- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
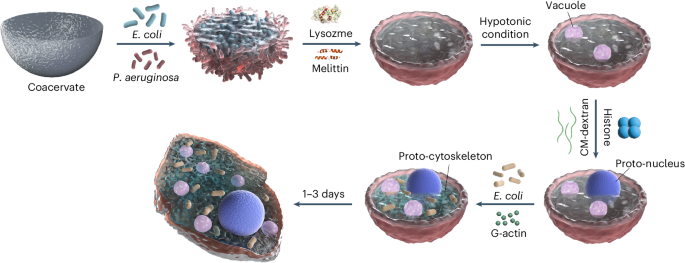
ABSTRACT Protocell research offers diverse opportunities to understand cellular processes and the foundations of life and holds attractive potential applications across various fields.
However, it is still a formidable task to construct a true-to-life synthetic cell with high organizational and functional complexity. Here we present a protocol for constructing
bacteriogenic protocells by employing prokaryotes as on-site repositories of compositional, functional and structural building blocks to address this challenge. This approach is based on the
capture and processing of two spatially segregated bacterial colonies within individual coacervate microdroplets to produce membrane-bounded, molecularly crowded, compositionally,
structurally and functionally complex synthetic cells. The bacteriogenic protocells inherit sufficient biological components from their bacterial building units to exhibit highly integrated
life-like properties, including biocatalysis, glycolysis and gene expression. The protocells can be endogenously remodeled to acquire diverse proto-organelles including a spatially
partitioned nucleus-like DNA/histone-based condensate to store genetic material, membrane-bounded water vacuoles to adjust cellular osmotic pressure, a three-dimensional network of F-actin
proto-cytoskeleton to support structural stability and proto-mitochondria to generate endogenous ATP as source of energy. The protocells ultimately develop a nonspherical morphology due to
the continuous biogeneration of metabolic products by implanted living bacteria cells. This protocol provides a novel living material assembly strategy for the construction of functional
protoliving microdevices and offers opportunities for potential applications in engineered synthetic biology and biomedicine. The protocol takes ~27 d to complete and requires expertise in
microbiology, phase separation, biochemistry and molecular biology related techniques. KEY POINTS * Membrane-bounded, molecularly crowded, compositionally, structurally and morphologically
complex bacteriogenic protocells are constructed on the basis of the capture and on-site processing of spatially segregated bacterial colonies within individual coacervate microdroplets. *
Bacteriogenic protocells are endogenously remodeled to acquire diverse proto-organelles and ultimately develop an amoeba-like nonspherical morphology. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only
$21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS LIVING MATERIAL ASSEMBLY OF
BACTERIOGENIC PROTOCELLS Article 14 September 2022 PROGRAMMED SPATIAL ORGANIZATION OF BIOMACROMOLECULES INTO DISCRETE, COACERVATE-BASED PROTOCELLS Article Open access 08 December 2020 A DE
NOVO MATRIX FOR MACROSCOPIC LIVING MATERIALS FROM BACTERIA Article Open access 21 September 2022 DATA AVAILABILITY The main data discussed in this protocol are available in the supporting
primary research paper17. All other data are available for research purposes from the corresponding authors upon reasonable request. Source data are provided with this paper. REFERENCES *
Dzieciol, A. J. & Mann, S. Designs for life: protocell models in the laboratory. _Chem. Soc. Rev._ 41, 79–85 (2012). Article CAS PubMed Google Scholar * van Stevendaal, M. H. M. E.,
van Hest, J. C. M. & Mason, A. F. Functional interactions between bottom-up synthetic cells and living matter for biomedical applications. _ChemSystemsChem_ 3, e2100009 (2021). Article
Google Scholar * Jeong, S., Nguyen, H. T., Kim, C. H., Ly, M. N. & Shin, K. Toward artificial cells: novel advances in energy conversion and cellular motility. _Adv. Funct. Mater._ 30,
1907182 (2020). Article CAS Google Scholar * Toparlak, O. D. & Mansy, S. S. Progress in synthesizing protocells. _Exp. Biol. Med._ 244, 304–313 (2018). Article Google Scholar *
Yewdall, N. A., Mason, A. F. & van Hest, J. C. M. The hallmarks of living systems: towards creating artificial cells. _Interface Focus_ 8, 20180023 (2018). Article PubMed PubMed
Central Google Scholar * Kurihara, K. et al. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. _Nat. Chem._ 3, 775–781 (2011). Article
CAS PubMed Google Scholar * Dora Tang, T. Y. et al. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. _Nat. Chem._ 6, 527–533 (2014).
Article PubMed Google Scholar * Li, M., Harbron, R. L., Weaver, J. V. M., Binks, B. P. & Mann, S. Electrostatically gated membrane permeability in inorganic protocells. _Nat. Chem._
5, 529–536 (2013). Article PubMed Google Scholar * Rodríguez-Arco, L., Li, M. & Mann, S. Phagocytosis-inspired behaviour in synthetic protocell communities of compartmentalized
colloidal objects. _Nat. Mater._ 17, 857–863 (2017). Google Scholar * Qiao, Y., Li, M., Booth, R. & Mann, S. Predatory behaviour in synthetic protocell communities. _Nat. Chem._ 9,
110–119 (2016). PubMed Google Scholar * Koga, S., Williams, D. S., Perriman, A. W. & Mann, S. Peptide–nucleotide microdroplets as a step towards a membrane-free protocell model. _Nat.
Chem._ 3, 720–724 (2011). Article CAS PubMed Google Scholar * Huang, X. et al. Interfacial assembly of protein–polymer nano-conjugates into stimulus-responsive biomimetic protocells.
_Nat. Commun._ 4, 2239 (2013). Article PubMed Google Scholar * Dora Tang, T. Y., van Swaay, D., deMello, A., Ross Anderson, J. L. & Mann, S. In vitro gene expression within
membrane-free coacervate protocells. _Chem. Commun._ 51, 11429–11432 (2015). Article CAS Google Scholar * Li, M., Green, D. C., Anderson, J. L. R., Binks, B. P. & Mann, S. In vitro
gene expression and enzyme catalysis in bio-inorganic protocells. _Chem. Sci._ 2, 1739–1745 (2011). Article CAS Google Scholar * Walde, P. & Ichikawa, S. Enzymes inside lipid
vesicles: preparation, reactivity and applications. _Biomol. Eng._ 18, 143–177 (2001). Article CAS PubMed Google Scholar * Walde, P., Goto, A., Monnard, P.-A., Wessicken, M. & Luisi,
P. L. Oparin’s reactions revisited: Enzymic synthesis of Poly(adenylic acid) in micelles and self-reproducing vesicles. _J Am. Chem. Soc._ 116, 7541–7547 (1994). Article CAS Google
Scholar * Xu, C., Martin, N., Li, M. & Mann, S. Living material assembly of bacteriogenic protocells. _Nature_ 609, 1029–1037 (2022). Article CAS PubMed Google Scholar * Mann, S.
Systems of creation: the emergence of life from nonliving matter. _Acc. Chem. Res._ 45, 2131–2141 (2012). Article CAS PubMed Google Scholar * Xu, C., Hu, S. & Chen, X. Artificial
cells: from basic science to applications. _Mater. Today_ 19, 516–532 (2016). Article CAS Google Scholar * Martino, C. & deMello, A. J. Droplet-based microfluidics for artificial cell
generation: a brief review. _Interface Focus_ 6, 20160011 (2016). Article PubMed PubMed Central Google Scholar * Solé, R. V., Munteanu, A., Rodriguez-Caso, C. & Macía, J. Synthetic
protocell biology: from reproduction to computation. _Philos. Trans. R. Soc. Lond. B_ 362, 1727–1739 (2007). Article Google Scholar * Jaffe, J. D. et al. The complete genome and proteome
of _Mycoplasma_ mobile. _Genome Res._ 14, 1447–1461 (2004). Article CAS PubMed PubMed Central Google Scholar * Thornburg, Z. R. et al. Fundamental behaviors emerge from simulations of a
living minimal cell. _Cell_ 185, 345–360.e328 (2022). Article CAS PubMed PubMed Central Google Scholar * Williams, D. S. et al. Polymer/nucleotide droplets as bio-inspired functional
micro-compartments. _Soft Matter_ 8, 6004–6014 (2012). Article CAS Google Scholar * Weibull, C. The isolation of protoplasts from _Bacillus megaterium_ by controlled treatment with
lysozyme. _J. Bacteriol._ 66, 688–695 (1953). Article CAS PubMed PubMed Central Google Scholar * Birdsell, D. C. & Cota-Robles, E. H. Production and ultrastructure of lysozyme and
ethylenediaminetetraacetate-lysozyme spheroplasts of _Escherichia coli_. _J. Bacteriol._ 93, 427–437 (1967). Article CAS PubMed PubMed Central Google Scholar * van den Bogaart, G.,
Guzman, J. V., Mika, J. T. & Poolman, B. On the mechanism of pore formation by melittin. _J. Biol. Chem._ 283, 33854–33857 (2008). Article PubMed PubMed Central Google Scholar *
Pandidan, S. & Mechler, A. Nano-viscosimetry analysis of the membrane disrupting action of the bee venom peptide melittin. _Sci. Rep._ 9, ARTN 10841 (2019). Article Google Scholar *
Oberholzer, T., Wick, R., Luisi, P. L. & Biebricher, C. K. Enzymatic RNA replication in self-reproducing vesicles—an approach to a minimal cell. _Biochem. Bioph. Res. Co._ 207, 250–257
(1995). Article CAS Google Scholar * Oberholzer, T., Albrizio, M. & Luisi, P. L. Polymerase chain-reaction in liposomes. _Chem. Biol._ 2, 677–682 (1995). Article CAS PubMed Google
Scholar * Ishikawa, K., Sato, K., Shima, Y., Urabe, I. & Yomo, T. Expression of a cascading genetic network within liposomes. _Febs. Lett._ 576, 387–390 (2004). Article CAS PubMed
Google Scholar * Carlson, E. D., Gan, R., Hodgman, C. E. & Jewett, M. C. Cell-free protein synthesis: applications come of age. _Biotechnol. Adv._ 30, 1185–1194 (2012). Article CAS
PubMed Google Scholar * Khosla, C. & Keasling, J. D. Metabolic engineering for drug discovery and development. _Nat. Rev. Drug Discov._ 2, 1019–1025 (2003). Article CAS PubMed
Google Scholar * Skruzny, M. et al. Molecular basis for coupling the plasma membrane to the actin cytoskeleton during clathrin-mediated endocytosis. _Proc. Natl Acad. Sci. USA_ 109,
E2533–E2542 (2012). Article CAS PubMed PubMed Central Google Scholar * Emelyanov, V. V. Mitochondrial connection to the origin of the eukaryotic cell. _Eur. J. Biochem._ 270, 1599–1618
(2003). Article CAS PubMed Google Scholar * Vellai, T. & Vida, G. The origin of eukaryotes: the difference between prokaryotic and eukaryotic cells. _Proc. R. Soc. Lond. B_ 266,
1571–1577 (1999). Article CAS Google Scholar * Drew, B. & Leeuwenburgh, C. Method for measuring ATP production in isolated mitochondria: ATP production in brain and liver mitochondria
of Fischer-344 rats with age and caloric restriction. _Am. J. Physiol._ 285, R1259–R1267 (2003). CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Y. Takebayashi and J.
Spencer for help with bacterial cultures; H. Sun, C. Berger-Schaffitzel and E. Bragginton for help with gel electrophoresis and western blot analysis; A. Coutable and J. L. R. Anderson for
providing the plasmid pEXP5-NT/deGFP; A. Leard from the Wolfson Bioimaging Facility for help with confocal imaging; and K. Heesom from the Proteomics Facility for proteomics analysis.
Special thanks to Wolfson Bioimaging Facility for providing the microscopes. C.X. was funded by China 2024 Excellent Young Scientists Fund Program (Overseas) (20241B4435) and Shanghai
Pujiang Program (23PJ1406000). N.M. and S.M. were funded by the ERC Advanced Grant Scheme (EC-2016-674 ADG 740235). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Materials Science
and Engineering, Shanghai Jiao Tong University, Shanghai, P. R. China Can Xu, Mei Li & Stephen Mann * Institute of Medical Robotics, Shanghai Jiao Tong University, Shanghai, P. R. China
Can Xu * Centre for Protolife Research and Centre for Organized Matter Chemistry, School of Chemistry, University of Bristol, Bristol, UK Mei Li & Stephen Mann * Univ. Bordeaux, CNRS,
Centre de Recherche Paul Pascal, Pessac, France Nicolas Martin * Max Planck-Bristol Centre for Minimal Biology, School of Chemistry, University of Bristol, Bristol, UK Stephen Mann *
Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, P. R. China Stephen Mann Authors * Can Xu View author publications You can also search for this author
inPubMed Google Scholar * Mei Li View author publications You can also search for this author inPubMed Google Scholar * Nicolas Martin View author publications You can also search for this
author inPubMed Google Scholar * Stephen Mann View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.X., M.L. and S.M. conceived the protocol
design. C.X. and N.M. performed the experimental procedures. C.X. and M.L. prepared the figures. C.X. and S.M. wrote the manuscript. M.L. and N.M. performed the revision. S.M. supervised the
work. CORRESPONDING AUTHORS Correspondence to Can Xu or Stephen Mann. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION
_Nature Protocols_ thanks Yan Qiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. KEY REFERENCE Xu, C. et al. _Nature_ 609, 1029–1037 (2022):
https://doi.org/10.1038/s41586-022-05223-w SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–9. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 5 Statistical Source
Data. SOURCE DATA FIG. 6 Statistical Source Data. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a
publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing
agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xu, C., Li, M., Martin, N. _et al._ Construction of complex bacteriogenic protocells from living
material assembly. _Nat Protoc_ (2025). https://doi.org/10.1038/s41596-025-01148-6 Download citation * Received: 08 July 2024 * Accepted: 13 January 2025 * Published: 05 March 2025 * DOI:
https://doi.org/10.1038/s41596-025-01148-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








