- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
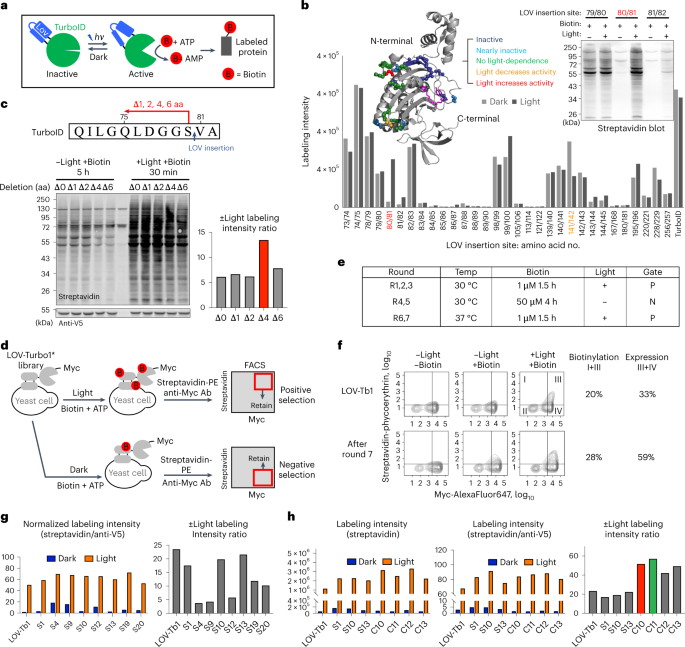
The incorporation of light-responsive domains into engineered proteins has enabled control of protein localization, interactions and function with light. We integrated optogenetic control
into proximity labeling, a cornerstone technique for high-resolution proteomic mapping of organelles and interactomes in living cells. Through structure-guided screening and directed
evolution, we installed the light-sensitive LOV domain into the proximity labeling enzyme TurboID to rapidly and reversibly control its labeling activity with low-power blue light.
‘LOV-Turbo’ works in multiple contexts and dramatically reduces background in biotin-rich environments such as neurons. We used LOV-Turbo for pulse-chase labeling to discover proteins that
traffic between endoplasmic reticulum, nuclear and mitochondrial compartments under cellular stress. We also showed that instead of external light, LOV-Turbo can be activated by
bioluminescence resonance energy transfer from luciferase, enabling interaction-dependent proximity labeling. Overall, LOV-Turbo increases the spatial and temporal precision of proximity
labeling, expanding the scope of experimental questions that can be addressed with proximity labeling.
The data associated with this study are available in the article and the Supplementary Information. The original mass spectra, spectral library and the protein sequence database used for
searches have been deposited in the public proteomics repository MassIVE (http://massive.ucsd.edu) and are accessible at ftp://massive.ucsd.edu/MSV000090683/. Additional data beyond that
provided in the figures and Supplementary Information are available from the corresponding author on request.
Rat cortical neurons were a kind gift from M. Lin (Stanford University). We thank J. Reinstein (Max Planck Institute) for helpful feedback. This work was supported by the NIH (grant nos.
R01-DK121409 and RC2DK129964 to A.Y.T., R01-OD026223 to A.Y.T. and S.A.C. and T32GM007276 to J.S.C.), the Stanford Wu Tsai Neurosciences Institute (A.Y.T.), the National Science Foundation
(NeuroNex grant no. 2014862 to A.Y.T. and GRFP DGE-1656518 to J.S.C.), the National Research Foundation of Korea grant no. NRF-2019R1A6A3A03033677 (S.-Y.L.), the Stanford Gerald J. Lieberman
Fellowship (J.S.C.) and the Burroughs Wellcome Fund grant no. CASI 1019469 (C.K.K.). A.Y.T. is a Chan Zuckerberg Biohub – San Francisco Investigator.
Present address: Center for Neuroscience and Department of Neurology, University of California, Davis, CA, USA
Present address: Amgen Research, South San Francisco, CA, USA
These authors contributed equally: Song-Yi Lee, Joleen S. Cheah.
Department of Genetics, Stanford University, Stanford, CA, USA
Song-Yi Lee, Boxuan Zhao, Christina K. Kim, Kelvin F. Cho & Alice Y. Ting
Department of Biology, Stanford University, Stanford, CA, USA
Department of Chemistry, Stanford University, Stanford, CA, USA
Chan Zuckerberg Biohub–San Francisco, San Francisco, CA, USA
S.-Y.L. and A.Y.T. conceived this project. S.-Y.L., J.S.C. and A.Y.T. designed experiments and analyzed all the data except those noted. S.-Y.L. and J.S.C. performed all experiments, unless
otherwise noted. N.D.U., C.X. and S.A.C. performed post-streptavidin-enrichment sample processing, mass spectrometry, and initial data analysis. B.Z. and S.-Y.L. performed the mouse brain
experiments. C.K.K., K.F.C. and S.-Y.L. performed cultured rat cortical neuron experiments. H.R. and S.-Y.L. performed BRET experiments. S.-Y.L., J.S.C. and A.Y.T. wrote the paper with input
from all authors.
S.-Y.L., J.S.C. and A.Y.T. have filed a patent application covering some aspects of this work (US Provisional Patent Application No. 63/488,940; CZ SF ref. CZB-273S-P1; Stanford ref.
S22-487; KT ref. 110221-1361830-009500PR). The remaining authors declare no competing interests.
Nature Methods thanks Angelos Constantinou, Tatsuya Sawasaki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rita
Strack, in collaboration with the Nature Methods team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Figs. 1–8, Table 1, Legends of Tables 2–4, Note 1, Methods, Antibodies list, Genetic constructs list and References.
Table 2: Proteomic data for ERM to nucleus pulse-chase experiment. Table 3: Proteomic data for ERM to mitochondria pulse-chase experiment. Table 4: Proteomic data at peptide level for ERM to
nucleus pulse-chase experiment.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author
self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Anyone you share the following link with will be able to read this content:






