- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Prime editors have a broad range of potential research and clinical applications. However, methods to delineate their genome-wide editing activities have generally relied on
indirect genome-wide editing assessments or the computational prediction of near-cognate sequences. Here we describe a genome-wide approach for the identification of potential prime editor
off-target sites, which we call PE-tag. This method relies on the attachment or insertion of an amplification tag at sites of prime editor activity to allow their identification. PE-tag
enables genome-wide profiling of off-target sites in vitro using extracted genomic DNA, in mammalian cell lines and in the adult mouse liver. PE-tag components can be delivered in a variety
of formats for off-target site detection. Our studies are consistent with the high specificity previously described for prime editor systems, but we find that off-target editing rates are
influenced by prime editing guide RNA design. PE-tag represents an accessible, rapid and sensitive approach for the genome-wide identification of prime editor activity and the evaluation of
prime editor safety. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12
print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be
subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR
CONTENT BEING VIEWED BY OTHERS MISMATCH PRIME EDITING GRNA INCREASED EFFICIENCY AND REDUCED INDELS Article Open access 02 January 2025 A WEB TOOL FOR THE DESIGN OF PRIME-EDITING GUIDE RNAS
Article 28 September 2020 PRIMEDESIGN SOFTWARE FOR RAPID AND SIMPLIFIED DESIGN OF PRIME EDITING GUIDE RNAS Article Open access 15 February 2021 DATA AVAILABILITY Illumina sequencing data
have been submitted to the Sequence Read Archive. mm10 and hg38 were used as reference genome. These datasets are available under BioProject accession number PRJNA811252. The authors declare
that all other data supporting the findings of this study are available within the paper and its Supplementary Information files. Backbone plasmids used for pegRNA and sgRNA cloning are
available from Addgene. Source data are provided with this paper. CODE AVAILABILITY The software used for data analysis is available at Github (Supplementary Note 6;
https://github.com/umasstr/GS-Preprocess and https://rdrr.io/github/LihuaJulieZhu/GUIDEseq/man/PEtagAnalysis.html). REFERENCES * Anzalone, A. V. et al. Search-and-replace genome editing
without double-strand breaks or donor DNA. _Nature_ 576, 149–157 (2019). CAS PubMed PubMed Central Google Scholar * Lin, Q. et al. Prime genome editing in rice and wheat. _Nat.
Biotechnol._ 38, 582–585 (2020). CAS PubMed Google Scholar * Liu, P. et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. _Nat. Commun._
12, 2121 (2021). CAS PubMed PubMed Central Google Scholar * Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime
editors. _Nat. Biotechnol._ 38, 824–844 (2020). CAS PubMed Google Scholar * Chen, P. J. et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes.
_Cell_ 184, 5635–5652 e5629 (2021). CAS PubMed PubMed Central Google Scholar * Nelson, J. W. et al. Engineered pegRNAs improve prime editing efficiency. _Nat. Biotechnol_. 40, 402–410
(2021). * Jang, H. et al. Application of prime editing to the correction of mutations and phenotypes in adult mice with liver and eye diseases. _Nat. Biomed. Eng._ 6, 181–194 (2021). *
Zheng, C. et al. A flexible split prime editor using truncated reverse transcriptase improves dual-AAV delivery in mouse liver. _Mol. Ther._ 30, 1343–1351 (2022). CAS PubMed PubMed Central
Google Scholar * Sheridan, C. CRISPR therapies march into clinic, but genotoxicity concerns linger. _Nat. Biotechnol._ 39, 897–899 (2021). CAS PubMed Google Scholar * Frock, R. L. et
al. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. _Nat. Biotechnol._ 33, 179–186 (2015). CAS PubMed Google Scholar * Tsai, S. Q. et al. GUIDE-seq
enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. _Nat. Biotechnol._ 33, 187–197 (2015). CAS PubMed Google Scholar * Kosicki, M., Tomberg, K. & Bradley, A.
Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. _Nat. Biotechnol._ 36, 765–771 (2018). CAS PubMed PubMed Central Google Scholar
* Bao, X. R., Pan, Y., Lee, C. M., Davis, T. H. & Bao, G. Tools for experimental and computational analyses of off-target editing by programmable nucleases. _Nat. Protoc._ 16, 10–26
(2021). CAS PubMed Google Scholar * Kim, D. Y., Moon, S. B., Ko, J. H., Kim, Y. S. & Kim, D. Unbiased investigation of specificities of prime editing systems in human cells. _Nucleic
Acids Res._ 48, 10576–10589 (2020). CAS PubMed PubMed Central Google Scholar * Kim, H. K. et al. Predicting the efficiency of prime editing guide RNAs in human cells. _Nat. Biotechnol._
39, 198–206 (2021). CAS PubMed Google Scholar * Tang, L. Prime editing progress. _Nat. Methods_ 18, 592 (2021). CAS PubMed Google Scholar * Liang, S. Q. et al. Genome-wide detection of
CRISPR editing in vivo using GUIDE-tag. _Nat. Commun._ 13, 437 (2022). CAS PubMed PubMed Central Google Scholar * Giannoukos, G. et al. UDiTaS, a genome editing detection method for
indels and genome rearrangements. _BMC Genomics_ 19, 212 (2018). PubMed PubMed Central Google Scholar * Petri, K. et al. CRISPR prime editing with ribonucleoprotein complexes in zebrafish
and primary human cells. _Nat. Biotechnol._ 40, 189–193 (2021). PubMed PubMed Central Google Scholar * Tomer, G., Buermeyer, A. B., Nguyen, M. M. & Liskay, R. M. Contribution of
human mlh1 and pms2 ATPase activities to DNA mismatch repair. _J. Biol. Chem._ 277, 21801–21809 (2002). CAS PubMed Google Scholar * Akcakaya, P. et al. In vivo CRISPR editing with no
detectable genome-wide off-target mutations. _Nature_ 561, 416–419 (2018). CAS PubMed PubMed Central Google Scholar * Wienert, B. et al. Unbiased detection of CRISPR off-targets in vivo
using DISCOVER-Seq. _Science_ 364, 286–289 (2019). CAS PubMed PubMed Central Google Scholar * Myerowitz, R. & Costigan, F. C. The major defect in Ashkenazi Jews with Tay-Sachs
disease is an insertion in the gene for the alpha-chain of beta-hexosaminidase. _J. Biol. Chem._ 263, 18587–18589 (1988). CAS PubMed Google Scholar * Kerem, B. S. et al. Identification of
mutations in regions corresponding to the two putative nucleotide (ATP)-binding folds of the cystic fibrosis gene. _Proc. Natl Acad. Sci. USA_ 87, 8447–8451 (1990). CAS PubMed PubMed
Central Google Scholar * Krishnaraj, R., Ho, G. & Christodoulou, J. RettBASE: Rett syndrome database update. _Hum. Mutat._ 38, 922–931 (2017). PubMed Google Scholar * Geurts, M. H.
et al. Evaluating CRISPR-based prime editing for cancer modeling and CFTR repair in organoids. _Life Sci. Alliance_ 4, e202000940 (2021). CAS PubMed PubMed Central Google Scholar * Cho,
S. W. et al. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. _Genome Res._ 24, 132–141 (2014). CAS PubMed PubMed Central Google Scholar *
Cameron, P. et al. Mapping the genomic landscape of CRISPR-Cas9 cleavage. _Nat. Methods_ 14, 600–606 (2017). CAS PubMed Google Scholar * Gao, R. et al. Genomic and transcriptomic analyses
of prime editing guide RNA-independent off-target effects by prime editors. _CRISPR J._ 5, 276–293 (2022). CAS PubMed Google Scholar * Liu, B. et al. A split prime editor with untethered
reverse transcriptase and circular RNA template. _Nat. Biotechnol._ 40, 1388–1393 (2022). CAS PubMed Google Scholar * Grunewald, J. et al. Engineered CRISPR prime editors with compact,
untethered reverse transcriptases. _Nat. Biotechnol._ 41, 337–343 (2022). PubMed Google Scholar * Yu, Z. et al. PEAC-seq adopts prime editor to detect CRISPR off-target and DNA
translocation. _Nat. Commun._ 13, 7545 (2022). CAS PubMed PubMed Central Google Scholar * Kwon, J. et al. TAPE-seq is a cell-based method for predicting genome-wide off-target effects of
prime editor. _Nat. Commun._ 13, 7975 (2022). CAS PubMed PubMed Central Google Scholar * Wang, D., Zhang, F. & Gao, G. CRISPR-based therapeutic genome editing: strategies and in
vivo delivery by AAV vectors. _Cell_ 181, 136–150 (2020). CAS PubMed PubMed Central Google Scholar * Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific
mRNA delivery and CRISPR-Cas gene editing. _Nat. Nanotechnol._ 15, 313–320 (2020). CAS PubMed PubMed Central Google Scholar * Petri, K. et al. Global-scale CRISPR gene editor specificity
profiling by ONE-seq identifies population-specific, variant off-target effects. Preprint at _bioRxiv_ https://doi.org/10.1101/2021.04.05.438458 (2021). * Shapiro, J. et al. Increasing
CRISPR efficiency and measuring its specificity in HSPCs using a clinically relevant system. _Mol. Ther. Methods Clin. Dev._ 17, 1097–1107 (2020). CAS PubMed PubMed Central Google Scholar
* Cancellieri, S. et al. Human genetic diversity alters off-target outcomes of therapeutic gene editing. _Nat. Genet._ 55, 34–43 (2023). CAS PubMed Google Scholar * Valley, H. C. et al.
Isogenic cell models of cystic fibrosis-causing variants in natively expressing pulmonary epithelial cells. _J. Cyst. Fibros._ 18, 476–483 (2019). CAS PubMed Google Scholar * Liu, P. et
al. Enhanced Cas12a editing in mammalian cells and zebrafish. _Nucleic Acids Res._ 47, 4169–4180 (2019). CAS PubMed PubMed Central Google Scholar * Giannoukos, G. et al. UDiTaS™, a
genome editing detection method for indels and genome rearrangements. _BMC Genomics_ 19, 212 (2018). PubMed PubMed Central Google Scholar * Rodriguez, T. C. et al. Genome-wide detection
and analysis of CRISPR-Cas off-targets. _Prog. Mol. Biol. Transl. Sci._ 181, 31–43 (2021). CAS PubMed Google Scholar * Zhu, L. J. et al. GUIDEseq: a bioconductor package to analyze
GUIDE-Seq datasets for CRISPR-Cas nucleases. _BMC Genomics_ 18, 379 (2017). PubMed PubMed Central Google Scholar * Clement, K. et al. CRISPResso2 provides accurate and rapid genome
editing sequence analysis. _Nat. Biotechnol._ 37, 224–226 (2019). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank members of the Xue Lab and Wolfe
Lab for helpful discussions. We thank H. Valley and M. Mense at the Cystic Fibrosis Foundation Therapeutic Lab for providing HBE cells. W.X. was supported by grants from the National
Institutes of Health (DP2HL137167, P01HL158506 and UH3HL147367) and the Cystic Fibrosis Foundation. S.A.W., P.L. and K.P. were supported in part by the National Institutes of Health (grants
R01HL120669 and UG3TR002668) and the Rett Syndrome Research Trust. C.K. and P.C. were funded by the Synthetic Biology Platform at the Wyss Institute for Biologically Inspired Engineering and
by the MIT Media Lab consortia of sponsors. AUTHOR INFORMATION Author notes * These authors contributed equally: Shun-Qing Liang, Pengpeng Liu. AUTHORS AND AFFILIATIONS * RNA Therapeutics
Institute, University of Massachusetts Chan Medical School, Worcester, MA, USA Shun-Qing Liang, Zexiang Chen, Erik J. Sontheimer & Wen Xue * Department of Molecular, Cell and Cancer
Biology, University of Massachusetts Chan Medical School, Worcester, MA, USA Pengpeng Liu, Karthikeyan Ponnienselvan, Sneha Suresh, Lihua Julie Zhu, Wen Xue & Scot A. Wolfe * Wyss
Institute, Harvard Medical School, Boston, MA, USA Christian Kramme & Pranam Chatterjee * Media Lab, Massachusetts Institute of Technology, Cambridge, MA, USA Pranam Chatterjee *
Department of Biomedical Engineering, Duke University, Durham, NC, USA Pranam Chatterjee * Department of Molecular Medicine, University of Massachusetts Chan Medical School, Worcester, MA,
USA Lihua Julie Zhu, Erik J. Sontheimer & Wen Xue * Program in Bioinformatics and Integrative Biology, University of Massachusetts Chan Medical School, Worcester, MA, USA Lihua Julie Zhu
* Li Weibo Institute for Rare Diseases Research, University of Massachusetts Chan Medical School, Worcester, MA, USA Erik J. Sontheimer, Wen Xue & Scot A. Wolfe Authors * Shun-Qing
Liang View author publications You can also search for this author inPubMed Google Scholar * Pengpeng Liu View author publications You can also search for this author inPubMed Google Scholar
* Karthikeyan Ponnienselvan View author publications You can also search for this author inPubMed Google Scholar * Sneha Suresh View author publications You can also search for this author
inPubMed Google Scholar * Zexiang Chen View author publications You can also search for this author inPubMed Google Scholar * Christian Kramme View author publications You can also search
for this author inPubMed Google Scholar * Pranam Chatterjee View author publications You can also search for this author inPubMed Google Scholar * Lihua Julie Zhu View author publications
You can also search for this author inPubMed Google Scholar * Erik J. Sontheimer View author publications You can also search for this author inPubMed Google Scholar * Wen Xue View author
publications You can also search for this author inPubMed Google Scholar * Scot A. Wolfe View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
S.-Q.L. and P.P.L. performed experiments, analyzed data and wrote the manuscript with co-authors. K.P. prepared protein. S.S. generated the HEK293T1278+TATC cell line. Z.C. prepared PE2
mRNA. C.K. and P.C. generated the HEK293TT158M cell line. L.J.Z. performed bioinformatic analysis. E.J.S., W.X. and S.A.W. supervised the study and wrote the manuscript with all co-authors.
CORRESPONDING AUTHORS Correspondence to Pengpeng Liu, Wen Xue or Scot A. Wolfe. ETHICS DECLARATIONS COMPETING INTERESTS University of Massachusetts has filed a patent application (serial no.
63/328076) on PE-tag in this work. S.A.W. is a consultant for Chroma Medicine and serves on the S.A.B. for Graphite Bio. W.X. is a consultant for the Cystic Fibrosis Foundation Therapeutics
Lab. All remaining authors declare that the research was conducted in the absence of commercial or financial conflict of interest. The authors declare no competing nonfinancial interests.
PEER REVIEW PEER REVIEW INFORMATION _Nature Methods_ thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary
Handling Editors: Lei Tang and Madhura Mukhopadhyay, in collaboration with the _Nature Methods_ team. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
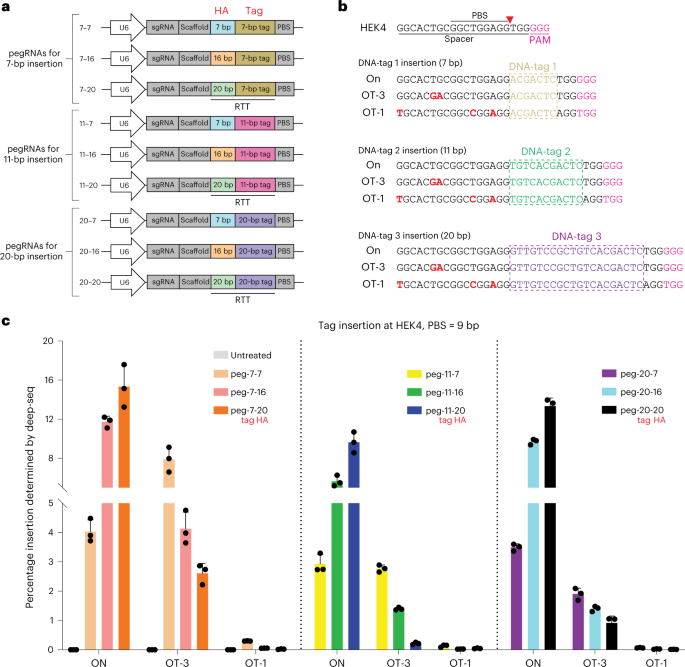
jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 DNA-TAG INTEGRATION AT TARGET SITE AND OFF TARGET SITES BY PE2. a, Comparison of
the PE2 prime editing efficiency as a function of different tag and HA lengths within the pegRNA at the Pcsk9 target site and OT-1 in Hepa1-6 cells, where prime editing components were
delivered by transient transfection. Insertion rates at the target site are precise edits, whereas percent editing at OT1 captures indels as well as tag insertions. Frequencies of editing
were quantified by deep sequencing from PCR amplicons spanning each locus. Results were obtained from three independent experiments and presented as mean ± SD. b, Comparison of the prime
editing efficiency for different tag and HA lengths inserted by PE2 at the VEGFA target site and OT-1 in HEK293T cells. Editing rates are determined by Illumina sequencing PCR amplicons
spanning each locus. Frequencies of editing were quantified by deep sequencing from PCR amplicons spanning each locus. Results were obtained from three independent experiments and presented
as mean ± SD. Source data EXTENDED DATA FIG. 2 BIOCHEMICAL CONDITIONS FOR _IN VITRO_ PE-TAG. a, Schematic overview of quantification of 3′ flap generation by qRT-PCR at the _HEK4_ locus.
HEK293T gDNA was treated with PE2 RNP to introduce the 3′ flap and then the editing efficiency was quantified by qRT-PCR with a tag-specific primer and a locus-specific primer. A pair of
primers located ~2000 bp upstream of the target site serve as an internal control for gDNA normalization. b, HEK293T gDNA was treated with different concentrations of PE2 RNP to introduce
the 3′ flap and then the editing efficiency was quantified by qRT-PCR. c, HEK293T gDNA was treated with 50 pmol of PE2 RNP to introduce the 3′ flap for different reaction times and then the
editing efficiency was quantified by qRT-PCR. Results were obtained from three independent experiments and presented as mean ± SD. **** P < 0.0001 by one-way ANOVA with Tukey’s multiple
comparisons test. d, HEK293T gDNA was treated with 50 pmol of PE2 RNP to introduce the 3′ flap in a buffer containing different concentrations of dNTPs and then the editing efficiency was
quantified by qRT-PCR. Results were obtained from three independent experiments and presented as mean ± SD. **** P < 0.0001 by one-way ANOVA with Tukey’s multiple comparisons test. e,
HEK293T gDNA was treated with 50 pmol of PE2 RNP to introduce the 3′ flap at different reaction temperatures and then the editing efficiency was quantified by qRT-PCR. Results were obtained
from three independent experiments and presented as mean ± SD. ** P < 0.01 and *** P < 0.001 by one-way ANOVA with Tukey’s multiple comparisons test. f, HEK293T gDNA was treated with
50 pmol of PE2 RNP to introduce the 3′ flap for two different reaction times (2 hrs and 24 hrs) and then the editing efficiency was quantified by qRT-PCR on target site and two OTs for the
_HEK4_ site. Results were obtained from three independent experiments and presented as mean ± SD. **** P < 0.0001 by unpaired, two-tailed Student’s t-test. Source data EXTENDED DATA FIG.
3 THE PRIME EDITING EFFICIENCY OF 3′ FLAP GENERATION WITH A SERIES OF PEGRNAS. a, The prime editing efficiency of 3′ flap generation with a series of pegRNAs which contain either one or two
mismatches in the PBS region. HEK293T gDNA was treated with PE2 RNP containing the _HEK4_ 20-7 pegRNA to introduce the 3′ flap for 2 hours, and then the flap incorporation efficiency was
quantified by qRT-PCR with a tag-specific primer and a locus-specific primer. A pair of primers located ~2000 bp upstream of the target site serve as an internal control for data analysis.
b, The efficiency of 3′ flap generation at HEK OT3 with a series of _HEK4_ 20-7 pegRNAs which contain either one or two mismatches in the PBS region. HEK293T gDNA was treated with PE2 RNP to
introduce the 3′ flap, and then the editing efficiency was quantified by qRT-PCR with a tag-specific primer and a locus-specific primer. A pair of primers located ~2000 bp upstream of the
target site serve as an internal control for data analysis. Where shown, bar charts indicate the mean and error bars are s.d. of n = 3 independent qRT-PCR experiments. Source data EXTENDED
DATA FIG. 4 _IN VITRO_ PE-TAG ON PURIFIED GDNA. a-b, Subset of potential off-target (OT) sites identified by _in vitro_ PE-tag in PE2 RNP treated HEK293T gDNA at CDH4 locus (a) and VEGFA
locus (b; Supplementary Data 1). Mismatches in the PBS and HA region of potential off-target sites relative to the target site (On) are shown in red and blue, respectively. UMI counts for
each site are shown. c, Venn diagram of overlap between off-target sites discovered by _in vitro_ PE-tag (UMI > 1) and previously described GUIDE-seq data for VEGFA site 211. Source data
EXTENDED DATA FIG. 5 PRIME EDITING AT ON TARGET SITE AND TWO OFF TARGET SITES IN HEK293T CELLS. a, (left) Comparison of precise editing efficiency for nucleotide substitution, targeted 1-bp
deletion, and 1-bp insertion with PE2 at _HEK4_ (ON) target site and (right) indel rates at two off-target sites (OT-1 and OT-3) in HEK293T cells after co-transfecting pegRNA and PE2
expression plasmids. pegRNA sequence composition and type of sequence modification encoded is indicated in the legend, where the terminal numbers indicate the different HA lengths within the
RTT. Frequencies of precise editing or indel rates were quantified by deep sequencing from PCR amplicons spanning each locus. Mock on target site editing represents all indels. Results were
obtained from three independent experiments and presented as mean ± SD. *_P_ < 0.05, ** _P_ < 0.01 and *** _P_ < 0.001 by unpaired, two-tailed Student’s t-test. To adjust for
multiple comparisons, _p_-values were adjusted using the Benjamini-Hochberg (BH) method. b, indel rates for nucleotide substitution, targeted 1-bp deletion, and 1-bp insertion pegRNAs with
PE2 at 6 additional potential off-target sites identified by PE-tag for _HEK4_ locus pegRNA in HEK293T cells. pegRNA sequence composition and type of sequence modification encoded is
indicated in the legend, where the terminal numbers indicate the different HA lengths within the RTT. Frequencies of precise editing were quantified by deep sequencing from PCR amplicons
spanning each locus. Results were obtained from three independent experiments and presented as mean ± SD. *_P_ < 0.05, ** _P_ < 0.01 and *** _P_ < 0.001 by unpaired, two-tailed
Student’s t-test. To adjust for multiple comparisons, _p_-values were adjusted using the Benjamini-Hochberg (BH) method. Source data EXTENDED DATA FIG. 6 OFF TARGET SITES VALIDATION IN
HEK293T CELLS. a, Indel rates for nucleotide substitution, targeted 1-bp deletion, and 1-bp insertion pegRNAs with PE2 at 8 potential off-target sites of top 20 OTs identified by GUIDE-seq
that overlap with _in vitro_ PE-tag for _HEK4_ locus pegRNA in HEK293T cells. Frequencies of editing were quantified by deep sequencing from PCR amplicons spanning each locus. Results were
obtained from three independent experiments and presented as mean ± SD. *_P_ < 0.05, ** _P_ < 0.01 and *** _P_ < 0.001 by two-way ANOVA with Tukey’s multiple comparisons test. b,
Indel rates for nucleotide substitution, targeted 1-bp deletion, and 1-bp insertion pegRNAs with PE2 at 12 potential off-target sites of top 20 OTs identified by GUIDE-seq but absent in the
_in vitro_ PE-tag for _HEK4_ locus pegRNA in HEK293T cells. pegRNA sequence composition and type of sequence modification encoded is indicated in the legend, where the terminal numbers
indicate the different HA lengths within the RTT. Frequencies of editing were quantified by deep sequencing from PCR amplicons spanning each locus. Results were obtained from three
independent experiments and presented as mean ± SD. *_P_ < 0.05, ** _P_ < 0.01 and *** _P_ < 0.001 by two-way ANOVA with Tukey’s multiple comparisons test. c, Editing outcomes with
PE2 and 1-bp deletion pegRNA at _HEK4_ MISS-2 and MISS-7 in HEK293T cells. Frequencies of editing were quantified by deep sequencing of PCR amplicons spanning the locus. CRISPResso output
shown for sequencing data. Source data EXTENDED DATA FIG. 7 CAS9 H840A AND MMLV RT PROTEINS ARE FUNCTIONALLY INDEPENDENTLY _IN VITRO_ IN PE-TAG SYSTEM. a, Schematic overview of the _in
vitro_ tag attachment in the human genome by purified PE2 or purified Cas9 H840A nickase and MMLV RT. gDNA is isolated from HEK293T cells and treated with indicated protein and a 20-7
pegRNA, resulting in a 20 bp tag attachment in the protospacer of on-target site. b, PE-tag was carried out _in vitro_ on purified HEK293T gDNA with three different protein cocktails: 1)
purified PE2 protein (Wolfe lab purified); 2) purified Cas9 H840A nickase and MMLV RT as separate proteins (Wolfe lab purified); 3) purified Cas9 H840A nickase (IDT) and MMLV RT
(Thermofisher) as separate proteins using the _HEK4_ 20-7 pegRNA for PE-tag. Locus specific primers (deep sequencing primer) were used to detect tag incorporation at the target site
(on-target) and off-target site 3 (OT-3). All three systems were able to incorporate the sequencing tag into the target locus demonstrating that the MMLV RT can function in trans to the
SpCas9 nickase for _in vitro_ reactions. * indicates the expected PCR product size. Results were obtained from three independent experiments and representative results are shown. c, Venn
diagram of overlap of PE potential off-target sites (UMI ≥ 1) discovered by three different protein cocktails. d, Subset of _in vitro_ off-target (OT) sites identified. Mismatches in the PBS
and HA region of potential off-target sites relative to the target site (On) are shown in red and blue, respectively. UMI counts for each site are shown. Source data EXTENDED DATA FIG. 8
PE-TAG IN HEK293T CELLS. a and b, Subset of potential off-target (OT) sites identified by PE-tag using PE2 RNP or expression plasmid treated cells at VEGFA locus (a) and CDH4 locus (b;
Supplementary Data 1). Mismatches in the PBS and HA region for potential off-target sites relative to the target site (On) are shown in red and blue, respectively. UMI counts for each site
are shown for each treatment. c, Indel rates for tag insertion pegRNAs with PE2 at top 5 potential off-target sites identified by _in vitro_ PE-tag for VEGFA locus pegRNA in HEK293T cells.
Indel frequencies were quantified by deep sequencing from PCR amplicons spanning each locus. Results were obtained from three independent experiments and presented as mean ± SD. ** _P_ <
0.01 and *** _P_ < 0.001 by unpaired, two-tailed Student’s t-test. To adjust for multiple comparisons, _p_-values were adjusted using the Benjamini-Hochberg (BH) method. Source data
EXTENDED DATA FIG. 9 PRIME EDITING AT A SUBSET OF OFF-TARGET SITES FOR THREE PATHOGENIC CORRECTING PEGRNAS. a, Venn diagram of overlap between potential off-target sites (UMI > 1)
discovered by _in vitro_ PE-tag and potential off-target sites discovered by GUIDE-tag in cell lines containing the pathogenic sequences treated with SpCas9 RNP and DSB tagging
oligonucleotide. b-c, Comparison of editing rates by prime editor programmed with pegRNA to correct pathogenic sequence at a subset of potential OT sites identified by PE-tag in cells
transfected with PE2 mRNA and pegRNA, plasmids expressing PE2 and pegRNA, or plasmids expressing PE2 and epegRNA at the CFTR locus (b) and MECP2 locus (c), respectively. Frequencies of
editing rates were quantified by deep sequencing. Results were obtained from three independent experiments and presented as mean ± SD. *_P_ < 0.05, ** _P_ < 0.01 and *** _P_ < 0.001
by unpaired, two-tailed Student’s t-test. To adjust for multiple comparisons, _p_-values were adjusted using the Benjamini-Hochberg (BH) method. Source data EXTENDED DATA FIG. 10 DOT PLOT
OF UMI COUNT PERCENTAGE. Dot plot of UMI count percentage (UMI%) associated with the target site and discovered potential off-target sites for 5 pegRNAs (a) and 2 pegRNAs (b) analyzed by _in
vitro_ PE-tag, PE-tag in cells by PE2 plasmid delivery and PE-tag in cells by PE2 RNP or mRNA delivery. Red symbols indicate the target site. Blue or green symbols indicate the top
off-target site with the remainder as black symbols. Source data file is provided. Source data SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Notes 1–6, Supplementary
Figs. 1–12 and Supplementary Tables 1–4. REPORTING SUMMARY PEER REVIEW FILE SUPPLEMENTARY DATA 1 Off-target sites identified by PE-tag at different sites. SOURCE DATA SOURCE DATA FIGS. 1–5
AND EXTENDED DATA FIGS. 1–10 Statistical source data for Figs. 1–5 and Extended Data Fig. 1–10. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds
exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely
governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liang, SQ., Liu, P., Ponnienselvan, K. _et al._
Genome-wide profiling of prime editor off-target sites in vitro and in vivo using PE-tag. _Nat Methods_ 20, 898–907 (2023). https://doi.org/10.1038/s41592-023-01859-2 Download citation *
Received: 13 April 2022 * Accepted: 17 March 2023 * Published: 08 May 2023 * Issue Date: June 2023 * DOI: https://doi.org/10.1038/s41592-023-01859-2 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative