PubMed PubMed Central Google Scholar * Chen, Y. _et al_. G
protein-coupled receptor kinase 2 promotes high-level Hedgehog signaling by regulating the active state of Smo through kinase-dependent and kinase-independent mechanisms in Drosophila.
_Genes Dev_ 24, 2054–2067, https://doi.org/10.1101/gad.1948710 (2010). Article CAS PubMed PubMed Central Google Scholar * Jia, J., Tong, C., Wang, B., Luo, L. & Jiang, J. Hedgehog
signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. _Nature_ 432, 1045–1050, https://doi.org/10.1038/nature03179 (2004). Article ADS CAS
PubMed Google Scholar * Zhou, Z. _et al_. Deubiquitination of Ci/Gli by Usp7/HAUSP Regulates Hedgehog Signaling. _Developmental cell_ 34, 58–72,
https://doi.org/10.1016/j.devcel.2015.05.016 (2015). Article CAS PubMed PubMed Central Google Scholar * Theodosiou, N. A. & Xu, T. Use of FLP/FRT system to study Drosophila
development. _Methods_ 14, 355–365, https://doi.org/10.1006/meth.1998.0591 (1998). Article CAS PubMed Google Scholar * Mullor, J. L., Calleja, M., Capdevila, J. & Guerrero, I.
Hedgehog activity, independent of decapentaplegic, participates in wing disc patterning. _Development_ 124, 1227–1237 (1997). CAS PubMed Google Scholar * Lee, J. J., von Kessler, D. P.,
Parks, S. & Beachy, P. A. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. _Cell_ 71, 33–50 (1992). Article
CAS PubMed Google Scholar * Kondo, S. & Ueda, R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. _Genetics_ 195, 715–721,
https://doi.org/10.1534/genetics.113.156737 (2013). Article CAS PubMed PubMed Central Google Scholar * Liu, Q. X. _et al_. Evolutionarily conserved transcription factor Apontic controls
the G1/S progression by inducing cyclin E during eye development. _Proc Natl Acad Sci USA_ 111, 9497–9502, https://doi.org/10.1073/pnas.1407145111 (2014). Article ADS CAS PubMed PubMed
Central Google Scholar * Jiang, J. & Hui, C. C. Hedgehog signaling in development and cancer. _Developmental cell_ 15, 801–812, https://doi.org/10.1016/j.devcel.2008.11.010 (2008).
Article CAS PubMed Google Scholar * Guerrero, I. & Ruiz i Altaba, A. Development. Longing for ligand: hedgehog, patched, and cell death. _Science_ 301, 774–776,
https://doi.org/10.1126/science.1088625 (2003). Article CAS PubMed Google Scholar * Hwang, H. C. & Clurman, B. E. Cyclin E in normal and neoplastic cell cycles. _Oncogene_ 24,
2776–2786, https://doi.org/10.1038/sj.onc.1208613 (2005). Article CAS PubMed Google Scholar * McMahon, A. P. More surprises in the Hedgehog signaling pathway. _Cell_ 100, 185–188 (2000).
Article CAS PubMed Google Scholar * Nakayama, T., Shimojima, T. & Hirose, S. The PBAP remodeling complex is required for histone H3.3 replacement at chromatin boundaries and for
boundary functions. _Development_ 139, 4582–4590, https://doi.org/10.1242/dev.083246 (2012). Article CAS PubMed Google Scholar * Zhou, Z. _et al_. Stability of HIB-Cul3 E3 ligase adaptor
HIB Is Regulated by Self-degradation and Availability of Its Substrates. _Scientific reports_ 5, 12709, https://doi.org/10.1038/srep12709 (2015). Article ADS CAS PubMed PubMed Central
Google Scholar * Rudrapatna, V. A., Bangi, E. & Cagan, R. L. Caspase signalling in the absence of apoptosis drives Jnk-dependent invasion. _EMBO Rep_ 14, 172–177,
https://doi.org/10.1038/embor.2012.217 (2013). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Denise J. Montell, Tetsuya Tabata, Jiong
Chen, Yasushi Hiromi, Shigeo Hayashi, MichelleStarz-Gaiano, Ryu Ueda, Shu Kondo, Hua Tang, Qiang Wang, Tsinghua Fly Center, NIG-Fly, Kyoto stock and Bloomington Stock Center for providing
antibodies, fly strains and technical advice. This work was supported by the National Natural Science Foundation of China (31571502, 31602011) and Funds of “Shandong Double Tops” Program
(SYL2017YSTD09). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratory of Developmental Genetics, Shandong Agricultural University, Tai’an, Shandong, 271018, China Xian-Feng Wang, Yang
Shen, Qian Cheng, Chong-Lei Fu, Zi-Zhang Zhou & Qing-Xin Liu * Department of Developmental Genetics, National Institute of Genetics, Mishima, Shizuoka, 411-8540, Japan Susumu Hirose
Authors * Xian-Feng Wang View author publications You can also search for this author inPubMed Google Scholar * Yang Shen View author publications You can also search for this author
inPubMed Google Scholar * Qian Cheng View author publications You can also search for this author inPubMed Google Scholar * Chong-Lei Fu View author publications You can also search for this
author inPubMed Google Scholar * Zi-Zhang Zhou View author publications You can also search for this author inPubMed Google Scholar * Susumu Hirose View author publications You can also
search for this author inPubMed Google Scholar * Qing-Xin Liu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.F. Wang, S. Hirose and Q.X.
Liu designed research, X.F. Wang, Y. Shen, Q. Cheng and C.L. Fu performed experiments and X.F. Wang, Z.Z. Zhou, S. Hirose and Q.X. Liu analyzed data and wrote the manuscript. CORRESPONDING
AUTHORS Correspondence to Susumu Hirose or Qing-Xin Liu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION
PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY
INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution
and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if
changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the
material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Wang, XF., Shen, Y., Cheng, Q. _et al._ Apontic directly activates _hedgehog_ and _cyclin E_ for proper organ growth and patterning. _Sci Rep_ 7, 12470 (2017).
https://doi.org/10.1038/s41598-017-12766-w Download citation * Received: 04 April 2017 * Accepted: 14 September 2017 * Published: 29 September 2017 * DOI:
https://doi.org/10.1038/s41598-017-12766-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




