- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Oil-in-water emulsions are harmful to both humankind and environment. Frequent oil spill disasters make it urgent to develop low cost and high-efficiency materials for the treatment
of oil-in-water emulsions. In this study, we report the facile template-free synthesis of macroporous polystyrene (PS) monolith from PS solution using a thermally-induced phase separation
(TIPS) technique. The fabricated monolith showed high hydrophobicity, superoleophilicity, and macroporous structure. Moreover, the monolith exhibited high removal efficiency toward different
oil-in-water emulsions. The monolith can be fabricated from cheap and commonly-used plastic. Thus, we anticipate that this research will contribute to both the recycling of PS and the
treatment of oil spill accidents. SIMILAR CONTENT BEING VIEWED BY OTHERS PREPARATION OF ASYMMETRIC JANUS HOLLOW SILICA MICROPARTICLE AND ITS APPLICATION ON OILY WASTEWATERS Article Open
access 13 March 2023 FABRICATION OF WELL-DEFINED MAGNETIC MICROPOROUS POLYMERIC MONOLITHS USING SIMPLE NON-AQUEOUS EMULSIFICATION TECHNIQUE Article Open access 03 March 2025 DESIGNING
SUPER-FAST TRIMODAL SPONGES USING RECYCLED POLYPROPYLENE FOR ORGANICS CLEANUP Article Open access 29 August 2023 INTRODUCTION Oil spill accidents can cause serious environmental pollution to
ocean or coastal water. An example is the Gulf of Mexico oil spill accident occurred in 2010. In this accident, plenty of crude oil has been released to the environment. Without any
treatment, the spilled oil can last for many years, making incessant damage to the environment1. Compared with the oil that forms a separated layer from water, oil-in-water emulsion is much
more difficult to be removed. Hence, there are increasing requirements to develop an economic, facile, and effective method to remove oil-in-water emulsion. Some engineering techniques have
been developed for the oil spill clean-up, such as, _in situ_ burning2,3,4, dredging5, bioremediation6,7,8, skimming9, dispersants10, 11, and solidifying. However, these methods are less
effective for the removal of oil-in-water emulsion, and face the problem of high side effect toward the environment. This inspired material scientists to develop new absorption materials
that have the potential for the treatment of oil spill accident. The new material should be hydrophobic12,13,14 to avoid the invading of water. In another hand, the new material should be
oleophilic to adsorb the oils. Some new materials have been developed for example, superhydrophobic melamine sponge15, marshmallow-like macroporous gel16, 17, flexible polypropylene
sponge18, carbon nanofiber aerogel19, 20, carbon nanotube sponge21, 22, spongy graphene23, and antifouling membrane24. Polystyrene (PS) is one of the most widely used plastics, with an
industrial production of several billion kilograms per year. PS is a major component of plastic debris in the ocean, and a main reason for white pollution. Because of its hydrophobic and
oleophilic properties, PS shows high potential to be used as oil absorbent. A monolithic PS material with high surface area is highly demanded in the absorption application. To our best
knowledge, porous PS monolith has never been synthesized from its polymers. The reported PS monoliths were synthesized from monomers (styrene) using template25. Apart from its high cost, the
current method cannot contribute to the recycling of used PS. To solve the above problems, herein we present for the first time the fabrication of a PS monolith using the thermally-induced
phase separation (TIPS) technique26,27,28,29,30,31. The monolith was applied for the removal of oil-in-water emulsion in the low concentration range. We anticipated this new fabrication
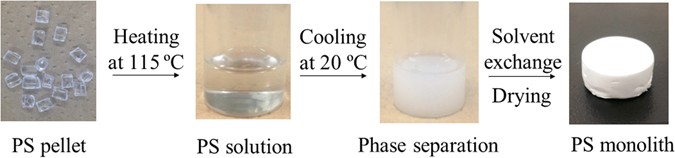
method will contribute to both the recycling of PS and the treatment of oil spill accidents. RESULTS AND DISCUSSION FABRICATION OF PS MONOLITH A mixture of decalin and 1-butanol was used as
the solvent for the fabrication of PS monolith (Fig. 1). PS pellet was dissolved in the solvent at 115 °C to form a homogenous solution. Subsequently, the solution was cooled at 20 °C. In
the cooling process, the solution transferred from transparent to white color, which indicated the formation of the monolith. The PS monolith was immersed into 2-propanol to remove the
embedded molecule and dried under vacuum. For the industrial application, cheap raw materials and a simple fabrication process are highly demanded. In this research, the polystyrene is low
cost, and the fabrication process is simple and convenient, indicating that the method is very suitable for industrial production. Both the solvent ratio and polymer concentration have great
effects on the formation of a PS monolith32. A porous monolith can be formed when the decalin ratio range from 40 to 70%. In this paper, all the samples were prepared in the mixed solvent
with fifty percent of decalin. The PS monolith can be formed if the polymer concentration is from 100 to 170 mg/mL. In case of a low concentration, the amount of polymer chains in a defined
space are not enough to form a firmly connected structure. If the polymer concentration is too high, the high viscosity of the polymer solution makes it hard to form a porous structure. To
get faster removal, a porous structure with high surface area is quite important. The morphology of the PS monolith was observed by SEM. As shown in Fig. 2, the monolith had relatively
uniform macrospores, with average pore sizes of 10 μm. The enlarged image (Fig. 2D) shows that the skeleton of the monolith was quite thin. The ultrathin skeleton and macroporous structure
indicate that the monoliths had low density. The density was measured to be 0.096 g/cm3 and the porosity was determined as 92% by gravimetry using the equation described in the literature33.
The macropore structure of the monolith is useful for the fast removal of oil. The mechanical property of the monolith is also important for the actual applications. The mechanical property
of the obtained PS monolith is good enough for the absorption application (data not shown). The monolith is free-standing and can sustain the stirring force in the absorption process,
indicating that the mechanical property of the monolith can fulfill the practical applications. The wetting property is also quite important for the oil absorption application. Figure 3B and
C show the contact angles of the PS monolith toward oil and water, respectively. The water contact angle was measured as 120°, indicating the high hydrophobicity of the monolith. The high
hydrophobicity demonstrates that the monolith can selectively repel water phase. At the same time, the oil contact angle was determined as 10°, indicating that the present monolith has the
absorption ability toward oil. SEPARATION OF OIL-IN-WATER EMULSION BY PS MONOLITH The specific wetting property of the PS monolith provides outstanding basis for the removal of oil-in-water
emulsion. Figure 4 shows the separation result of toluene-in-water emulsions. The milky white color of the feed solution indicates the existence of colloidal toluene (Fig. 4B). The optical
microscopy image illustrates that the sizes of colloids are around several micrometers (Fig. 4A). After absorption, the solution became transparent, similar to the color of pure water (Fig.
4C). In addition, no droplet was observed in the optical microscopy image (Fig. 4D), strongly implying that the toluene in the emulsion has been successfully removed. The UV absorption
spectra were measured to check the removal of colloidal toluene. As shown in Fig. 4E, before absorption, the characteristic absorption peaks of toluene were observed. However, none of the
peaks was observed in the spectrum of the removed aqueous solution, strongly indicating the high efficiency of the removal process. GC was applied to accurately measure the residual
concentration of toluene. The toluene content in the filtrate was as low as 40 ppm. The solubility of toluene in water at room temperature is 50 ppm. Those results strongly demonstrate that
not only the colloidal toluene but also part of dissolved toluene was removed by the PS monolith, indicating the high absorption efficiency. The high efficiency of the PS monolith depends on
the strong interaction of the polymer toward the oils. To determine the general absorption ability of the PS monolith, several common organic liquids were selected to prepare their
corresponding colloidal emulsions. For all the above organic liquids, colloidal emulsions were formed with the droplets of several micrometers. Figure 5 shows the removal efficiency of the
PS monolith toward different emulsions. In all the cases, The PS monolith exhibited high removal efficiency (more than 99%), strongly indicating that the absorption is general and the
monolith is useful for the treatment of practical spilled oil which always contains several types of oils. To demonstrate the possibility to reuse the monolith, the monolith was immersed in
plenty of 2-propanol. Subsequently, the monolith was dried under vacuum for 4 hours. After drying, the monolith was used for another cycle of absorption, we found that the monolith can be
used for another time, and can recover the oil in high efficiency. In a similar way, the monolith can remove the colloid toluene for more than 5 times, still with high efficiency (Fig. 6).
This means the combination of solvent exchange and vacuum drying is an effective method to remove the absorbed oils and release the polystyrene for another absorption cycle. The polystyrene
is a polymer that has strong interaction with the oils, making the monolith high affinity to adsorb them, leaving the residual oil of a quite low concentration, which is significant for the
high-efficient treatment of oil spill accident. However, the monolith cannot be used to absorb a large amount of oils at one time, in which case, the structure of the monolith will be
destroyed. This issue should be dissolved by further crosslinking of the monolith using some crosslinking method, such as the electron beam crosslinking. The further study is under
development in our group. CONCLUSIONS We have developed a facile and template-free method to synthesize a PS monolith with macroporous structure from its polymer solution. The fascinating
properties of the fabricated monolith including macroporous structure, high hydrophobicity, and superoleophilicity, are suitable for colloidal oil treatment. Because of its strong
interaction with the oils, the monolith can efficiently remove colloidal oils. Considering that the monolith is fabricated from polymer (PS) but not from monomers (styrene), the monolith
should contribute to the recycle of waste PS plastic. We believe that the present PS monolith will provide us a new tool for oil spill treatment and will contribute to the industrial
application. MATERIALS AND METHODS REAGENTS Decalin was supplied from Kanto Chemicals Co Inc. Polystyrene (Degree of polymerization: 2000), acetone, oil red, and 1-butanol were purchased
from Wako Co. All reagents were used as received without further purification. FABRICATION OF PS MONOLITH We prepared the PS monolith by the following procedure (Fig. 1). Three hundred
milligram of PS was dissolved in a mixed solvent containing 1 mL of decalin and 1 mL of 1-butanol at 115 °C. Subsequently, the solution was put at 20 °C to induce phase separation. The
obtained monolith was immersed into 2-propanol to remove the embedded solvent. The monolith was dried under vacuum for further characterization and application. EMULSION ABSORPTION
EXPERIMENT The oil-in-water emulsions were prepared by adding a certain amount of organic solvents (1 wt%) to water. Subsequently, the mixtures were emulsified for 5 minutes by a tip
sonicator (580 W, 30% of amplification). The obtained emulsion is stable for at least 1 hour without any significant phase separation. To carry the absorption measurement, 0.5 g of PS
monolith was cut into several pieces and was placed in a glass beaker filled with 5 ml of emulsions. The solution was stirred at a constant low speed. After absorption, the residual oil
concentration in the water was determined using gas chromatography. To demonstrate the recovery of the monolith, the monolith together with the absorbed oils was immersed in plenty of
2-propanol for solvent exchange. Subsequently, the monolith was put into the oven. After vacuum drying for 4 hours, the monolith was used for another cycle of adsorption. CHARACTERIZATIONS
The scanning electron microscope (SEM, Hitachi S-4800) was used to observe the morphology of the PS monolith at an accelerating voltage of 10 kV. The monoliths were fractured to small pieces
with the assistance of liquid nitrogen and fixed on a SEM sample stage. Subsequently, a thin layer of Pt film was sputtered onto the upper surface of the monoliths. Contact angles were
measured using an OCA20 contact-angle system (Data-physics, Germany). The concentration of residual oils in the aqueous phase was measured using gas chromatography (Shimadzu, GC-2014).
Optical microscopy images of the emulsions were taken using a BX 51TF Instec H601 (Olympus, Japan). REFERENCES * Bianchi, T. S. _et al_. Deepwater horizon oil in gulf of mexico waters after
2 years: transformation into the dissolved organic matter pool. _Environ. Sci. Technol._ 48, 9288–9297 (2014). Article ADS CAS PubMed Google Scholar * Evans, D. D., Mulholland, G. W.,
Baum, H. R., Walton, W. D. & McGrattan, K. B. _In situ_ burning of oil spills. _J. Res. Nat. Inst. Stand. Technol._ 106, 231–278 (2001). Article CAS Google Scholar * Lin, Q. X. _et
al_. _In-situ_ burning of oil in coastal marshes. 2. Oil spill cleanup efficiency as a function of oil type, marsh type, and water depth. _Environ. Sci. Technol._ 39, 1855–1860 (2005).
Article ADS CAS PubMed Google Scholar * Mullin, J. V. & Champ, M. A. Introduction/overview to _in situ_ burning of oil spills. _Spill Sci. Technol. Bull._ 8, 323–330 (2003). Article
CAS Google Scholar * Lt. George, H. & Burns, I. _et al_. Recovery of submergen oil at san juan, puerto rico 1994. _Int. Oil Spill Conf. Proc._ 1995, 551–557 (1995). Article Google
Scholar * Atlas, R. M. Microbial hydrocarbon degradation-bioremediation of oil-spill. _J. Chem. Technol. Biotechnol._ 52, 149–156 (1991). Article CAS Google Scholar * Bragg, J. R.,
Prince, R. C., Harner, E. J. & Atlas, R. M. Effectiveness of bioremediation for exxon-valdez oil-spill. _Nature_ 368, 413–418 (1994). Article ADS CAS Google Scholar * Prince, R. C.
Petroleum spill bioremediation in marine enviroments. _Crit. Rev. in Microbiol._ 19, 217–242 (1993). Article CAS Google Scholar * Fujita, I. & Yoshie, M. An onboard vacuum suction oil
skimming system. _Sea Technol._ 47, 49–52 (2006). Google Scholar * Kujawinski, E. B. _et al_. Fate of dispersants associated with the deepwater horizon oil spill. _Environ. Sci. Technol._
45, 1298–1306 (2011). Article ADS CAS PubMed Google Scholar * Lessard, R. R. & Demarco, G. The significance of oil spill dispersants. _Spill Sci. Technol. Bull._ 6, 59–68 (2000).
Article CAS Google Scholar * Hao, C. _et al_. Superhydrophobic-like tunable droplet bouncing on slippery liquid interfaces. _Nat_. _comm_. 7986 (2015). * Zhang, M. _et al_.
Superhydrophobic surface with hierarchical architecture and bimetallic composition for enhanced antibacterial activity. _ACS Appl. Mater. Inter._ 6, 22108–22115 (2014). Article CAS Google
Scholar * Liu, Y. _et al_. Pancake bouncing on superhydrophobic surfaces. _Nat. phys._ 10, 515–519 (2014). Article CAS PubMed PubMed Central Google Scholar * Ruan, C., Ai, K., Li, X.
& Lu, L. A superhydrophobic sponge with excellent absorbency and flame retardancy. _Angew. Chem. Int. Ed._ 53, 5556–5560 (2014). Article CAS Google Scholar * Hayase, G. _et al_. A
superamphiphobic macroporous silicone monolith with marshmallow-like flexibility. _Angew. Chem. Int. Ed._ 52, 10788–10791 (2013). Article CAS Google Scholar * Hayase, G., Kanamori, K.,
Fukuchi, M., Kaji, H. & Nakanishi, K. Facile synthesis of marshmallow-like macroporous gels usable under harsh conditions for the separation of oil and water. _Angew. Chem. Int. Ed._ 52,
1986–1989 (2013). Article CAS Google Scholar * Wang, G. & Uyama, H. Facile synthesis of flexible macroporous polypropylene sponges for separation of oil and water. _Sci. Rep._ 6,
21265 (2016). Article ADS PubMed PubMed Central Google Scholar * Bi, H. _et al_. Carbon microbelt aerogel prepared by waste paper: an efficient and recyclable sorbent for oils and
organic solvents. _Small_ 10, 3544–3550 (2014). Article CAS PubMed Google Scholar * Wu, Z.-Y., Li, C., Liang, H.-W., Chen, J.-F. & Yu, S.-H. Ultralight, flexible, and fire-resistant
carbon nanofiber aerogels from bacterial cellulose. _Angew. Chem. Int. Ed._ 52, 2925–2929 (2013). Article CAS Google Scholar * Gui, X. _et al_. Carbon nanotube sponges. _Adv. Mater._ 22,
617 (2010). Article CAS PubMed Google Scholar * Gui, X. _et al_. Recyclable carbon nanotube sponges for oil absorption. _Acta Mater._ 59, 4798–4804 (2011). Article CAS Google Scholar
* Bi, H. _et al_. Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. _Adv. Funct. Mater._ 22, 4421–4425 (2012). Article CAS Google Scholar * Zhao,
Y., Zhang, M. & Wang, Z. Underwater superoleophobic membrane with enhanced oil–water separation, antimicrobial, and antifouling activities. _Adv. Mater. inter._ 3, 1500664 (2016).
Article Google Scholar * Jing, P., Fang, X., Yan, J., Guo, J. & Fang, Y. Ultra-low density porous polystyrene monolith: facile preparation and superior application. _J_. _Mater_.
_Chem_. _A_ 1 (2013). * Okada, K. _et al_. Fabrication of mesoporous polymer monolith: a template-free approach. _Chem. Commun._ 47, 7422–7424 (2011). Article CAS Google Scholar * Wang,
G., Yoshikawa, H., Tamiya, E. & Uyama, H. Mesoporous poly(ethylene-co-vinyl alcohol) monolith captured with silver nanoparticles as a SERS substrate: facile fabrication and ultra-high
sensitivity. _RSC Adv._ 5, 25777–25780 (2015). Article CAS Google Scholar * Wang, G. & Uyama, H. Reactive poly(ethylene-co-vinyl alcohol) monoliths with tunable pore morphology for
enzyme immobilization. _Colloid Polym. Sci._ 293, 2429–2435 (2015). Article CAS Google Scholar * Wang, G., Xin, Y. & Uyama, H. Facile fabrication of mesoporous poly(ethylene-co-vinyl
alcohol)/chitosan blend monoliths. _Carbohydr. Polym._ 132, 345–350 (2015). Article CAS PubMed Google Scholar * Wang, G., Kundu, D. & Uyama, H. One-pot fabrication of palladium
nanoparticles captured in mesoporous polymeric monoliths and their catalytic application in C-C coupling reactions. _J. Colloid Interf. Sci._ 451, 184–188 (2015). Article ADS CAS Google
Scholar * Wang, G., Xin, Y., Han, W. & Uyama, H. Immobilization of catalase onto hydrophilic mesoporous poly(ethylene-co-vinyl alcohol) monoliths. _J. Appl. Polym. Sci._ 132, 42556
(2015). Google Scholar * Xin, Y., Fujimoto, T. & Uyama, H. Facile fabrication of polycarbonate monolith by non-solvent induced phase separation method. _Polymer_ 53, 2847–2853 (2012).
Article CAS Google Scholar * Ikeda, R. _et al_. The effect of porosity and mechanical property of a synthetic polymer scaffold on repair of osteochondral defects. _Int. Orthop._ 33,
821–828 (2009). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study is financially supported by a Nanshan District Key lab for Biopolymers and safety evaluation
(No.KC2014ZDZJ0001A). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Nanshan District Key Lab for Biopolymers and Safety Evaluation, Shenzhen Key Laboratory of Polymer Science and Technology,
Guangdong Research Center for Interfacial Engineering of Functional Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518060, P.R. China Guowei Wang
& Shiguo Chen * Key Laboratory of Optoelectronic Devices and Systems of Ministry of Education and Guangdong Province, College of Optoelectronic Engineering, Shenzhen University,
Shenzhen, 518060, P.R. China Guowei Wang & Bin Yu * Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, 565-0871, Japan Guowei Wang & Hiroshi
Uyama Authors * Guowei Wang View author publications You can also search for this author inPubMed Google Scholar * Bin Yu View author publications You can also search for this author
inPubMed Google Scholar * Shiguo Chen View author publications You can also search for this author inPubMed Google Scholar * Hiroshi Uyama View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS G.W. designed this research. B.Y. and G.W. performed the experiments and prepared figures. G.W., S.G., and H.U. wrote the manuscript.
CORRESPONDING AUTHOR Correspondence to Shiguo Chen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S
NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, G., Yu, B., Chen, S. _et al._ Template-free synthesis of
polystyrene monoliths for the removal of oil-in-water emulsion. _Sci Rep_ 7, 6534 (2017). https://doi.org/10.1038/s41598-017-06572-7 Download citation * Received: 12 October 2016 * Accepted:
08 June 2017 * Published: 26 July 2017 * DOI: https://doi.org/10.1038/s41598-017-06572-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative









