- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Mammalian cells sense and react to the mechanics of their immediate microenvironment. Therefore, the characterization of the biomechanical properties of tissues with high spatial
resolution provides valuable insights into a broad variety of developmental, homeostatic and pathological processes within living organisms. The biomechanical properties of the basement
membrane (BM), an extracellular matrix (ECM) substructure measuring only ∼100–400 nm across, are, among other things, pivotal to tumor progression and metastasis formation. Although the
precise assignment of the Young’s modulus _E_ of such a thin ECM substructure especially in between two cell layers is still challenging, biomechanical data of the BM can provide information
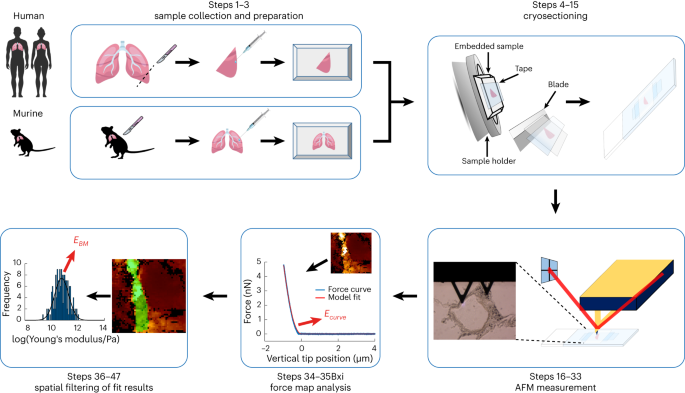
of eminent diagnostic potential. Here we present a detailed protocol to quantify the elastic modulus of the BM in murine and human lung tissue, which is one of the major organs prone to
metastasis. This protocol describes a streamlined workflow to determine the Young’s modulus _E_ of the BM between the endothelial and epithelial cell layers shaping the alveolar wall in lung
tissues using atomic force microscopy (AFM). Our step-by-step protocol provides instructions for murine and human lung tissue extraction, inflation of these tissues with cryogenic cutting
medium, freezing and cryosectioning of the tissue samples, and AFM force-map recording. In addition, it guides the reader through a semi-automatic data analysis procedure to identify the
pulmonary BM and extract its Young’s modulus _E_ using an in-house tailored user-friendly AFM data analysis software, the Center for Applied Tissue Engineering and Regenerative Medicine
processing toolbox, which enables automatic loading of the recorded force maps, conversion of the force versus piezo-extension curves to force versus indentation curves, calculation of
Young’s moduli and generation of Young’s modulus maps, where the pulmonary BM can be identified using a semi-automatic spatial filtering tool. The entire protocol takes 1–2 d. KEY POINTS *
The function of pulmonary alveoli is dependent on their mechanical robustness and response to external forces. The Young’s modulus _E_ (stiffness) of their basement membranes is higher than
that of the surrounding cell layers. * This protocol describes how to prepare lung sections from humans or mice and perform atomic force microscopy experiments. Challenges in data
analysis—including filtering to focus specifically on basement membrane values—are addressed using the Center for Applied Tissue Engineering and Regenerative Medicine processing toolbox.
Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54
other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and
online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS SPATIAL MAPPING OF THE COLLAGEN DISTRIBUTION IN HUMAN AND MOUSE TISSUES BY FORCE VOLUME ATOMIC FORCE MICROSCOPY Article Open access 24 September 2020 INTEGRATED COMPUTATIONAL AND
EXPERIMENTAL PIPELINE FOR QUANTIFYING LOCAL CELL–MATRIX INTERACTIONS Article Open access 12 August 2021 DEVELOPMENT OF A NOVEL AIR–LIQUID INTERFACE AIRWAY TISSUE EQUIVALENT MODEL FOR IN
VITRO RESPIRATORY MODELING STUDIES Article Open access 22 June 2023 DATA AVAILABILITY All raw data and derived data used to generate graphs presented in this manuscript are available from
the corresponding authors upon reasonable request. The force maps are available at https://figshare.com/articles/journal_contribution/Force_Maps/24591198. CODE AVAILABILITY All codes used in
this manuscript are available in an open-source repository on GitHub: https://github.com/CANTERhm/CANTER_Processing_Tool. REFERENCES * Chaudhuri, O., Cooper-White, J., Janmey, P. A.,
Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. _Nature_ 584, 535–546 (2020). Article CAS PubMed PubMed Central Google Scholar *
Eyckmans, J., Boudou, T., Yu, X. & Chen, C. S. A hitchhiker’s guide to mechanobiology. _Dev. Cell_ 21, 35–47 (2011). Article CAS PubMed PubMed Central Google Scholar * Holle, A. W.
et al. Cell–extracellular matrix mechanobiology: forceful tools and emerging needs for basic and translational research. _Nano Lett._ 18, 1–8 (2018). Article CAS PubMed Google Scholar *
Iskratsch, T., Wolfenson, H. & Sheetz, M. P. Appreciating force and shape-the rise of mechanotransduction in cell biology. _Nat. Rev. Mol. Cell Biol._ 15, 825–833 (2014). Article CAS
PubMed PubMed Central Google Scholar * Ladoux, B. & Mege, R. M. Mechanobiology of collective cell behaviours. _Nat. Rev. Mol. Cell Biol._ 18, 743–757 (2017). Article CAS PubMed
Google Scholar * Wang, J. H. & Thampatty, B. P. An introductory review of cell mechanobiology. _Biomech. Model. Mechanobiol._ 5, 1–16 (2006). Article CAS PubMed Google Scholar *
Barriga, E. H., Franze, K., Charras, G. & Mayor, R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. _Nature_ 554, 523–527 (2018). Article
CAS PubMed PubMed Central Google Scholar * Murphy, W. L., McDevitt, T. C. & Engler, A. J. Materials as stem cell regulators. _Nat. Mater._ 13, 547–557 (2014). Article CAS PubMed
PubMed Central Google Scholar * Vining, K. H. & Mooney, D. J. Mechanical forces direct stem cell behaviour in development and regeneration. _Nat. Rev. Mol. Cell Biol._ 18, 728–742
(2017). Article CAS PubMed PubMed Central Google Scholar * Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. _Science_ 341,
1240104 (2013). Article PubMed PubMed Central Google Scholar * Koser, D. E. et al. Mechanosensing is critical for axon growth in the developing brain. _Nat. Neurosci._ 19, 1592–1598
(2016). Article CAS PubMed PubMed Central Google Scholar * Prein, C. et al. Structural and mechanical properties of the proliferative zone of the developing murine growth plate
cartilage assessed by atomic force microscopy. _Matrix Biol._ 50, 1–15 (2016). Article CAS PubMed Google Scholar * Alvey, C. et al. Mechanosensing of solid tumors by cancer-attacking
macrophages. _Biophys. J._ 114, 654a–654a (2018). Article Google Scholar * Bras, M. M., Radmacher, M., Sousa, S. R. & Granja, P. L. Melanoma in the eyes of mechanobiology. _Front. Cell
Dev. Biol._ 8, 54 (2020). Article PubMed PubMed Central Google Scholar * Bras, M. M., Sousa, S. R., Carneiro, F., Radmacher, M. & Granja, P. L. Mechanobiology of colorectal cancer.
_Cancers_ https://doi.org/10.3390/cancers14081945 (2022). * Fleischhauer, L. et al. Nano-scale mechanical properties of the articular cartilage zones in a mouse model of post-traumatic
osteoarthritis. _Appl. Sci._ 12, 2596 (2022). Article CAS Google Scholar * Rianna, C., Radmacher, M. & Kumar, S. Direct evidence that tumor cells soften when navigating confined
spaces. _Mol. Biol. Cell_ 31, 1726–1734 (2020). Article CAS PubMed PubMed Central Google Scholar * Streitberger, K. J. et al. How tissue fluidity influences brain tumor progression.
_Proc. Natl Acad. Sci. USA_ 117, 128–134 (2020). Article CAS PubMed Google Scholar * Stylianou, A., Lekka, M. & Stylianopoulos, T. AFM assessing of nanomechanical fingerprints for
cancer early diagnosis and classification: from single cell to tissue level. _Nanoscale_ 10, 20930–20945 (2018). Article CAS PubMed Google Scholar * Suresh, S. Biomechanics and
biophysics of cancer cells. _Acta Biomater._ 3, 413–438 (2007). Article PubMed PubMed Central Google Scholar * Rudiger, D. et al. Cell-based strain remodeling of a nonfibrous matrix as
an organizing principle for vasculogenesis. _Cell Rep._ 32, 108015 (2020). Article PubMed Google Scholar * Bertalan, G. et al. Mechanical behavior of the hippocampus and corpus callosum:
An attempt to reconcile ex vivo with in vivo and micro with macro properties. _J. Mech. Behav. Biomed. Mater._ 138, 105613 (2023). Article PubMed Google Scholar * Franze, K. Atomic force
microscopy and its contribution to understanding the development of the nervous system. _Curr. Opin. Genet. Dev._ 21, 530–537 (2011). Article CAS PubMed Google Scholar * Koser, D. E.,
Moeendarbary, E., Hanne, J., Kuerten, S. & Franze, K. CNS cell distribution and axon orientation determine local spinal cord mechanical properties. _Biophys. J._ 108, 2137–2147 (2015).
Article CAS PubMed PubMed Central Google Scholar * Schaeffer, J., Weber, I. P., Thompson, A. J., Keynes, R. J. & Franze, K. Axons in the Chick embryo follow soft pathways through
developing somite segments. _Front. Cell Dev. Biol._ 10, 917589 (2022). Article PubMed PubMed Central Google Scholar * Fritsch, A. et al. Are biomechanical changes necessary for tumour
progression? _Nat. Phys._ 6, 730–732 (2010). Article CAS Google Scholar * Guck, J. et al. Optical deformability as an inherent cell marker for testing malignant transformation and
metastatic competence. _Biophys. J._ 88, 3689–3698 (2005). Article CAS PubMed PubMed Central Google Scholar * Ilina, O. et al. Cell-cell adhesion and 3D matrix confinement determine
jamming transitions in breast cancer invasion. _Nat. Cell Biol._ 22, 1103–1115 (2020). Article CAS PubMed PubMed Central Google Scholar * Irianto, J., Pfeifer, C. R., Ivanovska, I. L.,
Swift, J. & Discher, D. E. Nuclear lamins in cancer. _Cell. Mol. Bioeng._ 9, 258–267 (2016). Article CAS PubMed Google Scholar * Reuten, R. et al. Basement membrane stiffness
determines metastases formation. _Nat. Mater._ 20, 892–903 (2021). Article CAS PubMed Google Scholar * Seltmann, K., Fritsch, A. W., Kas, J. A. & Magin, T. M. Keratins significantly
contribute to cell stiffness and impact invasive behavior. _Proc. Natl Acad. Sci. USA_ 110, 18507–18512 (2013). Article CAS PubMed PubMed Central Google Scholar * Alsteens, D. et al.
Atomic force microscopy-based characterization and design of biointerfaces. _Nat. Rev. Mater_. https://doi.org/10.1038/natrevmats.2017.8 (2017). * Domke, J. & Radmacher, M. Measuring the
elastic properties of thin polymer films with the atomic force microscope. _Langmuir_ 14, 3320–3325 (1998). Article CAS Google Scholar * Huth, S., Sindt, S. & Selhuber-Unkel, C.
Automated analysis of soft hydrogel microindentation: Impact of various indentation parameters on the measurement of Young’s modulus. _PLoS ONE_ 14, e0220281 (2019). Article CAS PubMed
PubMed Central Google Scholar * Krieg, M. et al. Atomic force microscopy-based mechanobiology. _Nat. Revi. Phys._ 1, 41–57 (2019). Article Google Scholar * Radmacher, M., Fritz, M.,
Kacher, C. M., Cleveland, J. P. & Hansma, P. K. Measuring the viscoelastic properties of human platelets with the atomic force microscope. _Biophys. J._ 70, 556–567 (1996). Article CAS
PubMed PubMed Central Google Scholar * Radmacher, M., Tillamnn, R. W., Fritz, M. & Gaub, H. E. From molecules to cells: imaging soft samples with the atomic force microscope.
_Science_ 257, 1900–1905 (1992). Article CAS PubMed Google Scholar * Stolz, M. et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force
microscopy. _Nat. Nanotechnol._ 4, 186–192 (2009). Article CAS PubMed Google Scholar * van de Vijver, M. J. et al. A gene-expression signature as a predictor of survival in breast
cancer. _N. Engl. J. Med._ 347, 1999–2009 (2002). Article PubMed Google Scholar * Higgins, J. P. et al. Gene expression in the normal adult human kidney assessed by complementary DNA
microarray. _Mol. Biol. Cell_ 15, 649–656 (2004). Article CAS PubMed PubMed Central Google Scholar * Zhao, H. et al. Gene expression profiling predicts survival in conventional renal
cell carcinoma. _PLoS Med._ 3, e13 (2006). Article PubMed Google Scholar * Nicolau, M., Tibshirani, R., Borresen-Dale, A. L. & Jeffrey, S. S. Disease-specific genomic analysis:
identifying the signature of pathologic biology. _Bioinformatics_ 23, 957–965 (2007). Article CAS PubMed Google Scholar * CANTER Processing Toolbox v. 5.6.0 _GitHub_ (2022). * Binnig, G.
Atomic force microscope and method for imaging surfaces with atomic resolution. USA patent US4724318A (1986). * Binnig, G., Quate, C. F. & Gerber, C. Atomic force microscope. _Phys.
Rev. Lett._ 56, 930–933 (1986). Article CAS PubMed Google Scholar * Florin, E. L., Moy, V. T. & Gaub, H. E. Adhesion forces between individual ligand-receptor pairs. _Science_ 264,
415–417 (1994). Article CAS PubMed Google Scholar * Hansma, P. K. et al. Tapping mode atomic force microscopy in liquids. _Appl. Phys. Lett._ 64, 1738–1740 (1994). Article CAS Google
Scholar * Moy, V. T., Florin, E. L. & Gaub, H. E. Intermolecular forces and energies between ligands and receptors. _Science_ 266, 257–259 (1994). Article CAS PubMed Google Scholar
* Radmacher, M., Fritz, M., Hansma, H. G. & Hansma, P. K. Direct observation of enzyme activity with the atomic force microscope. _Science_ 265, 1577–1579 (1994). Article CAS PubMed
Google Scholar * Radmacher, M., Fritz, M. & Hansma, P. K. Imaging soft samples with the atomic force microscope: gelatin in water and propanol. _Biophys. J._ 69, 264–270 (1995). Article
CAS PubMed PubMed Central Google Scholar * Rief, M., Clausen-Schaumann, H. & Gaub, H. E. Sequence-dependent mechanics of single DNA molecules. _Nat. Struct. Biol._ 6, 346–349
(1999). Article CAS PubMed Google Scholar * Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J. M. & Gaub, H. E. Reversible unfolding of individual titin immunoglobulin domains by
AFM. _Science_ 276, 1109–1112 (1997). Article CAS PubMed Google Scholar * Drake, B. et al. Imaging crystals, polymers, and processes in water with the atomic force microscope. _Science_
243, 1586–1589 (1989). Article CAS PubMed Google Scholar * Loparic, M. et al. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy:
validation with a gel-microfiber composite. _Biophys. J._ 98, 2731–2740 (2010). Article CAS PubMed PubMed Central Google Scholar * Rotsch, C. & Radmacher, M. Drug-induced changes of
cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. _Biophys. J._ 78, 520–535 (2000). Article CAS PubMed PubMed Central Google Scholar *
Clausen-Schaumann, H., Rief, M., Tolksdorf, C. & Gaub, H. E. Mechanical stability of single DNA molecules. _Biophys. J._ 78, 1997–2007 (2000). Article CAS PubMed PubMed Central
Google Scholar * Lekka, M. et al. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. _Eur. Biophys. J._ 28, 312–316 (1999). Article CAS PubMed
Google Scholar * Weisenhorn, A. L., Khorsandi, M., Kasas, S., Gotzos, V. & Butt, H. J. Deformation and height anomaly of soft surfaces studied with an AFM. _Nanotechnology_ 4, 106
(1993). Article CAS Google Scholar * Rotsch, C., Jacobson, K. & Radmacher, M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by
using atomic force microscopy. _Proc. Natl Acad. Sci. USA_ 96, 921–926 (1999). Article CAS PubMed PubMed Central Google Scholar * Goldmann, W. H. & Ezzell, R. M. Viscoelasticity in
wild-type and vinculin-deficient (5.51) mouse F9 embryonic carcinoma cells examined by atomic force microscopy and rheology. _Exp. Cell Res._ 226, 234–237 (1996). Article CAS PubMed
Google Scholar * Radmacher, M. Measuring the elastic properties of biological samples with the AFM. _IEEE Eng. Med. Biol. Mag._ 16, 47–57 (1997). Article CAS PubMed Google Scholar *
Kinney, J. H., Balooch, M., Marshall, S. J., Marshall, G. W. Jr & Weihs, T. P. Atomic force microscope measurements of the hardness and elasticity of peritubular and intertubular human
dentin. _J. Biomech. Eng._ 118, 133–135 (1996). Article CAS PubMed Google Scholar * Lundkvist, A. et al. Viscoelastic properties of healthy human artery measured in saline solution by
AFM-based indentation technique. _MRS Online Proc. Library_ 436, 353–358 (1996). Article Google Scholar * Tao, N. J., Lindsay, S. M. & Lees, S. Measuring the microelastic properties of
biological material. _Biophys. J._ 63, 1165–1169 (1992). Article CAS PubMed PubMed Central Google Scholar * Aro, E. et al. Severe extracellular matrix abnormalities and
chondrodysplasia in mice lacking collagen prolyl 4-hydroxylase isoenzyme II in combination with a reduced amount of isoenzyme I. _J. Biol. Chem._ 290, 16964–16978 (2015). Article CAS
PubMed PubMed Central Google Scholar * Plodinec, M. et al. The nanomechanical signature of breast cancer. _Nat. Nanotechnol._ 7, 757–765 (2012). Article CAS PubMed Google Scholar *
Stolz, M. et al. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. _Biophys. J._
86, 3269–3283 (2004). Article CAS PubMed PubMed Central Google Scholar * Akhtar, R., Sherratt, M. J., Cruickshank, J. K. & Derby, B. Characterizing the elastic properties of
tissues. _Mater. Today_ 14, 96–105 (2011). Article CAS Google Scholar * Junior, C. et al. Baseline stiffness modulates the non-linear response to stretch of the extracellular matrix in
pulmonary fibrosis. _Int. J. Mol. Sci_. https://doi.org/10.3390/ijms222312928 (2021). * Junior, C. et al. Multi-step extracellular matrix remodelling and stiffening in the development of
idiopathic pulmonary fibrosis. _Int. J. Mol. Sci_. https://doi.org/10.3390/ijms24021708 (2023). * Sicard, D., Fredenburgh, L. E. & Tschumperlin, D. J. Measured pulmonary arterial tissue
stiffness is highly sensitive to AFM indenter dimensions. _J. Mech. Behav. Biomed. Mater._ 74, 118–127 (2017). Article PubMed PubMed Central Google Scholar * Zemla, J. et al. AFM-based
nanomechanical characterization of bronchoscopic samples in asthma patients. _J. Mol. Recognit._ 31, e2752 (2018). Article PubMed Google Scholar * Becke, T. D. et al. Single molecule
force spectroscopy reveals two-domain binding mode of pilus-1 tip protein RrgA of _Streptococcus pneumoniae_ to fibronectin. _ACS Nano_ 12, 549–558 (2018). Article CAS PubMed Google
Scholar * Becke, T. D. et al. Pilus-1 backbone protein RrgB of _Streptococcus pneumoniae_ binds collagen I in a force-dependent way. _ACS Nano_ 13, 7155–7165 (2019). Article CAS PubMed
Google Scholar * Pill, M. F., East, A. L. L., Marx, D., Beyer, M. K. & Clausen-Schaumann, H. Mechanical activation drastically accelerates amide bond hydrolysis, matching enzyme
activity. _Angew. Chem. Int. Ed. Engl._ 58, 9787–9790 (2019). Article CAS PubMed Google Scholar * Schmidt, S. W., Filippov, P., Kersch, A., Beyer, M. K. & Clausen-Schaumann, H.
Single-molecule force-clamp experiments reveal kinetics of mechanically activated silyl ester hydrolysis. _ACS Nano_ 6, 1314–1321 (2012). Article CAS PubMed Google Scholar * Docheva, D.
et al. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. _J. Cell Mol. Med._ 12, 537–552
(2008). Article PubMed Google Scholar * Docheva, D., Padula, D., Schieker, M. & Clausen-Schaumann, H. Effect of collagen I and fibronectin on the adhesion, elasticity and cytoskeletal
organization of prostate cancer cells. _Biochem. Biophys. Res. Commun._ 402, 361–366 (2010). Article CAS PubMed Google Scholar * Kiderlen, S. et al. Age related changes in cell
stiffness of tendon stem/progenitor cells and a rejuvenating effect of ROCK-inhibition. _Biochem. Biophys. Res. Commun._ 509, 839–844 (2019). Article CAS PubMed Google Scholar * Reuten,
R. et al. Structural decoding of netrin-4 reveals a regulatory function towards mature basement membranes. _Nat. Commun._ 7, 13515 (2016). Article CAS PubMed PubMed Central Google
Scholar * Yin, H. et al. Three-dimensional self-assembling nanofiber matrix rejuvenates aged/degenerative human tendon stem/progenitor cells. _Biomaterials_ 236, 119802 (2020). Article CAS
PubMed Google Scholar * Ferreira, S. A. et al. Bi-directional cell-pericellular matrix interactions direct stem cell fate. _Nat. Commun._ 9, 4049 (2018). Article PubMed PubMed Central
Google Scholar * Norman, M. D. A., Ferreira, S. A., Jowett, G. M., Bozec, L. & Gentleman, E. Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels
using atomic force microscopy. _Nat. Protoc._ 16, 2418–2449 (2021). Article CAS PubMed PubMed Central Google Scholar * Alberton, P. et al. Aggrecan hypomorphism compromises articular
cartilage biomechanical properties and is associated with increased incidence of spontaneous osteoarthritis. _Int. J. Mol. Sci_. https://doi.org/10.3390/ijms20051008 (2019). * Alberton, P.
et al. Aggrecan is critical in maintaining the cartilage matrix biomechanics which in turn influences the correct development of the growth plate. _Osteoarthr. Cartil._ 27, S178–S178 (2019).
Article Google Scholar * Gronau, T. et al. Forced exercise-induced osteoarthritis is attenuated in mice lacking the small leucine-rich proteoglycan decorin. _Ann. Rheum. Dis._ 76, 442–449
(2017). Article CAS PubMed Google Scholar * Hartmann, B. et al. Early detection of cartilage degeneration: a comparison of histology, fiber bragg grating-based micro-indentation, and
atomic force microscopy-based nano-indentation. _Int. J. Mol. Sci_. https://doi.org/10.3390/ijms21197384 (2020). * Rellmann, Y. et al. ER Stress in ERp57 knockout knee joint chondrocytes
induces osteoarthritic cartilage degradation and osteophyte formation. _Int. J. Mol. Sci_.https://doi.org/10.3390/ijms23010182 (2021). * Kamper, M. et al. Early changes in morphology, bone
mineral density and matrix composition of vertebrae lead to disc degeneration in aged collagen IX −/−mice. _Matrix Biol._ 49, 132–143 (2016). Article CAS PubMed Google Scholar * Lin, D.
et al. Loss of tenomodulin expression is a risk factor for age-related intervertebral disc degeneration. _Aging Cell_ 19, e13091 (2020). Article CAS PubMed PubMed Central Google Scholar
* Dex, S. et al. Tenomodulin is required for tendon endurance running and Collagen I Fibril Adaptation to Mechanical Load. _EBioMedicine_ 20, 240–254 (2017). Article PubMed PubMed
Central Google Scholar * Li, P. et al. Mice lacking the matrilin family of extracellular matrix proteins develop mild skeletal abnormalities and are susceptible to age-associated
osteoarthritis. _Int. J. Mol. Sci_. https://doi.org/10.3390/ijms21020666 (2020). * Muschter, D. et al. Sensory neuropeptides are required for bone and cartilage homeostasis in a murine
destabilization-induced osteoarthritis model. _Bone_ 133, 115181 (2020). Article CAS PubMed Google Scholar * Seifer, P. et al. The Matrilin-3 T298M mutation predisposes for
post-traumatic osteoarthritis in a knock-in mouse model. _Osteoarthr. Cartil._ 29, 78–88 (2021). Article CAS Google Scholar * Westermann, L. M. et al. Imbalanced cellular metabolism
compromises cartilage homeostasis and joint function in a mouse model of mucolipidosis type III gamma. _Dis. Model Mech_.https://doi.org/10.1242/dmm.046425 (2020). * Franke, O. et al.
Mechanical properties of hyaline and repair cartilage studied by nanoindentation. _Acta Biomater._ 3, 873–881 (2007). Article CAS PubMed Google Scholar * Braet, F., Rotsch, C., Wisse, E.
& Radmacher, M. Comparison of fixed and living liver endothelial cells by atomic force microscopy. _Appl. Phys. A_ 66, S575–S578 (1998). Article CAS Google Scholar * Fiore, V. F. et
al. Mechanics of a multilayer epithelium instruct tumour architecture and function. _Nature_ 585, 433–439 (2020). Article CAS PubMed PubMed Central Google Scholar * Glentis, A. et al.
Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. _Nat. Commun._ 8, 924 (2017). Article PubMed PubMed Central Google Scholar
* Koester, J. et al. Niche stiffening compromises hair follicle stem cell potential during ageing by reducing bivalent promoter accessibility. _Nat. Cell Biol._ 23, 771–781 (2021). Article
CAS PubMed Google Scholar * Last, J. A., Liliensiek, S. J., Nealey, P. F. & Murphy, C. J. Determining the mechanical properties of human corneal basement membranes with atomic force
microscopy. _J. Struct. Biol._ 167, 19–24 (2009). Article CAS PubMed PubMed Central Google Scholar * Wareham, L. K. et al. Lysyl oxidase-like 1 deficiency alters ultrastructural and
biomechanical properties of the peripapillary sclera in mice. _Matrix Biol._ 16, 100120 (2022). Article CAS Google Scholar * Liyanage, S. et al. Optimization and validation of cryostat
temperature conditions for trans-reflectance mode FTIR microspectroscopic imaging of biological tissues. _MethodsX_ 4, 118–127 (2017). Article PubMed PubMed Central Google Scholar *
Ashkin, A., Dziedzic, J. M., Bjorkholm, J. E. & Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. _Opt. Lett._ 11, 288 (1986). Article CAS
PubMed Google Scholar * Marago, O. M., Jones, P. H., Gucciardi, P. G., Volpe, G. & Ferrari, A. C. Optical trapping and manipulation of nanostructures. _Nat. Nanotechnol._ 8, 807–819
(2013). Article CAS PubMed Google Scholar * Neuman, K. C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. _Nat.
Methods_ 5, 491–505 (2008). Article CAS PubMed PubMed Central Google Scholar * Marchi, G. et al. Microindentation sensor system based on an optical fiber Bragg grating for the
mechanical characterization of articular cartilage by stress-relaxation. _Sens. Actuators B Chem._ 252, 440–449 (2017). Article CAS Google Scholar * Wakitani, S. et al. Repair of large
full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. _Tissue Eng._ 4, 429–444 (1998). Article CAS PubMed Google Scholar * Moutos,
F. T., Freed, L. E. & Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. _Nat. Mater._ 6, 162–167 (2007). Article CAS
PubMed Google Scholar * Schwarz, S. et al. Contactless vibrational analysis of transparent hydrogel structures using laser-doppler vibrometry. _Exp. Mech._ 60, 1067–1078 (2020). Article
CAS Google Scholar * Bhave, G., Colon, S. & Ferrell, N. The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness. _Am. J. Physiol. Ren.
Physiol._ 313, F596–F602 (2017). Article CAS Google Scholar * Fisher, R. F. & Wakely, J. The elastic constants and ultrastructural organization of a basement membrane (lens capsule).
_Proc. R. Soc. Lond. B_ 193, 335–358 (1976). Article CAS PubMed Google Scholar * Wisdom, K. M. et al. Covalent cross-linking of basement membrane-like matrices physically restricts
invasive protrusions in breast cancer cells. _Matrix Biol._ 85-86, 94–111 (2020). Article CAS PubMed Google Scholar * Del Campo, L. et al. Vascular smooth muscle cell-specific progerin
expression in a mouse model of Hutchinson-Gilford progeria syndrome promotes arterial stiffness: therapeutic effect of dietary nitrite. _Aging Cell_ 18, e12936 (2019). Article PubMed
PubMed Central Google Scholar * Di Russo, J. et al. Endothelial basement membrane laminin 511 is essential for shear stress response. _EMBO J._ 36, 183–201 (2017). Article PubMed Google
Scholar * Steppan, J. et al. Lysyl oxidase-like 2 depletion is protective in age-associated vascular stiffening. _Am. J. Physiol. Heart Circ. Physiol._ 317, H49–H59 (2019). Article CAS
PubMed PubMed Central Google Scholar * Wenceslau, C. F. et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. _Am. J. Physiol. Heart
Circ. Physiol._ 321, H77–H111 (2021). Article CAS PubMed PubMed Central Google Scholar * Florin, E. L., Pralle, A., Horber, J. K. & Stelzer, E. H. Photonic force microscope based on
optical tweezers and two-photon excitation for biological applications. _J. Struct. Biol._ 119, 202–211 (1997). Article CAS PubMed Google Scholar * Catala-Castro, F., Schaffer, E. &
Krieg, M. Exploring cell and tissue mechanics with optical tweezers. _J. Cell Sci_. https://doi.org/10.1242/jcs.259355 (2022). * Cuthbertson, R. A. & Mandel, T. E. Anatomy of the mouse
retina. Capillary basement membrane thickness. _Invest. Ophthalmol. Vis. Sci._ 27, 1653–1658 (1986). CAS PubMed Google Scholar * Vracko, R., Thorning, D. & Huang, T. W. Basal lamina
of alveolar epithelium and capillaries: quantitative changes with aging and in diabetes mellitus. _Am. Rev. Respir. Dis._ 120, 973–983 (1979). CAS PubMed Google Scholar * Rico, F. et al.
Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. _Phys. Rev. E Stat. Nonlin. Soft Matter Phys._ 72, 021914 (2005). Article
PubMed Google Scholar * Chizhik, S. A., Wierzcholski, K., Trushko, A. V., Zhytkova, M. A. & Miszczak, A. Properties of cartilage on micro- and nanolevel. _Adv. Tribol._ 2010, 1–8
(2010). Article Google Scholar * Mak, A. F., Lai, W. M. & Mow, V. C. Biphasic indentation of articular cartilage–I. Theoretical analysis. _J. Biomech._ 20, 703–714 (1987). Article CAS
PubMed Google Scholar * Matzelle, T. R. et al. Micromechanical properties of “smart” gels: studies by scanning force and scanning electron microscopy of PNIPAAm. _J. Phys. Chem. B_ 106,
2861–2866 (2002). Article CAS Google Scholar * Bilodeau, G. G. Regular pyramid punch problem. _J. Appl. Mech._ 59, 519–523 (1992). Article Google Scholar * Dimitriadis, E. K., Horkay,
F., Maresca, J., Kachar, B. & Chadwick, R. S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. _Biophys. J._ 82, 2798–2810 (2002).
Article CAS PubMed PubMed Central Google Scholar * Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. _Science_ 313, 1642–1645 (2006). Article CAS
PubMed Google Scholar * Hell, S. W. Far-field optical nanoscopy. _Science_ 316, 1153–1158 (2007). Article CAS PubMed Google Scholar * Hell, S. W. Microscopy and its focal switch. _Nat.
Methods_ 6, 24–32 (2009). Article CAS PubMed Google Scholar * Friedl, P., Wolf, K., von Andrian, U. H. & Harms, G. Biological second and third harmonic generation microscopy. _Curr.
Protoc. Cell Biol._ 4, 4.15 (2007). Google Scholar * Evans, C. L. & Xie, X. S. Coherent anti-stokes Raman scattering microscopy: chemical imaging for biology and medicine. _Annu. Rev.
Anal. Chem._ 1, 883–909 (2008). Article CAS Google Scholar * Lu, F., Jin, M. & Belkin, M. A. Tip-enhanced infrared nanospectroscopy via molecular expansion force detection. _Nat.
Photonics_ 8, 307–312 (2014). Article CAS Google Scholar * Ruggeri, F. S. et al. Infrared nanospectroscopy reveals the molecular interaction fingerprint of an aggregation inhibitor with
single Abeta42 oligomers. _Nat. Commun._ 12, 688 (2021). Article CAS PubMed PubMed Central Google Scholar * Ruggeri, F. S., Mannini, B., Schmid, R., Vendruscolo, M. & Knowles, T. P.
J. Single molecule secondary structure determination of proteins through infrared absorption nanospectroscopy. _Nat. Commun._ 11, 2945 (2020). Article CAS PubMed PubMed Central Google
Scholar * Schillers, H. et al. Standardized nanomechanical atomic force microscopy procedure (SNAP) for measuring soft and biological samples. _Sci. Rep._ 7, 5117 (2017). Article PubMed
PubMed Central Google Scholar * Hutter, J. L. & Bechhoefer, J. Calibration of atomic‐force microscope tips. _Rev. Sci. Instrum._ 64, 1868–1873 (1993). Article CAS Google Scholar *
Bedzhov, I. & Zernicka-Goetz, M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. _Cell_ 156, 1032–1044 (2014). Article CAS PubMed
PubMed Central Google Scholar * Kyprianou, C. et al. Basement membrane remodelling regulates mouse embryogenesis. _Nature_ 582, 253–258 (2020). Article CAS PubMed PubMed Central Google
Scholar * Saraswathibhatla, A., Indana, D. & Chaudhuri, O. Cell–extracellular matrix mechanotransduction in 3D. _Nat. Rev. Mol. Cell Biol_. https://doi.org/10.1038/s41580-023-00583-1
(2023). * Sherwood, D. R. Basement membrane remodeling guides cell migration and cell morphogenesis during development. _Curr. Opin. Cell Biol._ 72, 19–27 (2021). Article CAS PubMed
PubMed Central Google Scholar * Candiello, J. et al. Biomechanical properties of native basement membranes. _FEBS J._ 274, 2897–2908 (2007). Article CAS PubMed Google Scholar *
Halfter, W. et al. The bi-functional organization of human basement membranes. _PLoS ONE_ 8, e67660 (2013). Article CAS PubMed PubMed Central Google Scholar * Halfter, W. et al. New
concepts in basement membrane biology. _FEBS J._ 282, 4466–4479 (2015). Article CAS PubMed Google Scholar * Henrich, P. B. et al. Nanoscale topographic and biomechanical studies of the
human internal limiting membrane. _Invest. Ophthalmol. Vis. Sci._ 53, 2561–2570 (2012). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS B.H., L.F. and H.C.-S.
acknowledge funding from the Bavarian State Ministry of Science and the Arts through the Bavarian Research Focus ‘Herstellung und biophysikalische Charakterisierung von dreidimensionalen
Geweben (CANTER)’ and the Bavarian Academic Forum (BayWISS)—Doctoral Consortium ‘Health Research’. The development of the data analysis software CANTER processing toolbox was funded by the
German Research Foundation as part of subproject 1 (CL 409/4-1/2) of the research consortium ‘Exploring articular cartilage and subchondral bone degeneration and regeneration in
osteoarthritis – ExCarBon’ (FOR2407-1/2). H.C.-S. acknowledges funding from the German Research Foundation through the major instrumentation campaign GGA-HAW (INST 99/38-1). This work was
further supported by the Danish Cancer Society (R204-A12454 (R.R.)). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Munich University of Applied Sciences, Center for Applied Tissue
Engineering and Regenerative Medicine – CANTER, Munich, Germany Bastian Hartmann, Lutz Fleischhauer & Hauke Clausen-Schaumann * Center for Nanoscience, Munich, Germany Bastian Hartmann,
Lutz Fleischhauer & Hauke Clausen-Schaumann * Experimental and Clinical Pharmacology and Toxicology, Medical Faculty, University of Freiburg, Freiburg, Germany Monica Nicolau &
Raphael Reuten * Department of Obstetrics and Gynecology, Medical Center, University of Freiburg, Freiburg, Germany Monica Nicolau, Florin-Andrei Taran & Raphael Reuten * Faculty of
Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark Thomas Hartvig Lindkær Jensen * Department of Pathology, Rigshospitalet, Copenhagen, Denmark Thomas Hartvig Lindkær
Jensen Authors * Bastian Hartmann View author publications You can also search for this author inPubMed Google Scholar * Lutz Fleischhauer View author publications You can also search for
this author inPubMed Google Scholar * Monica Nicolau View author publications You can also search for this author inPubMed Google Scholar * Thomas Hartvig Lindkær Jensen View author
publications You can also search for this author inPubMed Google Scholar * Florin-Andrei Taran View author publications You can also search for this author inPubMed Google Scholar * Hauke
Clausen-Schaumann View author publications You can also search for this author inPubMed Google Scholar * Raphael Reuten View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS All authors developed experimental protocols and designed experiments. B.H., L.F. and T.H.L.J. conducted the experiments. B.H., L.F. and M.N. developed the data
analysis tools. B.H., M.N. and F.-A.T. analyzed the data. H.C.-S. and R.R. conceived the ideas and contributed to experimental interpretation. B.H., H.C.-S. and R.R. wrote the manuscript.
All authors revised the manuscript. CORRESPONDING AUTHORS Correspondence to Hauke Clausen-Schaumann or Raphael Reuten. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Protocols_ thanks the anonymous reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS Key reference using this protocol
Reuten, R. et al. _Nat. Mater_. 20, 892–903 (2021): https://doi.org/10.1038/s41563-020-00894-0 Key data used in this protocol Reuten, R. et al. _Nat. Mater_. 20, 892–903 (2021):
https://doi.org/10.1038/s41563-020-00894-0 EXTENDED DATA EXTENDED DATA FIG. 1 BM’S YOUNG’S MODULUS LEVELS OF NET4 WILD TYPE VERSUS KNOCKOUT STRATIFIED INTO FEMALE AND MALE. Figure created
with BioRender.com. Source data EXTENDED DATA FIG. 2 HUMAN TISSUES WITH SIMILAR BASEMENT MEMBRANE ANATOMY. Figure created with BioRender.com and adapted with permission from ref. 30,
Springer Nature Limited. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Suplementary discussion and Figs. 1–20. SOURCE DATA SOURCE DATA FIG. 5 Young’s modulus values histograms. SOURCE
DATA FIG. 6 Log-transformed Young’s modulus values used to generate the histograms and the QQ-plot. SOURCE DATA FIG. 7 Log-transformed Young’s modulus values of the histograms. Young’s
modulus values (individual values and summary values) of the box plots in Fig. 7d. Standard deviation values of the box plots in Fig. 7e. SOURCE DATA EXTENDED DATA FIG. 1 Young’s modulus
values from Net4 WT and KO mice splitted into female and male (individual values and summary values) of the box plots in Extended Data Fig. 1. RIGHTS AND PERMISSIONS Springer Nature or its
licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the
accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Hartmann, B., Fleischhauer, L., Nicolau, M. _et al._ Profiling native pulmonary basement membrane stiffness using atomic force microscopy. _Nat Protoc_ 19, 1498–1528 (2024).
https://doi.org/10.1038/s41596-024-00955-7 Download citation * Received: 20 February 2023 * Accepted: 27 November 2023 * Published: 01 March 2024 * Issue Date: May 2024 * DOI:
https://doi.org/10.1038/s41596-024-00955-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative