- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
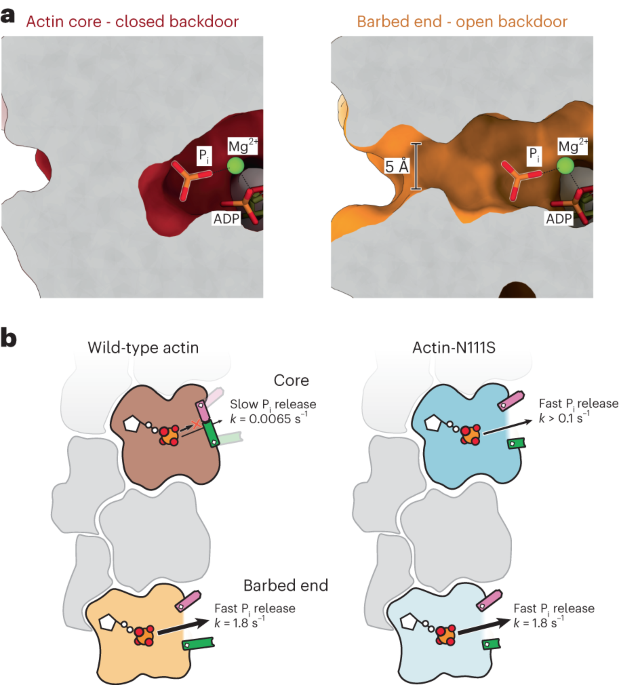
The release of inorganic phosphate (Pi) from actin marks old actin filaments for disassembly. By combining cryo-electron microscopy (cryo-EM) with in vitro reconstitution and molecular
dynamics simulations, we show how actin filaments release Pi through a ‘molecular backdoor’ and demonstrate that this arrangement is distorted in a disease-linked actin variant. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other
Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online
access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Merino, F., Pospich, S.
& Raunser, S. Towards a structural understanding of the remodeling of the actin cytoskeleton. _Semin. Cell Dev. Biol._ 102, 51–64 (2020). A REVIEW ARTICLE THAT DESCRIBES THE FUNDAMENTAL
FUNCTIONS OF ACTIN FILAMENTS IN CELLS AND HOW THEY ARE REGULATED BY ABPS. Article CAS PubMed Central Google Scholar * Oosterheert, W. et al. Structural basis of actin filament assembly
and aging. _Nature_ 611, 374–379 (2022). THIS PAPER, WHICH MOTIVATED OUR PI RELEASE STUDY, REVEALS HIGH RESOLUTION STRUCTURAL INSIGHTS INTO THE ASSEMBLY AND AGING OF ACTIN FILAMENTS. Article
CAS PubMed PubMed Central Google Scholar * Carlier, M. F. & Pantaloni, D. Direct evidence for ADP–Pi–F-actin as the major intermediate in ATP–actin polymerization. Rate of
dissociation of Pi from actin filaments. _Biochemistry_ 25, 7789–7792 (1986). THIS PIONEERING STUDY PROVIDES EVIDENCE THAT ADP-PI-BOUND F-ACTIN REPRESENTS A DISTINCT, METASTABLE STATE OF THE
FILAMENT. Article CAS PubMed Google Scholar * Jégou, A. et al. Individual actin filaments in a microfluidic flow reveal the mechanism of ATP hydrolysis and give insight into the
properties of profilin. _PLoS Biol._ 9, e1001161 (2011). THIS PAPER PROVIDES CONCLUSIVE EVIDENCE THAT PI RELEASE FROM F-ACTIN IS A STOCHASTIC PROCESS AND SHOWS THAT PI ESCAPES MUCH FASTER
FROM ACTIN SUBUNITS AT THE BARBED END THAN FROM THOSE AT THE FILAMENT CORE. Article PubMed PubMed Central Google Scholar * Lappalainen, P., Kotila, T., Jégou, A. & Romet-Lemonne, G.
Biochemical and mechanical regulation of actin dynamics. _Nat. Rev. Mol. Cell Biol._ 23, 836–852 (2022). A RECENT REVIEW ARTICLE THAT DISCUSSES HOW ACTIN FILAMENT TURNOVER IS REGULATED IN
DYNAMIC CELLULAR ACTIN NETWORKS. Article CAS PubMed Google Scholar Download references ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. THIS IS A SUMMARY OF: Oosterheert, W. et al. Molecular mechanisms of inorganic-phosphate release from the core and
barbed end of actin filaments. _Nat. Struct. Mol. Biol_. https://doi.org/10.1038/s41594-023-01101-9 (2023). RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE The great escape — how inorganic phosphate is released from actin filaments. _Nat Struct Mol Biol_ 30, 1621–1622 (2023). https://doi.org/10.1038/s41594-023-01102-8 Download citation
* Published: 29 September 2023 * Issue Date: November 2023 * DOI: https://doi.org/10.1038/s41594-023-01102-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative





