- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The role of the P2Y6 receptor in bladder function has recently attracted a great deal of attention in lower urinary tract research. We conducted this study to determine
contributions of the P2Y6 receptor in lower urinary tract function of normal phenotypes by comparing P2Y6-deficient mice and wild-type mice. In _in vivo_ experiments, P2Y6-deficient mice had
more frequent micturition with smaller bladder capacity compared to wild-type mice; however, there was no difference between these groups in bladder-filling pressure/volume relationships
during cystometry under decerebrate, unanaesthetized conditions. Analysis of _in vivo_ bladder contraction revealed significant difference between the 2 groups, with P2Y6-deficient mice
presenting markedly shorter bladder contraction duration but no difference in peak contraction pressure. However, analysis of _in vitro_ experiments showed no P2Y6 involvements in
contraction and relaxation of bladder muscle strips and in ATP release by mechanical stimulation of primary-cultured urothelial cells. These results suggest that the P2Y6 receptor in the
central nervous system, dorsal root ganglion, or both is involved in inhibition of bladder afferent signalling or sensitivity in the pontine micturition centre and that the receptor in the
detrusor may be implicated in facilitation to sustain bladder contraction force. SIMILAR CONTENT BEING VIEWED BY OTHERS TGR5 AGONISTS INDUCE PERIPHERAL AND CENTRAL HYPERSENSITIVITY TO
BLADDER DISTENSION Article Open access 15 June 2022 BLADDER OUTLET OBSTRUCTION DISRUPTS CIRCADIAN BLADDER FUNCTION IN MICE Article Open access 14 July 2020 EXCITATORY PURINERGIC AND
CHOLINERGIC EXPRESSION CHANGED IN A PARTIAL BLADDER OUTLET OBSTRUCTION-INDUCED OVERACTIVE BLADDER RAT MODEL Article Open access 26 October 2023 INTRODUCTION P2Y receptors are
G-protein-coupled receptors for extracellular nucleotides1, which are classified into eight P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11–14)2,3,4. P2Y receptors are found in various
animal species, implying their development early in evolution5. The P2Y6 receptor is a uridine diphosphate (UDP)-preferring receptor and is partially responsive to uridine-5′-triphosphate
(UTP) and adenosine diphosphate (ADP) but not responsive to adenosine triphosphate (ATP)4, 6. The receptor couples to Gq and induces inositol lipid signalling through phospholipase C-β
isozymes6. The P2Y6 receptor is widely distributed in various tissues including placenta, thymus, spleen, kidney, vascular smooth muscle, lung, small intestine, bone, fat cells, spinal cord
and brain2, 4, 7, and is expressed in many kinds of cells such as intestinal epithelial cells, T cells (affected T cells), monocytes, microglia, vascular endothelial cells, cardiomyocytes,
smooth muscle cells and neurones2, 4, 7,8,9. Thus, this receptor is implicated in a variety of pathophysiological conditions such as immune responses, pro-inflammatory responses, astrocyte
apoptosis, effector T cell activation, phagocytosis, neuropathic pain2, 4, 10,11,12. Extensive studies have suggested that the use of P2Y6 antagonists may be a useful strategy to treat
diseases related to inflammation, neurodegeneration and nociception10, 12,13,14,15. The potential role of the P2Y6 receptor in lower urinary tract function has been raised by recent studies
that have demonstrated functional involvements of the receptor in urothelial signalling16, 17 and detrusor contractility18 of the urinary bladder. Carneiro and co-workers showed that an
activation of the urothelial P2Y6 receptor increases voiding frequency during cystometry in the anaesthetized rat by facilitating ATP release from the bladder urothelium to stimulate
suburothelial afferent nerves16. Yu and co-workers demonstrated that the P2Y6 activation by UDP enhances P2X-mediated bladder smooth muscle contractility18. While these studies have been
conducted to determine functions of the P2Y6 receptor in the bladder, roles of the receptor in the central nervous system (CNS) and the peripheral nervous system (PNS) (i.e., in the reflex
micturition circuitry) are still unknown. While blockade of the P2Y6 receptor in the bladder has been proposed to be therapeutically useful for alleviating persistent storage symptoms
induced by pathological bladder19, the effect of the systemic receptor blockade on physiological functions needs clarification. Thus, we performed the present study using P2Y6-knockout (KO)
mice to determine the contributions of the P2Y6 receptor in lower urinary tract function of wild-type (WT) phenotypes. Recently, we have developed dual voiding function analysis of both
voluntary voiding behaviour in metabolic cage and unanaesthetized reflex micturition during cystometry20. A combination of a genetically modified mouse and this novel approach has allowed us
to perform detailed evaluation of targeted-gene phenotypes in lower urinary tract function of the small rodent. First, we evaluated _in vivo_ lower urinary tract activity by dual voiding
function analysis and found significant differences between P2Y6-KO mice and WT mice. Second, to determine whether the differences in the _in vivo_ experiment results were attributable to
bladder local mechanisms, we compared the 2 groups by examining the contraction and relaxation of _in vitro_ bladder muscle strips in response to pharmacologic treatments and by evaluating
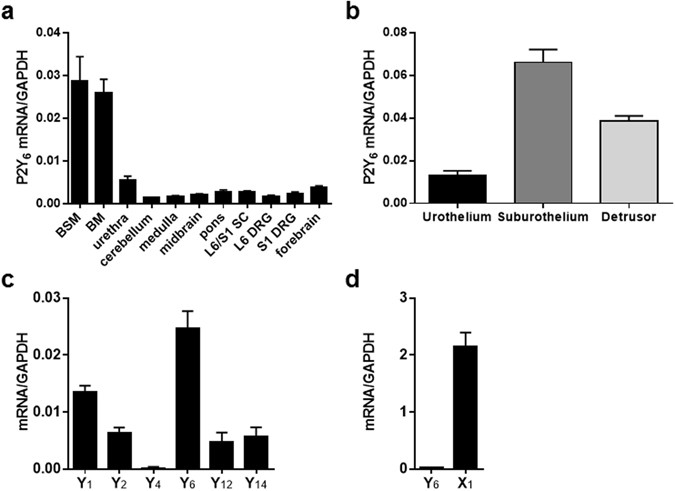
ATP release from primary-cultured urothelial cells in response to mechanical stimulation. RESULTS MRNA EXPRESSION OF P2Y6 AND OTHER P2 SUBTYPES Real-time RT-PCR analysis was performed to
examine P2Y6 mRNA expression in body tissues associated with the control of lower urinary tract function in normal (i.e., WT) mice. The expression of P2Y6 mRNA was widely distributed in the
central nervous system (CNS), L6/S1 dorsal root ganglion (DRG), bladder and urethra (Fig. 1a). Gene expression was abundant in the lower urinary tract, especially in the bladder. The rank
order of P2Y6 mRNA expression in the bladder was as follows: suburothelium > detrusor > urothelium (Fig. 1b). Comparisons of mRNA expression levels of P2Y6 and other purinergic
receptor subtypes were performed in the detrusor, where we were primarily intrigued by a previous study that examined the role of P2Y6 in the modulation of bladder muscle tone18. As shown in
Fig. 1c, P2Y6 mRNA was expressed to the greatest extent among P2Y subtypes. Furthermore, the expression level of P2X1 mRNA was nearly 100 times higher than that of P2Y6 mRNA (Fig. 1d).
Western blotting and immunostaining to determine protein expression and localization, respectively, of P2Y6 receptors were reserved because currently commercially available antibodies to the
P2Y6 receptor are unreliable and lack specificity for the receptor21. ANALYSIS OF VOLUNTARY VOIDING BEHAVIOUR IN METABOLIC CAGES Figure 2a shows representative recording charts of 48-h
voluntary voiding behaviour and water intake in a WT mouse (top) and a P2Y6-KO mouse (bottom). Water intake (Fig. 2b), urine output volume (Fig. 2c), and voiding frequency (Fig. 2d) were
evaluated for 24 h (12 h in the dark period and 12 h in the light period). While there were no differences between the 2 groups in water intake in the light period and in 24 h, water intake
in the dark period was greater in P2Y6-KO mice than in WT mice (Fig. 2b). No differences in urine output in any period were found between the 2 groups (Fig. 2c). In all periods, the voiding
frequency of P2Y6-KO mice was markedly higher than that of WT mice (Fig. 2d). Comparisons between WT and P2Y6-KO mice in urine volume/voiding (Fig. 2e), voiding duration (Fig. 2f), and mean
uroflow rate (Fig. 2g) (i.e., voiding related variables) were performed. Urine volume/voiding in P2Y6-KO mice was significantly less than that in WT mice (Fig. 2e). There were no differences
in urine volume/voiding between the dark period and the light period in P2Y6-KO mice (126.5 ± 14.5 µl and 136.1 ± 26.4 µl, respectively; P = 0.57, by a Wilcoxon matched-pairs signed rank
test, n = 9) and WT mice (313.8 ± 47.8 µl and 413.4 ± 83.8 µl, respectively; P = 0.11, n = 7). Voiding duration in P2Y6-KO mice was significantly shorter than that in WT mice (Fig. 2f).
There were no differences in mean uroflow rates between the 2 groups (P = 0.24, by Mann-Whitney test; Fig. 2g). EVALUATION OF UNANAESTHETIZED REFLEX ACTIVITY OF THE LOWER URINARY TRACT
DURING CYSTOMETRY Figure 3a shows representative cystometry recordings of a WT mouse (top) and a P2Y6-KO mouse (bottom). Cystometry parameters evaluated in this study were described in a
previously published article20. In addition, to provide a better evaluation of bladder contractions, we defined other parameters as shown in Fig. 3b. P2Y6-KO mice were compared with WT mice
in parameters associated with the bladder-filling phase and bladder contraction phase, as shown in Table 1. There were no differences between the 2 groups in increases of bladder contraction
pressure such as maximal voiding pressure (MVP) and closing peak pressure (CPP; P = 0.07 and P = 0.11, respectively, unpaired t-test), whereas the bladder contraction duration (BCD) was
markedly reduced in P2Y6-KO mice compared with WT mice (Table 1). Figure 3c shows bladder contraction traces enlarged in the abscissa of a WT mouse (top) and a P2Y6-KO mouse (bottom).
Detailed analysis of a bladder contraction phase (Fig. 3b) showed that p1, p2 and p4 of 4 phases in BCD were markedly shorter in P2Y6-KO mice than those in WT mice (Table 2). P2Y6-KO mice
had smaller voided volume (VV) and smaller volume thresholds for inducing micturition contraction (VT) compared with WT mice (Table 1). Post-void residual volume (RV) and voiding efficiency
(VE) were also smaller in P2Y6-KO mice than in WT mice (Table 1). The small but statistically significant reduction of VE in P2Y6-KO mice, despite smaller RV in P2Y6-KO mice compared with WT
mice, was due to a greater reduction ratio in VV (59% smaller) than in VT (58% smaller). Pressure thresholds for inducing micturition contraction (PT) and bladder compliance (BCP) in
P2Y6-KO mice were lower than those in WT mice. However, these 2 variables were dependent on intravesical volume at the time-point of examination (Fig. 3d,e), thus suggesting there was no
difference between the 2 groups in the relationship of bladder pressure or BCP against intravesical volume. Moreover, VT and VV were correlated with MVP in WT (Fig. 3f) and P2Y6-KO mice
(Fig. 3g), showing that the bladder contraction pressure during micturition is urination volume-dependent. The results are consistent with a previous study showing a significant correlation
between MVP and a variable substitute for VV or VT in male mice (but not in female mice)22. Small but statistically significant elevation in post-void resting pressure (RP) was detected in
P2Y6-KO mice compared with WT mice; however, the underlying mechanism for the difference is unknown. CONTRACTILE RESPONSE TO CARBACHOL, ELECTRICAL FIELD STIMULATION (EFS), OR ATP OF _IN
VITRO_ BLADDER MUSCLE STRIPS Bladders excised from WT mice and P2Y6-KO mice were examined by haematoxylin and eosin (H&E) staining, which revealed that the bladder of the P2Y6-KO mouse
presents no morphological abnormalities (e.g., thickening of muscle layer, edematous change, and granulation) in comparison with that of the WT mouse (n = 2 for each group) (Supplementary
Fig. 1S). Before preparation of detrusor strips for contraction experiments, each body of bladder was weighed: the weights of WT (body weight: 25.4 ± 0.6 g) and P2Y6-KO (28.1 ± 0.6 g) mice
were 11.6 ± 0.8 mg and 13.5 ± 1.0 mg, respectively, showing no difference between the two groups (n = 16 for each group, P = 0.14, unpaired t-test). Parasympathetic innervation to the
bladder muscle produces, with acetylcholine and ATP, a bladder contraction during which the micturition occurs. First, an effect of 60 mM KCl was examined to demonstrate the viability of
bladder muscle strips, showing no difference between WT and P2Y6-KO mice (Fig. 4a,b). To compare WT and P2Y6-KO mice in bladder muscle strip contractility responding to cholinergic
stimulation, the effect of carbachol (3 nM to 100 µM) was examined and the concentration-response curves were constructed (Fig. 4c,d). No difference was found between the 2 groups (P = 0.69,
F-test). Contractions were evoked by electrical field stimulations (at 1 Hz, 2 Hz, 4 Hz, 8 Hz, 16 Hz and 32 Hz) in the presence or absence of atropine (10 µM) to compare WT and P2Y6-KO mice
in muscarinic contributions to the generation of the contraction (Fig. 4e). No difference in EFS-evoked contractions in either the presence or absence of atropine was found between the 2
groups (Fig. 4f,g). In addition, there was no difference between the 2 groups in the degree of contraction force changed by atropine (Fig. 4h). Furthermore, a contraction evoked by ATP (1
mM) of the bladder muscle strip was compared between a WT mouse and a P2Y6-KO mouse (Fig. 4i). There were no differences between the 2 groups in peak contraction force (Fig. 4j) and in
duration between the time-points of the peak contraction force and the subsequent baseline, showing 33.1 ± 6.0 s for WT mice and 35.1 ± 6.4 s for P2Y6-KO mice (P = 0.96, by Mann-Whitney
test). RELAXING EFFECT OF Β-ADRENERGIC AGONIST ON A PRE-CONTRACTED _IN VITRO_ BLADDER MUSCLE STRIP Sympathetic innervation to the bladder smooth muscle facilitates storage accommodation
during bladder-filling _via_ β-adrenoceptors. To examine if P2Y6 interacts with β-adrenergic modulation, we compared WT and P2Y6-KO mice with respect to the relaxation of pre-contracted
bladder strips in response to isoproterenol (non-selective β-agonist) or BRL 37344 (selective β3-agonist). Isoproterenol (0.1 µM, 0.3 µM, 1 µM and 3 µM) relaxed 3 µM carbachol-induced
contractions of bladder muscle strips of WT and P2Y6-KO mice in a concentration-dependent manner (Fig. 5a). Likewise, BRL 37344 (3 µM and 10 µM) produced concentration-dependent relaxation
of bladder muscle strips in both groups (Fig. 5b). There was no difference between the 2 groups with respect to bladder muscle relaxation induced by either isoproterenol (P = 0.47, by
repeated measures two-way ANOVA) or BRL 37344 (P = 0.38; Fig. 5c). STRETCH-INDUCED ATP RELEASE FROM PRIMARY-CULTURED UROTHELIAL CELLS Bladder urothelium releases ATP upon stretch
stimulation, which mediates signalling to primary afferent neurons _via_ purinergic receptors23. To determine whether activation of P2Y6 is required for the stretch-induced ATP release from
urothelial cells, we measured the amount of extracellular ATP following mechanical cell stretch stimulation by using an ATP photon imaging system. Urothelial cells on a stretch chamber were
extended using the following conditions: stretch distance of 100 µm, 200 µm or 300 µm, and stretch speed of 100 µm/s. Upon stretch stimulation, prominent ATP release occurred in WT cells and
P2Y6-KO cells, both of which were comparable in amount (Fig. 6a,b). In addition, we compared the stretch-induced ATP release from WT cells between the presence and absence of a P2Y6
agonist, UDP (100 µM). As shown in Fig. 6c, the agonist did not affect the ATP release. DISCUSSION Compared with WT mice, P2Y6-KO mice showed markedly higher micturition frequency and
smaller urination volume/voiding during voluntary voiding behaviour. In reflex micturition cycles during cystometry under decerebrate, unanaesthetized conditions, P2Y6-KO mice had markedly
smaller bladder capacity than WT mice. No differences in the bladder pressure-volume relationship (i.e., during bladder-filling) and the peak contraction pressures (i.e., during bladder
contraction presenting micturition) were found between the 2 groups, whereas the bladder contraction duration was prominently reduced in P2Y6-KO mice. There were no differences between the 2
groups with respect to contraction of _in vitro_ bladder muscle strips induced by KCl, carbachol, ATP or EFS and with respect to relaxation by a β-adrenergic agonist of pre-contracted
bladder muscle strips. Also, no difference in ATP release evoked by stretch stimulation of primary-cultured urothelial cells was found between these groups. Thus, the _in vivo_ dual voiding
function analysis of voiding behaviour and reflex micturition demonstrated that the P2Y6-deficiency is associated with frequent micturition, decreased voiding volume and early attenuation of
bladder contractility, whereas the _in vitro_ experiments show no apparent involvement of the P2Y6 receptor either in contraction and relaxation of bladder smooth muscle or in ATP release
from urothelial cells in response to mechanical stimulation. In response to mechanical stimulation (i.e., bladder distension), urothelial cells in bladder urothelium release ATP that
activates P2X3 receptors in suburothelial nerve plexus to induce firing of the bladder afferent that conveys the mechanosensory signals to the CNS24,25,26. As an intravesical volume
increases, the afferent firing in the pelvic nerve from the bladder gradually increases, which activates spinobulbospinal reflex pathways that pass through the pontine micturition centre
(PMC) that functions as an “on-off” switch to trigger micturition27, 28. Thus, first of all, it should be considered that the cause of increased micturition frequency and decreased bladder
capacity are attributable to increased excitability of afferent sensory pathway or decreased threshold in activation of the PMC in the micturition reflex pathway. Urinary continence is
maintained by the sympathetic outflow in the hypogastric nerve from the thoracolumbar sympathetic nucleus to the bladder neck/proximal urethra and by the pudendal outflow from the
lumbosacral motor nucleus to the external urethral sphincter27, 28. Disruption of the continence mechanism causes early response in releasing the intravesical fluid. Therefore, secondly,
possible involvement of urethra function with respect to the frequent micturition and small voiding volume should be also considered. Our real-time RT-PCR analysis determined gene expression
of the P2Y6 subtype in urothelium, suburothelium and detrusor (Fig. 1). A previous immunolabelling study demonstrated that, of P2Y receptor subtypes, the P2Y6 receptor is most abundantly
expressed in urothelial cells of the guinea-pig bladder29. These results suggest the possibility that the receptor plays a role in homeostatic or pathophysiological function in the bladder.
The present experiments using a previously established ATP measurement technique30,31,32, however, showed that neither P2Y6-deficiency nor the P2Y6 agonist affects ATP release by stretch
stimulation of primary-cultured urothelial cells, suggesting that the urothelial P2Y6 receptor is not physiologically involved in mediation of ATP release in response to mechanical
stimulation. Thus, deletion of the P2Y6 receptor in the urothelium is not likely to be the cause of bladder overactivity in the _in vivo_ experiments. The bladder pressure-volume
relationship during cystometry indicated that the P2Y6 receptor is not involved in modulation of bladder tone during bladder-filling. _In vitro_ experiments examining relaxation of
pre-contracted bladder muscle strips in response to the β-adrenergic agonist showed that deletion of the P2Y6 receptor does not affect the postsynaptic, sympathetic regulation of bladder
muscle tone. These results suggest that the P2Y6 receptor is not associated with change in bladder muscle tension that might influence on the bladder afferent firing. The bladder efferent
limb (i.e., pathway that conveys signals from the PMC) that mediates bladder motility is not considered to directly participate in regulation of micturition frequency. ATP and acetylcholine
are the major transmitters from the bladder efferent nerve terminals to induce detrusor contraction25. It is unknown whether ATP or acetylcholine from the urothelium can directly stimulate
the detrusor to contract. Even if there is such mechanism, the present results showed that P2Y6-deficiency also does not change the contraction of _in vitro_ bladder muscle strips in
response to activation _via_ ATP or acetylcholine. The participation of suburothelial myofibroblasts _via_ the P2Y6 receptor in facilitating propagation of sensory signalling by enhancing
individual cellular responses has been proposed29, 33. However, it is not likely that this mechanism is involved in the bladder overactivity because deletion of the suburothelial P2Y6
receptor would be expected to reduce the bladder afferent firing. _In vivo_ micturition variables are greatly influenced, not only by bladder activity, but also by urethra function34,35,36.
In the present study, voluntary voiding behaviour analysis showed that there is no difference between WT and P2Y6-KO mice in uroflow rate (Fig. 2h) and cystometry evaluation revealed that
post-void residual volume is not increased in P2Y6-KO mice, compared with WT mice (Table 1). These results showed that the coordinated expelling function of the bladder and urethra is well
preserved in P2Y6-KO mice, thus suggesting that systemic deletion of the P2Y6 receptor does not disturb micturition. The previous study by Otomo and co-workers using dual recording of
isovolumetric bladder pressure and simultaneous urethral pressure in urethane-anaesthetized rats showed an intra-arterial (i.a.) injection of RB-2, a non-selective P2Y antagonist, increases
urethral resting pressure (i.e., urethral tone relative to bladder-filling phase during cystometry) but does not change maximal urethral relaxation (i.e., decreased urethra pressure relative
to the bladder contraction/voiding phase during cystometry)37, suggesting the possibility that blockade of P2Y receptors in urethra facilitates urinary continence and does not disturb
micturition. The latter (i.e., no disturbance in micturition) supports our present result and the former (i.e., facilitation in continence) suggests that blockade of the P2Y6 receptor in
urethra would not be involved in frequent micturition, although not only the P2Y6 but also other P2Y receptor subtypes are non-selectively blocked in their study. Taken together, our results
strongly suggest that the site responsible for the bladder activity caused by systemic deletion of the P2Y6 receptor is present, not in bladder and urethra, but in the CNS, dorsal root
ganglion (DRG) or both that is involved in the micturition reflex pathway. Previous studies from other laboratory showed that an intravesical infusion of PSB0474, a P2Y6 agonist, increases
micturition frequency during cystometry in urethane-anaesthetized rats16, 17. The proposed mechanism for the bladder overactivity was that ATP release from the urothelium facilitated by the
agonist activates P2X3 receptors in suburothelial afferent nerves and causes the hyperreflexia. Controversy may be raised with respect to the inconsistency between these studies with the
agonist and our present results using P2Y6-KO mice, both of which show bladder overactivity. However, the discrepancy can be attributable to the species difference because the results of our
experiments measuring stretch-induced ATP release from primary-cultured urothelial cells revealed that the P2Y6 receptor in the mouse urothelium is not important in mediation of the ATP
release. Also, in contrast with their studies showing that the prominent bladder overactivity is induced by activation of the P2Y6 receptor only in bladder and urethra, our results
demonstrating the hyperreflexia associated with systemic deletion of the P2Y6 receptor suggest the importance of the receptor involvement in the CNS or the PNS. Moreover, bladder
overactivity or instability generated by the locally-administered P2Y6 agonist may be owing to decrease in urethral tonus38,39,40, in addition to activation by the increased intravesical ATP
of the suburothelial P2X3-signalling. ATP induces relaxation of urethra smooth muscle in various animal species41,42,43,44,45. The previous study by Otomo and co-workers demonstrated that
i.a. injection of ATP decreases urethral resting pressure (i.e., urethra pressure to maintain continence) without affecting urethral relaxation during a bladder contraction37. Activation of
urethral afferent pathway by urine leakage into the proximal urethra/bladder neck facilitates a voiding reflex46, thus suggesting that a decrease in urethral tonus during bladder-filling
would result in early release of the bladder fluid. In agreement with this urethral mechanism, i.a. administration of sodium nitroprusside, a nitric oxide donor, which induces urethral
relaxation with neither affecting bladder contractility nor stimulating bladder afferent transmission, produced prominent bladder overactivity in conscious rats34. Similarly, α-bungarotoxin,
which produces urethral striated muscle relaxation without affecting autonomic bladder pathways, given i.v. markedly increased micturition frequency in decerebrate, unanaesthetized rats35.
Thus, it is suggested that intravesical ATP release facilitated by the P2Y6 agonist, which is perfused into intra-urethra during each voiding, induces urethral relaxation, resulting in
bladder overactivity. The P2Y6 receptor is likely to be involved in modulation of bladder contraction force and subsequent relaxation. The detailed analysis of bladder contraction during
cystometry revealed that P2Y6-KO mice had shorter durations at phases of bladder pressure increase that leads to micturition (i.e., p1 of BCD), bladder pressure decrease accompanying fluid
release (i.e., p2 of BCD), and bladder pressure decrease that terminates the contraction (i.e., p4 of BCD) compared with WT mice, whereas these mice showed no difference from WT mice at the
phase of bladder pressure increase immediately after expelling the fluid (i.e., p3 of BCD). Shorter duration in p1 or p4 is due to direct or indirect influence of P2Y6-deficiency on bladder
smooth muscle, whereas that in p2 is likely to be largely caused by smaller voiding volume in P2Y6-KO mice. The shorter p4 duration in P2Y6-KO mice is supported by the previous study by Yu
and co-workers18. The investigators showed that activation of the P2Y6 receptor enhances the P2X1-mediated contractile force and induces a sustained increase in _in vitro_ bladder smooth
muscle tone, further suggesting that the synergistic interaction between the P2X1 response and P2Y6 signalling is conducted through the phospholipase C (PLC)/inositol trisphosphate (IP3)
pathway. In agreement with their result18, the present cystometry experiments showed that bladders of P2Y6-KO mice are unable to sustain the contraction force for as long a duration as those
of WT mice. In addition, it is possible that the bladder relaxation during p4 of P2Y6-KO mice is indirectly enhanced by altered contributions of other P2Y subtypes due to the
P2Y6-deficiency. For example, P2Y1 mRNA is expressed in rat urinary bladders47 and an activation of P2Y1 inhibits acetylcholine release from parasympathetic, cholinergic nerve terminals48,
which would change bladder contractility. P2Y1 receptor mRNA is markedly increased in the aorta of P2Y6-KO mice49. Likewise, P2Y1 expression may be increased in urinary bladders of P2Y6-KO
mice, resulting in augmented implications of the receptor. Upon the use of genetically mutated animals, it is important to consider the possibility that other genes that have not been
deliberately manipulated can be subsequently affected. The results of the present study suggest that the P2Y6 phenotypes of the mouse lower urinary tract function are associated with an
inhibition of excitatory bladder afferent signalling or of sensitivity in the PMC and with a facilitation of sustaining bladder contraction force. The P2Y6 receptor in the CNS (not including
the forebrain), DRG or both is implicated in regulation of urinary frequency, whereas that in the detrusor is likely to be involved in modulation of bladder contractility. The P2Y6 receptor
has been found in the DRG12 and the spinal dorsal horn10, indicating that the receptor is involved in sensory transmission. In fact, previous studies showed central and peripheral
involvements of the P2Y6 receptor in development of neuropathic pain10, 12; however, it is unknown whether the receptor participates in normal sensory transmission. The use of P2Y6 receptor
antagonists may be promising in alleviating neuropathic pain and symptoms associated with inflammation and neurodegeneration10, 12,13,14,15; while, our study claims that great attention must
be paid to development of the pharmacotherapy for possible adverse impact of frequent urination. Further study is necessary to determine the P2Y6 site responsible for controlling the lower
urinary tract function. Moreover, it is of interest to investigate, in other species including humans, whether malfunction or blockade of the P2Y6 receptor generates lower urinary tract
symptom such as bladder overactivity. MATERIALS AND METHODS ANIMALS All animals in this study were obtained, housed, cared for and used in accordance with the “Guiding Principles in the Care
and Use of Animals in the Field of Physiologic Sciences” published by the Physiologic Society of Japan. In addition, all experimental protocols were approved by the Institutional Animal
Care and Use Committee of the University of Yamanashi (Chuo, Yamanashi, Japan). All efforts were made to minimize animal suffering and reduce the number of animals used. Experiments were
conducted using 8- to 12-week-old male C57BL/6N mice (SLC, Shizuoka, Japan). A pair of P2Y6-KO mice were generously provided by Dr. Bernard Robaye (Universite Libre de Bruxelles, Brussels,
Belgium). These mice were backcrossed (for more than 8 generations) on a C57BL/6N background. Before each experiment, the mice were housed under a 12:12-h light-dark cycle with controlled
humidity and temperature, and they were provided with food and water _ad libitum_. QUANTITATIVE REAL-TIME PCR Quantitative real-time PCR assays were performed on mouse specimens as
previously described50. The primer sequences for amplification are shown in Supplementary Table 1S. ANALYSIS OF VOLUNTARY VOIDING PATTERN IN METABOLIC CAGES Evaluations of voluntary voiding
behaviour were conducted according to a previously published method20. In brief, conscious mice were individually placed in novel, patented metabolic cages (Shinfactory Co. Ltd., Fukuoka,
Japan) constructed in a soundproof room at 25 °C with a 12:12-h light-dark cycle. Each mouse was provided with free access to food and water. After an acclimation period of 3 days in the
cage, data on voided urine (weight and timing) and water consumption (volume and timing) were continuously collected for each mouse over 2 days using a Power-Lab data-acquisition system (AD
Instruments, Colorado Springs, CO, USA). EVALUATION OF REFLEX MICTURITION CYCLE DURING CYSTOMETRY Urodynamic analysis was conducted in decerebrate, unanaesthetized mice according to a
previously published method20. Animals were anaesthetized with sevoflurane (2.5–5%) in O2 (flow rate: 0.2 l/min) during surgery. After a tracheal cannulation with a polyethylene tube (PE-90,
Clay-Adams, Parsippany, NJ) to facilitate respiration, the precollicular decerebration was performed51: both carotid arteries are ligated, followed by a midline incision of the head skin
with a scalpel and by removal of the skull and the forebrain with a fine rongeur and a blunt spatula, respectively. Sevoflurane was then discontinued. After no further intracranial
hemorrhage was detected visually, the lateral flaps of the incised head skin were sutured together. Cystometry was performed by a continuous infusion of room temperature physiological saline
(10 µl/min) into the bladder _via_ the dome to elicit repetitive voids, which allowed for data collection from a number of micturition cycles. Fluid voided from the urethral meatus was
collected and measured to determine voided volume. Once constant voided volumes were collected, the infusion was stopped at the beginning of a voiding contraction, and the last voided volume
was measured35. Immediately after the final voided volume measurement, the abdomen was opened by suture removal, and the bladder was exposed. The remaining intravesical content was expelled
by directly exerting pressure on the bladder with a curved forceps, and the collected fluid was estimated as residual volume. HISTOPATHOLOGICAL EXAMINATION Whole bladders excised from mice
were embedded in tissue-TEK OCT compound (Sakura Finetek, Tokyo, Japan), frozen in liquid nitrogen, and cut into 7 µm sections. The bladder tissues mounted on glass slides were fixed with 4%
paraformaldehyde, and then stained with haematoxylin and eosin (H&E) for microscopic examination. ISOMETRIC CONTRACTION OR RELAXATION OF _IN VITRO_ BLADDER MUSCLE STRIPS Body of bladder
was excised and then bladder strips approximately 5 to 6 mm wide and 7 to 10 mm long were prepared. The bladder strips with intact mucosa were suspended in a 15-ml organ bath (Panlab,
Barcelona, Spain) filled with Krebs solution composed of 119 mM NaCl, 4.6 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 15 mM NaHCO3, 1.2 mM NaH2PO4, and 5 mM glucose at 37 °C and gassed with 95%
O2/5% CO2. Changes in strip tension were measured with an MLT0210/D isometric force transducer (AD Instruments) and recorded with an ML118 and ML785 Power-Lab acquisition system (AD
Instruments). Strips were placed between two platinum electrodes in the organ baths containing Krebs solution and an initial tension of 1 g was applied to the strips, which were allowed to
equilibrate for at least 30 min before experiments. Strips were first challenged with 60 mM KCl to test tissue viability. In contraction experiments, the strips were stimulated by carbachol,
electrical field stimulation (EFS) at frequencies of 1 Hz, 2 Hz, 4 Hz, 8 Hz, 16 Hz, and 32 Hz with and without 10 µM atropine, and 1 mM ATP, in this order. Prior to each stimulation,
washout with Krebs solution was performed 3 times. Carbachol concentration-response curves were constructed by adding graded concentrations, which were expressed as the final concentration
of 3 nM to 100 mM in the organ bath. Contractile responses in strips were normalized to the weight of each strip. In relaxation experiments, prior to testing each drug concentration, the
strips were stimulated by 3 µM carbachol for evoking its contraction. When contraction became stable, a non-selective β-adrenoceptor agonist, isoproterenol (0.1 µM, 0.3 µM, 1 µM and 3 µM) or
a selective β3-adrenoceptor agonist, BRL 37344 (3 µM and 10 µM) was applied. Each strip was treated with either drug. Prior to each drug concentration, washout with Krebs solution was
performed 3 times. Relaxing responses in strips were normalized to the weight of each strip. Maximal relaxation of the tension was used for data analysis, which were expressed as a percent
inhibition of the carbachol-induced tension of the bladder strip. MEASUREMENT OF ATP RELEASE FROM PRIMARY-CULTURED UROTHELIAL CELLS Preparation of urothelial cultures, mechanical stretching
experiments and photon imaging of ATP release were performed as previously described30, 31. In brief, an elastic silicone stretch chamber (STB-CH-04, STREX, Osaka, Japan) and an extension
device (STB150, STREX) were set on a photon imaging system, and the chamber medium was replaced with extracellular solution containing a luciferase reagent (ATP bioluminescence assay kit CLS
II, Roche Diagnostics, Basel, Switzerland). ATP bioluminescence during stretch stimulation was detected and visualized with a VIM camera (C2400-30H, Hamamatsu Photonics, Hamamatsu, Japan).
The standard calibration curve yielded a correlation coefficient for bioluminescence _vs._ ATP concentration of 0.998 over a concentration range of 0 nM to 2 μM. DRUGS ATP (Sigma-Aldrich
Japan GK, Tokyo, Japan), atropine (Sigma-Aldrich Japan GK), carbachol (Sigma-Aldrich Japan GK), KCl (Nacalai Tesque, Inc., Kyoto, Japan), isoproterenol (Sigma-Aldrich Japan GK) and BRL 37344
(Sigma-Aldrich Japan GK) were used for _in vitro_ experiments using bladder muscle strips. UDP (Sigma-Aldrich Japan GK) was used for experiments measuring ATP release from primary-cultured
urothelial cells. Sevoflurane (Maruishi Pharmaceutical, Osaka, Japan) was used to anaesthetize animals during surgery for cystometry experiments. STATISTICAL ANALYSIS All values are
expressed as the mean ± SEM. The Wilcoxon matched-pairs signed rank test, Mann-Whitney test, unpaired t-test, F-test and repeated measures two-way analysis of variance (ANOVA) followed by
Tukey’s multiple comparisons test or Sidak’s multiple comparisons test were used for statistical analysis, if applicable. Coefficients (r) and P values (P) were calculated by Spearman’s
correlation analysis or by Pearson’s correlation analysis, if applicable, to examine correlations between variables. For all analyses, P values of <0.05 were considered significant.
REFERENCES * Burnstock, G. Discovery of purinergic signalling, the initial resistance and current explosion of interest. _Br J Pharmacol_ 167, 238–255 (2012). Article CAS PubMed PubMed
Central Google Scholar * von Kügelgen, I. & Wetter, A. Molecular pharmacology of P2Y-receptors. _Naunyn-Schmiedeberg’s Arch Pharmacol_ 362, 310–323 (2000). Article Google Scholar *
Abbracchio, M. P. _et al_. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy.
_Pharmacol Rev_ 58, 281–341 (2006). Article CAS PubMed PubMed Central Google Scholar * von Kügelgen, I. & Hoffmann, K. Pharmacology and structure of P2Y receptors.
_Neuropharmacology_ 104, 50–61 (2016). Article Google Scholar * Dranoff, J. A. _et al_. A primitive ATP receptor from the little skate _Raja erinacea_. _J Biol Chem_ 275, 30701–30706
(2000). Article CAS PubMed Google Scholar * Nicholas, R. A., Watt, W. C., Lazarowski, E. R., Li, Q. & Harden, K. Uridine nucleotide selectivity of three phospholipase C-activating P2
receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. _Mol Pharmacol_ 50, 224–229 (1996). CAS PubMed Google Scholar * Kobayashi, K. _et
al_. Neurons and glia cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. _J Comp Neurol_ 498, 443–454 (2006). Article CAS PubMed Google
Scholar * Xiang, Z. & Burnstock, G. Distribution of P2Y6 and P2Y12 receptor: their colocalization with calbindin, calretinin and nitric oxide synthase in the guinea pig enteric nervous
system. _Histochem Cell Biol_ 125, 327–336 (2006). Article CAS PubMed Google Scholar * Koizumi, S. _et al_. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis.
_Nature_ 446, 1091–1095 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Kobayashi, K., Yamanaka, H., Yanamoto, F., Okubo, M. & Noguchi, K. Multiple P2Y subtypes in
spinal microglia are involved in neuropathic pain after peripheral nerve injury. _Glia_ 60, 1529–1539 (2012). Article PubMed Google Scholar * Quintas, C. _et al_. Microglia P2Y6 receptors
mediate nitric oxide release and strocyte apoptosis. _J Neuroinflamm_ 11, 141 (2014). Article Google Scholar * Barragán-Iglesias, P. _et al_. Participation of peripheral P2Y1, P2Y6 and
P2Y11 receptors in formalin-induced inflammatory pain in rats. _Pharmacol Biochem Behav_ 128, 23–32 (2015). Article PubMed Google Scholar * Vieira, R. P. _et al_. Purinergic receptor type
6 contributes to airway inflammation and remodeling in experimental allergic airway inflammation. _Am J Respir Crit Care Med_ 184, 215–223 (2011). Article CAS PubMed Google Scholar *
Grbic, D. M. _et al_. P2Y6 receptor contributes to neutrophil recruitment to inflamed intestinal mucosa by increasing CXC chemokine ligand 8 expression in an AP-1-dependent manner in
epithelial cells. _Inflamm Bowel Dis_ 18, 1456–1469 (2012). Article PubMed Google Scholar * Uratsuji, H. _et al_. P2Y6 receptor signaling pathway mediates inflammatory responses induced
by monosodium urate crystals. _J Immunol_ 188, 436–444 (2012). Article CAS PubMed Google Scholar * Carneiro, I. _et al_. Activation of P2Y6 receptors increases the voiding frequency in
anaesthetized rats by releasing ATP from the bladder urothelium. _Br J Pharamcol_ 171, 3404–3419 (2014). Article CAS Google Scholar * Timóteo, M. A. _et al_. ATP released _via_ pannexin-1
hemichannels mediates bladder overactivity triggered by urothelial P2Y6 receptors. _Biochem Pharmacol_ 87, 371–379 (2014). Article PubMed Google Scholar * Yu, W., Sun, X., Robson, S. C.
& Hill, W. G. Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y6 activation of the phospholipase C/inositol trisphosphate pathway. _FASEB J_ 27,
1895–1903 (2013). Article CAS PubMed PubMed Central Google Scholar * Silva, I., Ferreirinha, F., Magalhães-Cardoso, M. T., Silva-Ramos, M. & Correia-de-Sá, P. Activation of P2Y6
receptors facilitates nonneuronal adenosine triphosphate and acetylcholine release from urothelium with the lamina propria of men with bladder outlet obstruction. _J Urol_ 194, 1146–1154
(2015). Article CAS PubMed Google Scholar * Yoshiyama, M. _et al_. Functional roles of TRPV1 and TRPV4 in control of lower urinary tract activity: dual analysis of behavior and reflex
during the micturition cycle. _Am J Physiol Renal Physiol_ 308, F1128–F1134 (2015). Article CAS PubMed Google Scholar * Yu, W. & Hill, W. G. Lack of specificity shown by P2Y6
receptor antibodies. _Naunyn-Schmiedeberg’s Arch Pharmacol_ 386, 885–891 (2013). Article CAS Google Scholar * Yoshiyama, M. _et al_. Sex-related differences in activity of lower urinary
tract in response to intravesical acid irritation in decerebrate unanesthetized mice. _Am J Physiol Regul Integr Comp Physiol_ 295, R954–R960 (2008). Article CAS PubMed Google Scholar *
Wang, E. C. Y. _et al_. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. _J Clin Invest_ 115, 2412–2422 (2005). Article CAS PubMed PubMed Central Google
Scholar * Araki, I. _et al_. Emerging families of ion channels involved in urinary bladder nociception. _Pharmaceuticals_ 3, 2248–2267 (2010). Article CAS PubMed PubMed Central Google
Scholar * Birder, L. _et al_. Neural control of the lower urinary tract: peripheral and spinal mechanisms. _Neurourol Urodyn_ 29, 128–139 (2010). Article CAS PubMed PubMed Central
Google Scholar * Birder, L. & Andersson, K.-E. Urothelial signaling. _Physiol Rev_ 93, 653–680 (2013). Article CAS PubMed PubMed Central Google Scholar * de Groat, W. C.
Integrative control of the lower urinary tract: preclinical perspective. _Br J Pharmacol_ 147, S25–S40 (2006). Article PubMed PubMed Central Google Scholar * de Groat, W. C., Griffiths,
D. & Yoshimura, N. Neural control of the lower urinary tract. _Compr Physiol_ 5, 327–396 (2015). PubMed PubMed Central Google Scholar * Sui, G.-P., Wu, C. & Fry, C. H.
Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. _BJU Int_ 97, 1327–1331 (2006). Article CAS PubMed Google Scholar * Mochizuki, T. _et al_.
The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. _J Biol Chem_ 284, 21257–21264 (2009). Article CAS PubMed PubMed Central
Google Scholar * Miyamoto, T. _et al_. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. _J Biol Chem_ 289, 16565–16575 (2014). Article
CAS PubMed PubMed Central Google Scholar * Nakagomi, H. _et al_. Urothelial ATP exocytosis: regulation of bladder compliance in the urine storage phase. _Sci Rep_ 6, 29761,
doi:10.1038/srep29761 (2016). Article ADS PubMed PubMed Central Google Scholar * Fry, C. H., Sui, G.-P., Kanai, A. J. & Wu, C. The function of suburothelial myofibroblasts in the
bladder. _Neurourol Urodyn_ 26, 914–919 (2007). Article CAS PubMed Google Scholar * Persson, K., Igawa, Y., Mattiasson, A. & Andersson, K.-E. Effects of inhibition of the
L-arginine/nitric oxide pathway in the rat lower urinary tract _in vivo_ and _in vitro_. _Br J Pharmacol_ 107, 178–184 (1992). Article CAS PubMed PubMed Central Google Scholar *
Yoshiyama, M., de Groat, W. C. & Fraser, M. O. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding
dysfunction in the rat with spinal cord injury. _Urology_ 55, 956–960 (2000). Article CAS PubMed Google Scholar * Yoshiyama, M., Roppolo, J. R., Takeda, M. & de Groat, W. C. Effects
of urethane on reflex activity of lower urinary tract in decerebrate unanesthetized rats. _Am J Physiol Renal Physiol_ 304, F390–F396 (2013). Article CAS PubMed Google Scholar * Otomo,
R. _et al_. The role of ATP-receptor in controlling the urinary bladder and urethral function in rats. _Nihon Hinyokika Gakkai Zasshi_ 90, 681–687 (1999). CAS PubMed Google Scholar *
Hindmarsh, J. R., Gosling, P. T. & Deane, A. M. Bladder instability. Is the primary defect in the urethra? _Br J Pharmacol_ 55, 648–651 (1983). CAS Google Scholar * Low, J. A.,
Armstrong, J. B. & Mauger, G. M. The unstable urethra in the female. _Obstet Gynecol_ 74, 69–74 (1989). CAS PubMed Google Scholar * Andersson, K.-E. Autonomic neurotransmission and
the unstable bladder. _Neurourol Urodyn_ 9, 555–557 (1990). Article Google Scholar * Ohnishi, N., Park, Y.-C., Kurita, T. & Kajimoto, N. Role of ATP and related purine compounds on
urethral relaxation in male rabbits. _Int J Urol_ 4, 191–197 (1997). Article CAS PubMed Google Scholar * Pinna, C., Ventura, S., Puglisi, L. & Burnstock, G. A pharmacological and
histochemical study of hamster urethra and the role of urothelium. _Br J Pharmacol_ 119, 655–662 (1996). Article CAS PubMed PubMed Central Google Scholar * Pinna, C., Puglisi, L. &
Burnstock, G. ATP and vasoactive intestinal polypeptide relaxant responses in hamster isolated proximal urethra. _Br J Pharmacol_ 124, 1069–1074 (1998). Article CAS PubMed PubMed Central
Google Scholar * Pinna, C. _et al_. Purine- and pyrimidine-induced responses and P2Y receptor characterization in the hamster proximal urethra. _Br J Pharmacol_ 144, 510–518 (2005).
Article CAS PubMed PubMed Central Google Scholar * Werkström, V. & Andersson, K.-E. ATP- and adenosine-induced relaxation of the smooth muscle of the pig urethra. _BJU Int_ 96,
1386–1391 (2005). Article PubMed Google Scholar * Jung, S. Y. _et al_. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress
incontinence and detrusor instability. _J Urol_ 162, 204–212 (1999). Article CAS PubMed Google Scholar * Obara, K., Lepor, H. & Walden, P. D. Localization of P2Y1 purinoceptor
transcript in the rat penis and urinary bladder. _J Urol_ 160, 587–591 (1998). Article CAS PubMed Google Scholar * Aronsson, P., Andersson, M., Ericsson, T. & Giglio, D. Assessment
and characterization of purinergic contractions and relaxations in the rat urinary bladder. _Basic & Clin Pharmacol Toxicol_ 107, 603–613 (2010). Article CAS Google Scholar * Bar, I.
_et al_. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. _Mol Pharmacol_ 74, 777–784 (2008). Article CAS PubMed Google
Scholar * Kobayashi, H., Yoshiyama, M., Zakoji, H., Takeda, M. & Araki, I. Sex differences in the expression profile of acid-sensing ion channels in the mouse urinary bladder: a
possible involvement in irritative bladder symptoms. _BJU Int_ 104, 1746–1751 (2009). Article CAS PubMed Google Scholar * Sapru, H. N. & Krieger, A. J. Procedure for the
decerebration of the rat. _Brain Res. Bull_ 3, 675–679 (1978). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to Dr. Bernard Robaye (Universite
Libre de Bruxelles, brussels, Belgium) for providing pairs of the P2Y6-KO mice. We also thank Ms. Mie Kanda for her technical assistance. This study was supported by CREST (to S. Koizumi),
JSPS KAKENHI on innovative Areas (25116512 & 25117003) (to S. Koizumi), Challenging Exploratory Research (26670699) (to M. Takeda), Scientific Research (22591787) (to M. Yoshiyama) and
Young Scientists (26861264) (to S. Kira). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Urology, University of Yamanashi Graduate School of Medical Science, Yamanashi,
409-3898, Japan Satoru Kira, Mitsuharu Yoshiyama, Sachiko Tsuchiya, Tatsuya Miyamoto, Hiroshi Nakagomi, Tsutomu Mochizuki & Masayuki Takeda * Department of Neuropharmacology, University
of Yamanashi Graduate School of Medical Science, Yamanashi, 409-3898, Japan Eiji Shigetomi, Keisuke Shibata & Schuichi Koizumi * Japan Science and Technology Agency, CREST, Tokyo,
102-0076, Japan Eiji Shigetomi, Keisuke Shibata & Schuichi Koizumi Authors * Satoru Kira View author publications You can also search for this author inPubMed Google Scholar * Mitsuharu
Yoshiyama View author publications You can also search for this author inPubMed Google Scholar * Sachiko Tsuchiya View author publications You can also search for this author inPubMed Google
Scholar * Eiji Shigetomi View author publications You can also search for this author inPubMed Google Scholar * Tatsuya Miyamoto View author publications You can also search for this author
inPubMed Google Scholar * Hiroshi Nakagomi View author publications You can also search for this author inPubMed Google Scholar * Keisuke Shibata View author publications You can also
search for this author inPubMed Google Scholar * Tsutomu Mochizuki View author publications You can also search for this author inPubMed Google Scholar * Masayuki Takeda View author
publications You can also search for this author inPubMed Google Scholar * Schuichi Koizumi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
S. Kira, M.Y., T. Miyamoto, H.N., T. Mochizuki and S. Koizumi, designed the research. M.Y., M.T. and S. Koizumi, supervised the study. S. Kira, M.Y. and S.T., performed the experiments. S.
Kira, M.Y., E.S., K.S., M.T. and S. Koizumi, discussed the data. S. Kira, M.Y., interpreted results of the experiments, prepared the figures, and wrote the main manuscript text. M.Y., edited
the manuscript. CORRESPONDING AUTHOR Correspondence to Mitsuharu Yoshiyama. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL
INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL
SUPPLEMENTARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution
and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if
changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the
material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Kira, S., Yoshiyama, M., Tsuchiya, S. _et al._ P2Y6-deficiency increases micturition frequency and attenuates sustained contractility of the urinary bladder in mice. _Sci Rep_ 7,
771 (2017). https://doi.org/10.1038/s41598-017-00824-2 Download citation * Received: 26 September 2016 * Accepted: 14 March 2017 * Published: 10 April 2017 * DOI:
https://doi.org/10.1038/s41598-017-00824-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative









