- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Microglia, the brain’s resident macrophages, help to regulate brain function by removing dying neurons, pruning non-functional synapses, and producing ligands that support neuronal
survival1. Here we show that microglia are also critical modulators of neuronal activity and associated behavioural responses in mice. Microglia respond to neuronal activation by suppressing
neuronal activity, and ablation of microglia amplifies and synchronizes the activity of neurons, leading to seizures. Suppression of neuronal activation by microglia occurs in a highly
region-specific fashion and depends on the ability of microglia to sense and catabolize extracellular ATP, which is released upon neuronal activation by neurons and astrocytes. ATP triggers
the recruitment of microglial protrusions and is converted by the microglial ATP/ADP hydrolysing ectoenzyme CD39 into AMP; AMP is then converted into adenosine by CD73, which is expressed on
microglia as well as other brain cells. Microglial sensing of ATP, the ensuing microglia-dependent production of adenosine, and the adenosine-mediated suppression of neuronal responses via
the adenosine receptor A1R are essential for the regulation of neuronal activity and animal behaviour. Our findings suggest that this microglia-driven negative feedback mechanism operates
similarly to inhibitory neurons and is essential for protecting the brain from excessive activation in health and disease. Access through your institution Buy or subscribe This is a preview
of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more
Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS:
* Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MICROGLIAL GI-DEPENDENT DYNAMICS REGULATE BRAIN NETWORK
HYPEREXCITABILITY Article 14 December 2020 RHOA BALANCES MICROGLIAL REACTIVITY AND SURVIVAL DURING NEUROINFLAMMATION Article Open access 20 October 2023 METABOLIC FLEXIBILITY ENSURES PROPER
NEURONAL NETWORK FUNCTION IN MODERATE NEUROINFLAMMATION Article Open access 22 June 2024 DATA AVAILABILITY The gene expression data related to this study are available at the NCBI Gene
Expression Omnibus (GEO) under accession number GSE149897. Source data are provided with this paper. CODE AVAILABILITY The code used for analysis of calcium transience in neurons to analyse
event rates, magnitude, spatial correlation and synchrony can be found at https://github.com/GradinaruLab/striatum2P. REFERENCES * Werneburg, S., Feinberg, P. A., Johnson, K. M. &
Schafer, D. P. A microglia-cytokine axis to modulate synaptic connectivity and function. _Curr. Opin. Neurobiol_. 47, 138–145 (2017). CAS PubMed PubMed Central Google Scholar * Li, Y.,
Du, X. F., Liu, C. S., Wen, Z. L. & Du, J. L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. _Dev. Cell_ 23, 1189–1202 (2012). CAS PubMed
Google Scholar * Eyo, U. B. et al. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. _J. Neurosci_.
34, 10528–10540 (2014). PubMed PubMed Central Google Scholar * Akiyoshi, R. et al. Microglia enhance synapse activity to promote local network synchronization. _eNeuro_ 5,
ENEURO.0088-18.2018 (2018). PubMed PubMed Central Google Scholar * Kato, G. et al. Microglial contact prevents excess depolarization and rescues neurons from excitotoxicity. _eNeuro_ 3,
ENEURO.0004-16.2016 (2016). PubMed PubMed Central Google Scholar * Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional
state of synapses in vivo and determine the fate of ischemic terminals. _J. Neurosci_. 29, 3974–3980 (2009). CAS PubMed PubMed Central Google Scholar * Peng, J. et al. Microglial P2Y12
receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice. _Mol. Brain_ 12, 71 (2019). PubMed PubMed Central Google Scholar * Cserép, C. et al. Microglia
monitor and protect neuronal function through specialized somatic purinergic junctions. _Science_ 367, 528–537 (2020). ADS PubMed Google Scholar * Bernier, L. P. et al. Nanoscale
surveillance of the brain by microglia via cAMP-regulated filopodia. _Cell Rep_. 27, 2895–2908.e4 (2019). CAS PubMed Google Scholar * Madry, C. et al. Microglial ramification,
surveillance, and interleukin-1β release are regulated by the two-pore domain K+ channel THIK-1. _Neuron_ 97, 299–312.e6 (2018). CAS PubMed PubMed Central Google Scholar * Liu, Y. U. et
al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. _Nat. Neurosci_. 22, 1771–1781 (2019). CAS PubMed PubMed Central Google
Scholar * Stowell, R. D. et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. _Nat. Neurosci_. 22,
1782–1792 (2019). CAS PubMed PubMed Central Google Scholar * Elmore, M. R. P. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a
microglia progenitor cell in the adult brain. _Neuron_ 82, 380–397 (2014). CAS PubMed PubMed Central Google Scholar * Bozzi, Y. & Borrelli, E. The role of dopamine signaling in
epileptogenesis. _Front. Cell. Neurosci_. 7, 157 (2013). PubMed PubMed Central Google Scholar * Chitu, V., Gokhan, Ş., Nandi, S., Mehler, M. F. & Stanley, E. R. Emerging roles for
CSF-1 receptor and its ligands in the nervous system. _Trends Neurosci_. 39, 378–393 (2016). CAS PubMed PubMed Central Google Scholar * Kana, V. et al. CSF-1 controls cerebellar
microglia and is required for motor function and social interaction. _J. Exp. Med_. 216, 2265–2281 (2019). CAS PubMed PubMed Central Google Scholar * Easley-Neal, C., Foreman, O.,
Sharma, N., Zarrin, A. A. & Weimer, R. M. CSF1R ligands IL-34 and CSF1 are differentially required for microglia development and maintenance in white and gray matter brain regions.
_Front. Immunol_. 10, 2199 (2019). CAS PubMed PubMed Central Google Scholar * Saunders, A. et al. Molecular diversity and specializations among the cells of the adult mouse brain. _Cell_
174, 1015–1030.e16 (2018). CAS PubMed PubMed Central Google Scholar * Wenzel, M., Hamm, J. P., Peterka, D. S. & Yuste, R. Acute focal seizures start as local synchronizations of
neuronal ensembles. _J. Neurosci_. 39, 8562–8575 (2019). CAS PubMed PubMed Central Google Scholar * Pankratov, Y., Lalo, U., Verkhratsky, A. & North, R. A. Vesicular release of ATP
at central synapses. _Pflugers Arch_. 452, 589–597 (2006). CAS PubMed Google Scholar * Pascual, O. et al. Neurobiology: astrocytic purinergic signaling coordinates synaptic networks.
_Science_ 310, 113–116 (2005). CAS ADS PubMed Google Scholar * Corkrum, M. et al. Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. _Neuron_ 105,
1036–1047.e5 (2020). CAS PubMed PubMed Central Google Scholar * Beamer, E., Conte, G. & Engel, T. ATP release during seizures—a critical evaluation of the evidence. _Brain Res.
Bull_. 151, 65–73 (2019). CAS PubMed Google Scholar * Haynes, S. E. et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. _Nat. Neurosci_. 9, 1512–1519
(2006). CAS PubMed Google Scholar * Ayata, P. et al. Epigenetic regulation of brain region-specific microglia clearance activity. _Nat. Neurosci_. 21, 1049–1060 (2018). CAS PubMed
PubMed Central Google Scholar * Madry, C. et al. Effects of the ecto-ATPase apyrase on microglial ramification and surveillance reflect cell depolarization, not ATP depletion. _Proc. Natl
Acad. Sci. USA_ 115, E1608–E1617 (2018). CAS PubMed PubMed Central Google Scholar * Dissing-Olesen, L. et al. Activation of neuronal NMDA receptors triggers transient ATP-mediated
microglial process outgrowth. _J. Neurosci_. 34, 10511–10527 (2014). PubMed PubMed Central Google Scholar * Robson, S. C., Sévigny, J. & Zimmermann, H. The E-NTPDase family of
ectonucleotidases: structure function relationships and pathophysiological significance. _Purinergic Signal_. 2, 409–430 (2006). CAS PubMed PubMed Central Google Scholar * Lanser, A. J.
et al. Disruption of the ATP/adenosine balance in CD39−/− mice is associated with handling-induced seizures. _Immunology_ 152, 589–601 (2017). CAS PubMed PubMed Central Google Scholar *
Dunwiddie, T. V. & Masino, S. A. The role and regulation of adenosine in the central nervous system. _Annu. Rev. Neurosci_. 24, 31–55 (2001). CAS PubMed Google Scholar * Zimmermann,
H., Zebisch, M. & Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. _Purinergic Signal_. 8, 437–502 (2012). CAS PubMed PubMed Central Google Scholar *
Flagmeyer, I., Haas, H. L. & Stevens, D. R. Adenosine A1 receptor-mediated depression of corticostriatal and thalamostriatal glutamatergic synaptic potentials in vitro. _Brain Res_. 778,
178–185 (1997). CAS PubMed Google Scholar * Yabuuchi, K. et al. Role of adenosine A1 receptors in the modulation of dopamine D1 and adenosine A2A receptor signaling in the neostriatum.
_Neuroscience_ 141, 19–25 (2006). CAS PubMed Google Scholar * Trusel, M. et al. Coordinated regulation of synaptic plasticity at striatopallidal and striatonigral neurons orchestrates
motor control. _Cell Rep_. 13, 1353–1365 (2015). CAS PubMed Google Scholar * Zhou, S. et al. Pro-inflammatory effect of downregulated CD73 expression in EAE astrocytes. _Front. Cell.
Neurosci_. 13, 233 (2019). CAS PubMed PubMed Central Google Scholar * Bateup, H. S. et al. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic
drugs. _Nat. Neurosci_. 11, 932–939 (2008). CAS PubMed PubMed Central Google Scholar * Wendeln, A. C. et al. Innate immune memory in the brain shapes neurological disease hallmarks.
_Nature_ 556, 332–338 (2018). CAS ADS PubMed PubMed Central Google Scholar * Süß, P. et al. Chronic peripheral inflammation causes a region-specific myeloid response in the central
nervous system. _Cell Rep_. 30, 4082–4095.e6 (2020). PubMed Google Scholar * Krasemann, S. et al. The TREM2–APOE pathway drives the transcriptional phenotype of dysfunctional microglia in
neurodegenerative diseases. _Immunity_ 47, 566–581.e9 (2017). CAS PubMed PubMed Central Google Scholar * Mildner, A., Huang, H., Radke, J., Stenzel, W. & Priller, J. P2Y12 receptor
is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. _Glia_ 65, 375–387 (2017). PubMed Google Scholar *
Palop, J. J. et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. _Neuron_ 55, 697–711 (2007).
CAS PubMed PubMed Central Google Scholar * Lam, A. D. et al. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. _Nat. Med_. 23, 678–680
(2017). CAS PubMed PubMed Central Google Scholar * Wohleb, E. S., Franklin, T., Iwata, M. & Duman, R. S. Integrating neuroimmune systems in the neurobiology of depression. _Nat.
Rev. Neurosci_. 17, 497–511 (2016). CAS PubMed Google Scholar * Spangenberg, E. E. et al. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β
pathology. _Brain_ 139, 1265–1281 (2016). PubMed PubMed Central Google Scholar * Bejar, R., Yasuda, R., Krugers, H., Hood, K. & Mayford, M. Transgenic calmodulin-dependent protein
kinase II activation: dose-dependent effects on synaptic plasticity, learning, and memory. _J. Neurosci_. 22, 5719–5726 (2002). CAS PubMed PubMed Central Google Scholar * Alexander, G.
M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. _Neuron_ 63, 27–39 (2009). CAS PubMed PubMed Central Google Scholar *
Stanley, S. et al. Profiling of glucose-sensing neurons reveals that ghrh neurons are activated by hypoglycemia. _Cell Metab_. 18, 596–607 (2013). CAS PubMed Google Scholar * Parkhurst,
C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. _Cell_ 155, 1596–1609 (2013). CAS PubMed PubMed Central Google Scholar *
Wang, Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. _Nat. Immunol_. 13, 753–760 (2012). CAS PubMed PubMed Central
Google Scholar * Tronche, F. et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. _Nat. Genet_. 23, 99–103 (1999). CAS PubMed Google
Scholar * Harris, S. E. et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. _Bone_ 50, 42–53 (2012). CAS PubMed Google Scholar * Rothweiler, S. et
al. Selective deletion of ENTPD1/CD39 in macrophages exacerbates biliary fibrosis in a mouse model of sclerosing cholangitis. _Purinergic Signal_. 15, 375–385 (2019). CAS PubMed PubMed
Central Google Scholar * Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. _Immunity_ 38, 79–91 (2013). CAS PubMed Google
Scholar * Scammell, T. E. et al. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. _J. Neurosci_. 23, 5762–5770 (2003). CAS PubMed PubMed
Central Google Scholar * Thompson, L. F. et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. _J. Exp. Med_. 200, 1395–1405 (2004). CAS PubMed PubMed
Central Google Scholar * André, P. et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. _J. Clin. Invest_. 112, 398–406 (2003).
PubMed PubMed Central Google Scholar * Oakley, H. et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease
mutations: potential factors in amyloid plaque formation. _J. Neurosci_. 26, 10129–10140 (2006). CAS PubMed PubMed Central Google Scholar * Casanova, E. et al. A CamKIIα iCre BAC allows
brain-specific gene inactivation. _Genesis_ 31, 37–42 (2001). CAS PubMed Google Scholar * Doyle, J. P. et al. Application of a translational profiling approach for the comparative
analysis of CNS cell types. _Cell_ 135, 749–762 (2008). CAS PubMed PubMed Central Google Scholar * Heiman, M. et al. A translational profiling approach for the molecular characterization
of CNS cell types. _Cell_ 135, 738–748 (2008). CAS PubMed PubMed Central Google Scholar * von Schimmelmann, M. et al. Polycomb repressive complex 2 (PRC2) silences genes responsible for
neurodegeneration. _Nat. Neurosci_. 19, 1321–1330 (2016). Google Scholar * Kim, D. & Salzberg, S. L. TopHat-Fusion: an algorithm for discovery of novel fusion transcripts. _Genome
Biol_. 12, R72 (2011). CAS PubMed PubMed Central Google Scholar * Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data.
_Bioinformatics_ 31, 166–169 (2015). CAS PubMed Google Scholar * Purushothaman, I. & Shen, L. SPEctRA: a scalable pipeline for RNA -seq ana lysis.
https://zenodo.org/record/60547#.X1khQDNKjIU (2016). * Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. _Genome Biol_.
15, 550 (2014). PubMed PubMed Central Google Scholar * Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. _Nature_ 541, 481–487 (2017). CAS ADS
PubMed PubMed Central Google Scholar * Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. _Nat. Neurosci_. 16, 1896–1905 (2013). CAS PubMed PubMed Central
Google Scholar * Howe, E. A., Sinha, R., Schlauch, D. & Quackenbush, J. RNA-seq analysis in MeV. _Bioinformatics_ 27, 3209–3210 (2011). CAS PubMed PubMed Central Google Scholar *
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. _BMC Bioinformatics_ 14, 128 (2013). PubMed PubMed Central Google Scholar * Kuleshov,
M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. _Nucleic Acids Res_. 44 (W1), W90−W97 (2016). CAS PubMed PubMed Central Google Scholar * Gokce,
O. et al. Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-seq. _Cell Rep_. 16, 1126–1137 (2016). CAS PubMed PubMed Central Google Scholar * Bohlen, C. J.,
Bennett, F. C. & Bennett, M. L. Isolation and culture of microglia. _Curr. Protoc. Immunol_. 125, e70 (2019). PubMed Google Scholar * Butovsky, O. et al. Identification of a unique
TGF-β-dependent molecular and functional signature in microglia. _Nat. Neurosci_. 17, 131–143 (2014). CAS PubMed Google Scholar * Gabriel, L. R., Wu, S. & Melikian, H. E. Brain slice
biotinylation: an ex vivo approach to measure region-specific plasma membrane protein trafficking in adult neurons. _J. Vis. Exp_. 86, e51240 (2014). Google Scholar * Crupi, M. J. F.,
Richardson, D. S. & Mulligan, L. M. Cell surface biotinylation of receptor tyrosine kinases to investigate intracellular trafficking. _Methods Mol. Biol_. 1233, 91–102 (2015). PubMed
Google Scholar * Sullivan, J. M. et al. Autism-like syndrome is induced by pharmacological suppression of BET proteins in young mice. _J. Exp. Med_. 212, 1771–1781 (2015). CAS PubMed
PubMed Central Google Scholar * Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. _J. Neurosci.
Methods_ 291, 83–94 (2017). CAS PubMed Google Scholar * Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at
https://www.biorxiv.org/content/10.1101/061507v2 (2016). * Yoder, N. C. peakfinder(x0, sel, thresh, extrema, includeEndpoints, interpolate).
https://www.mathworks.com/matlabcentral/fileexchange/25500-peakfinder-x0-sel-thresh-extrema-includeendpoints-interpolate (Matlab Central File Exchange, 2016). * Klaus, A. et al. The
spatiotemporal organization of the striatum encodes action space. _Neuron_ 95, 1171–1180.e7 (2017). CAS PubMed PubMed Central Google Scholar * Barbera, G. et al. Spatially compact neural
clusters in the dorsal striatum encode locomotion relevant information. _Neuron_ 92, 202–213 (2016). CAS PubMed PubMed Central Google Scholar * Kato, D. et al. in _Microglia. Methods in
Molecular Biology_ (eds. Garaschuk, O. & Verkhratsky A.) (Humana, 2019). * Thévenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity.
_IEEE Trans. Image Process_. 7, 27–41 (1998). ADS PubMed Google Scholar * Ting, J. T. et al. Preparation of acute brain slices using an optimized _N_-methyl-d-glucamine protective
recovery method. _J. Vis. Exp_. 132, e53825 (2018). Google Scholar * Fieblinger, T. et al. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced
dyskinesia. _Nat. Commun_. 5, 5316 (2014). CAS ADS PubMed Google Scholar * Graves, S. M. & Surmeier, D. J. Delayed spine pruning of direct pathway spiny projection neurons in a mouse
model of parkinson’s disease. _Front. Cell. Neurosci_. 13, 32 (2019). CAS PubMed PubMed Central Google Scholar * Wong, J. M. T. et al. Benzoyl chloride derivatization with liquid
chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples. _J. Chromatogr. A_ 1446, 78–90 (2016). CAS PubMed PubMed Central Google Scholar *
Gangarossa, G. et al. Convulsant doses of a dopamine D1 receptor agonist result in Erk-dependent increases in Zif268 and Arc/Arg3.1 expression in mouse dentate gyrus. _PLoS One_ 6, e19415
(2011). CAS ADS PubMed PubMed Central Google Scholar * Bunch, L. & Krogsgaard-Larsen, P. Subtype selective kainic acid receptor agonists: discovery and approaches to rational
design. _Med. Res. Rev_. 29, 3–28 (2009). CAS PubMed Google Scholar * Willoughby, J. O., Mackenzie, L., Medvedev, A. & Hiscock, J. J. Distribution of Fos-positive neurons in cortical
and subcortical structures after picrotoxin-induced convulsions varies with seizure type. _Brain Res_. 683, 73–87 (1995). CAS PubMed Google Scholar * Sipe, G. O. et al. Microglial P2Y12
is necessary for synaptic plasticity in mouse visual cortex. _Nat. Commun_. 7, 10905 (2016). CAS ADS PubMed PubMed Central Google Scholar * Racine, R. J. Modification of seizure
activity by electrical stimulation. II. Motor seizure. _Electroencephalogr. Clin. Neurophysiol_. 32, 281–294 (1972). CAS PubMed Google Scholar * Silverman, J. L., Yang, M., Lord, C. &
Crawley, J. N. Behavioural phenotyping assays for mouse models of autism. _Nat. Rev. Neurosci_. 11, 490–502 (2010). CAS PubMed PubMed Central Google Scholar * Langfelder, P. et al.
Integrated genomics and proteomics define huntingtin CAG length-dependent networks in mice. _Nat. Neurosci_. 19, 623–633 (2016). CAS PubMed PubMed Central Google Scholar * Wang, Y. et
al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. _Cell_ 160, 1061–1071 (2015). CAS PubMed PubMed Central Google Scholar * Keren-Shaul, H. et al.
A unique microglia type associated with restricting development of Alzheimer’s disease. _Cell_ 169, 1276–1290.e17 (2017). CAS PubMed Google Scholar * Sousa, C. et al. Single-cell
transcriptomics reveals distinct inflammation-induced microglia signatures. _EMBO Rep_. 19, e46171 (2018). PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We
thank P. Greengard and A. Nairn for sharing the DARPP32 antibodies; J. J. Badimon for the Ticagrelor and Clopidogrel; R. Greene for the _Adora1__fl/fl_ mice; M. Merad and F. Desland for the
_Csf1__fl/fl_; _Nestin__Cre_ mice, the MSSM FACS facility and J. Ochando, C. Bare, and G. Viavattene for assistance with flow cytometry analysis; A. Lopez and A. Watters for assistance with
microdialysis experiments; G. Milne and the Vanderbilt University Neurochemistry Core for LC–MS analysis; D. Wagenaar and CalTech Neurotechnology Laboratory for help with construction of the
two-photon system; and all Schaefer laboratory members and A. Tarakhovsky for discussions and critical comments on the manuscript. This work was supported by the National Institutes of
Health (NIH) Director New Innovator Award DP2 MH100012-01 (A.S.), NIH grants R01NS091574 (A.S.), R01MH118329 (A.S.), DA047233 (A.S.), R01NS106721 (A.S.) and U01AG058635 (A.S.), a Robin
Chemers Neustein Award (P.A.), NIH grant RO1AG045040 (J.X.J.), Welch Foundation Grant AQ-1507 (J.X.J.), NARSAD Young Investigator Award no. 25065 (P.A.), NIH grants T32AG049688 (A.B.),
T32AI078892 (A.T.C.), 1K99NS114111 (M.A.W.), T32CA207201 (M.A.W.), R01NS102807 (F.J.Q.), R01AI126880 (F.J.Q.), and R01ES025530 (F.J.Q.), a TCCI Chen Graduate Fellowship (X.C.), an A*STAR
National Science Scholarship (A.N.), the CZI Neurodegeneration Challenge Network (V.G.), NIH BRAIN grant RF1MH117069 (V.G.), NIH grants HL107152 (S.C.R.), HL094400 (S.C.R.), AI066331
(S.C.R.), GM-136429 (W.G.J.), GM-51477 (W.G.J.), GM-116162 (W.G.J.), HD-098363 (W.G.J.), DA042111 (E.S.C.), DA048931 (E.S.C.), funds from a VUMC Faculty Research Scholar Award (M.G.K.), the
Brain and Behavior Research Foundation (M.G.K. and E.S.C), the Whitehall Foundation (E.S.C.), and the Edward Mallinckrodt Jr. Foundation (E.S.C.). The Vanderbilt University Neurochemistry
Core is supported by the Vanderbilt Brain Institute and the Vanderbilt Kennedy Center (EKS NICHD of NIH Award U54HD083211). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Nash Family
Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, USA Ana Badimon, Hayley J. Strasburger, Pinar Ayata, Philip Hwang, Andrew T. Chan, Masago Ishikawa,
Yong-Hwee E. Loh, Paul J. Kenny & Anne Schaefer * Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, USA Ana Badimon, Hayley J. Strasburger, Pinar Ayata,
Philip Hwang, Andrew T. Chan & Anne Schaefer * Center for Glial Biology, Icahn School of Medicine at Mount Sinai, New York, NY, USA Ana Badimon, Hayley J. Strasburger, Pinar Ayata,
Philip Hwang, Andrew T. Chan & Anne Schaefer * Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai, New York, NY, USA Pinar Ayata & Anne Schaefer *
Division of Biology and Biological Engineering, California Institute of Technology, Pasadena, CA, USA Xinhong Chen, Aditya Nair, Anat Kahan & Viviana Gradinaru * Department of Anatomy
and Molecular Cell Biology, Nagoya University Graduate School of Medicine, Nagoya, Japan Ako Ikegami & Hiroaki Wake * Division of System Neuroscience, Kobe University Graduate School of
Medicine, Kobe, Japan Ako Ikegami & Hiroaki Wake * Department of Pharmacology, University of Minnesota, Minneapolis, MN, USA Steven M. Graves * Center for Brain Immunology and Glia,
Department of Neuroscience, University of Virginia, Charlottesville, VA, USA Joseph O. Uweru & Ukpong B. Eyo * Department of Surgery, Beth Israel Deaconess Medical Center, Harvard
Medical School, Boston, MA, USA Carola Ledderose & Wolfgang G. Junger * Department of Pharmacology, Vanderbilt University, Nashville, TN, USA Munir Gunes Kutlu & Erin S. Calipari *
Ann Romney Center for Neurologic Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA Michael A. Wheeler & Francisco J. Quintana * Department of Genetics and
Genomic Sciences, Icahn Institute of Data Science and Genomic Technology, Icahn School of Medicine at Mount Sinai, New York, NY, USA Ying-Chih Wang & Robert Sebra * Department of
Biochemistry and Structural Biology, University of Texas Health Science Center, San Antonio, TX, USA Jean X. Jiang * Department of Physiology, Feinberg School of Medicine, Northwestern
University, Chicago, IL, USA D. James Surmeier * Department of Anesthesia, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA Simon C. Robson * Department of
Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA Simon C. Robson * Vanderbilt Brain Institute, Vanderbilt University, Nashville, TN, USA Erin S.
Calipari * Vanderbilt Center for Addiction Research, Vanderbilt University, Nashville, TN, USA Erin S. Calipari * Department of Molecular Physiology and Biophysics, Vanderbilt University,
Nashville, TN, USA Erin S. Calipari * Department of Psychiatry and Behavioral Sciences, Vanderbilt University, Nashville, TN, USA Erin S. Calipari * Department of Pathology and Immunology,
Washington University School of Medicine, St. Louis, MO, USA Marco Colonna * The Broad Institute of MIT and Harvard, Cambridge, MA, USA Francisco J. Quintana Authors * Ana Badimon View
author publications You can also search for this author inPubMed Google Scholar * Hayley J. Strasburger View author publications You can also search for this author inPubMed Google Scholar *
Pinar Ayata View author publications You can also search for this author inPubMed Google Scholar * Xinhong Chen View author publications You can also search for this author inPubMed Google
Scholar * Aditya Nair View author publications You can also search for this author inPubMed Google Scholar * Ako Ikegami View author publications You can also search for this author inPubMed
Google Scholar * Philip Hwang View author publications You can also search for this author inPubMed Google Scholar * Andrew T. Chan View author publications You can also search for this
author inPubMed Google Scholar * Steven M. Graves View author publications You can also search for this author inPubMed Google Scholar * Joseph O. Uweru View author publications You can also
search for this author inPubMed Google Scholar * Carola Ledderose View author publications You can also search for this author inPubMed Google Scholar * Munir Gunes Kutlu View author
publications You can also search for this author inPubMed Google Scholar * Michael A. Wheeler View author publications You can also search for this author inPubMed Google Scholar * Anat
Kahan View author publications You can also search for this author inPubMed Google Scholar * Masago Ishikawa View author publications You can also search for this author inPubMed Google
Scholar * Ying-Chih Wang View author publications You can also search for this author inPubMed Google Scholar * Yong-Hwee E. Loh View author publications You can also search for this author
inPubMed Google Scholar * Jean X. Jiang View author publications You can also search for this author inPubMed Google Scholar * D. James Surmeier View author publications You can also search
for this author inPubMed Google Scholar * Simon C. Robson View author publications You can also search for this author inPubMed Google Scholar * Wolfgang G. Junger View author publications
You can also search for this author inPubMed Google Scholar * Robert Sebra View author publications You can also search for this author inPubMed Google Scholar * Erin S. Calipari View author
publications You can also search for this author inPubMed Google Scholar * Paul J. Kenny View author publications You can also search for this author inPubMed Google Scholar * Ukpong B. Eyo
View author publications You can also search for this author inPubMed Google Scholar * Marco Colonna View author publications You can also search for this author inPubMed Google Scholar *
Francisco J. Quintana View author publications You can also search for this author inPubMed Google Scholar * Hiroaki Wake View author publications You can also search for this author
inPubMed Google Scholar * Viviana Gradinaru View author publications You can also search for this author inPubMed Google Scholar * Anne Schaefer View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS A.S. and A.B. conceived and designed the study. A.B. did molecular, behavioural, FACS and imaging experiments. H.J.S. did primary
neuronal culture, microglia isolation, microglia culture, FACS and Axion microelectrode array experiments. P.A. did in vivo TRAP experiments. A.B., X.C., A.N., V.G. and A.S. designed
two-photon imaging experiments, which were performed by X.C. and A.N. A.K. built the customized two-photon system. A.B., A.I., H.W. and A.S. designed the two-photon imaging of microglial
protrusions, which was performed by A.I. A.T.C. and R.S. performed single-nucleus 10X sequencing. Y.-C.W. analysed single-nucleus 10X sequencing data. Y.-H.E.L. analysed bulk RNA-seq data
from TRAP experiments. A.S., D.J.S. and S.M.G. designed experiments to measure neuronal excitability that were conducted by S.M.G. A.B., M.I., P.J.K. and A.S. designed experiments to measure
sEPSCs that were conducted by M.I. A.S. and A.B. designed and P.H. performed molecular and imaging experiments. C.L. and W.G.J. conducted the HPLC analysis. M.G.K. and E.S.C. conducted the
microdialysis experiments. A.B., J.O.U. and U.B.E. conducted seizure susceptibility experiments on _P2ry12_−_/_− mice. S.C.R. generated _Cd39__fl/fl_ mice. J.X.J. generated _Csf1__fl/fl_
mice. M.C. generated _Il34__fl/fl_ mice. M.A.W. and F.J.Q. generated _Cd39__fl/fl__Cx3cr1__CreErt2/+(Jung)_ mice. A.B., M.A.W., F.J.Q. and A.S. designed behavioural experiments. A.S. and
A.B. wrote the manuscript. All authors discussed results, and provided input and edits on the manuscript. CORRESPONDING AUTHOR Correspondence to Anne Schaefer. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature_ thanks Ania Majewska and the other, anonymous, reviewer(s) for their
contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED
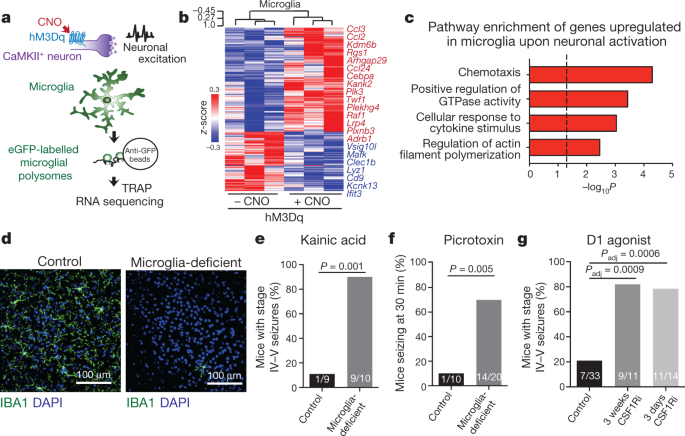
DATA FIGURES AND TABLES EXTENDED DATA FIG. 1 DREADD-BASED MOUSE MODELS TO STUDY MICROGLIA RESPONSES TO NEURONAL ACTIVATION AND INHIBITION REVEALS DISTINCT MICROGLIA RESPONSES. A, B,
Neuron-specific activation (A) and inhibition (B) has been achieved by the expression of the Gq-coupled (activating) hM3Dq or Gi-coupled (inhibiting) hM4Di in _CaMKII_+ forebrain neurons.
The _CaMKII-tTa_ mice were bred to either _tetO-CHRM3_ or _tetO-CHRM4_ mice to generate _CaMKII-tTa; tetO-CHRM3_ or CaMKII-tTa;_ tetO-CHRM4_ mice. hM3Dq or hM4Di were activated by i.p.
injection of clozapine-N-oxide (CNO) to activate (0.25 mg kg–1) or inhibit (1 mg kg–1) _CaMKII+_ neuronal activity, respectively. C-E, Validation of CNO-mediated neuronal activation and
inhibition: C, Heatmap (left) and violin plot (right) show RNA expression levels of 18 immediate early genes in total striatum 2 h after CNO-mediated neuronal inhibition (orange) or neuronal
activation (blue) as compared with controls (_n_ = 2 _CaMKII-tTa; tetO-CHRM4, n = 5_ control, and _n_ = _3 CaMKII-tTa; tetO-CHRM3_ mice) (right, _P_ = 0.0001, One-way ANOVA (Kruskal–Wallis
test) with Dunn’s multiple comparison test). D, Dot plot showing quantification of the average number of cFOS+ cells in the dorsal striatum of _CaMKII-tTa; tetO-CHRM4_ (orange, _n_ = 4
mice), control (black, _n_ = 6 mice), and _CaMKII-tTa; tetO-CHRM3_ (blue, _n_ = 4 mice) mice one hour after treatment with CNO (_P_ = 0.0004, One-way ANOVA with Tukey’s post hoc test). E,
Representative images showing cFOS+ cells (green) in the striatum of _CaMKII-tTa; tetO-CHRM4_ (top), control (middle), and _CaMKII-tTa; tetO-CHRM3_ (bottom) mice in response to CNO, DAPI
(blue) (image are representative of two independent cohorts of mice). F, To allow for the microglia-specific analysis of changes in ribosome-associated RNA levels following neuron
inhibition, the _CaMKII-tTa; tetO-CHRM4_ mice were bred to _Cx3cr1__CreErt2/+(Litt)__; Eef1a1__LSL.eGFPL10a/+_ mice followed by tamoxifen-induced Cre-mediated L10a-eGFP expression in
microglia. G, Changes in ribosome-bound mRNA levels in striatal microglia were determined using the TRAP-sequencing approach. The heatmap shows the variation in the expression levels of 135
upregulated and 220 downregulated genes (_z_-scored log2(RPKM) at 2 h following CNO-mediated neuronal inhibition. H, Selected gene ontology (using GO) annotations for upregulated genes
(using DESeq2) in striatal microglia in response to neuronal inhibition, GO analysis was performed using ENRICHR analysis69,70 (dotted line, _P_ = 0.05). I, Venn diagrams comparing
microglial genes up- and downregulated following _CaMKII__+_ neuronal activation and inhibition reveals highly differential microglia response. J, qPCR confirmation of increased mRNA
expression (lower ΔCT, normalized to _Gapdh_) in microglia upon neuronal activation (_Ccl3_, left, _n_ = 3 mice, _P_ = 0.059, unpaired two-tailed _t_-test) and neuronal inhibition (_Cd74_,
right, _n_ = 2 mice). K, Dot plots show lack of expression changes in selected genes in the striatum of wild type mice 2 h after saline, 0.25 mg kg–1 CNO injection, or 1 mg kg–1 CNO
injection (_n_ = 3, 3, and 4 mice; _Kdm6b: P =_ 0.70 _Adrb1: P =_ 0.22, _Ccl24: P =_ 0.54, _Ccl3: P =_ 0.43, _Kcnk13: P =_ 0.37, _Ikbkb, P =_ 0.62, One-way ANOVA with Tukey’s post hoc test).
RPKM: reads per kilobase of transcript per million mapped reads, TRAP: translating ribosome affinity purification; Data shown as mean ± s.e.m. Source data EXTENDED DATA FIG. 2 MICROGLIA
DEFICIENT MICE SHOW NORMAL BASELINE BEHAVIOURS BUT EXAGGERATED RESPONSES TO NEUROSTIMULANTS. A, Dot plots show the average number of microglia per mm2 in cortex, striatum, cerebellum and
hippocampus in control and microglia deficient mice (_n_ = 3 and 4 mice, cortex: _P_ < 0.0001, striatum: _P_ = 0.0003, cerebellum: _P_ = 0.0001, DG: _P_ < 0.0001, CA3: _P_ < 0.0001,
CA1: _P_ < 0.0001, unpaired two-tailed _t_-test). B-E, Behavioural characteristics of microglia deficient mice. B, Anxiety-like behaviour was measured by the ratio of time spent in the
open arms/closed arms in the elevated plus maze (_n_ = 10 mice, _P_ = 0.65, unpaired two-tailed _t_-test). C, Motor coordination was measured by latency to fall from the accelerating rotarod
(_n_ = 8 and 12 mice, interaction: _P_ = 0.89, time: _P_ = 0.13, treatment: _P_ = 0.36; subjects: _P_ < 0.0001, Two-way repeated measures ANOVA). D, Olfactory behaviour was measured by
the sniff test (_n_ = 21 and 13 mice, _P_ = 0.09, unpaired two-tailed _t_-test). E, Social behaviour was measured by using the classic three-chamber sociability task (Social preference:
mouse preference for sniffing another mouse over object, Control: _n_ = 7 mice; _P_ = 0.0002, microglia deficient: _n_ = 9 mice, _P_ < 0.0001; Social Memory: mouse preference for sniffing
novel mouse over familiar mouse, Control _n_ = 7 mice, _P_ = 0.0023, microglia deficient: _n_ = 7 mice, _P_ = 0.0009; paired two-tailed _t_-test). F, Representative images show brain-wide
gene expression patterns of receptors targeted by kainic acid (kainate and AMPA receptor), picrotoxin (GABAA receptor), and SKF81297 (D1 receptor) (Allen Institute). G, Number of stage IV-V
seizures (Racine scale92) per mouse visually recorded within one hour in response to kainic acid (18 mg kg–1, i.p.) are shown as a dot plot (_n_ = 9 and 10 mice, _P_ = 0.0008, unpaired
two-tailed _t_-test). H, Dot plot showing distance travelled in response to D1 agonist in one hour in the open field (SKF81297, 3 mg kg–1, i.p.)(_n_ = 14 and 8 mice, _P_ = 0.025, unpaired
two-tailed _t_-test). I, Representative cortical EEG traces during a tonic-clonic seizure event in response to D1 agonist treatment (SKF81297, 5 mg kg–1 i.p.) in control (top) and microglia
deficient (bottom) mice showing high amplitude and rhythmic discharges followed by EEG depression. DG: dentate gyrus; Data shown as mean ± s.e.m. Source data EXTENDED DATA FIG. 3 GENERATION
AND CHARACTERIZATION OF _IL34-_DEFICIENT AND _CSF1-_DEFICIENT MICE. A, Violin plots show the expression levels of cell-type specific representative marker genes across the 10 identified cell
types from striatum snRNA-seq data analysis. Black dots indicate mean expression of selected gene per cell type. B, In situ hybridization for _Il34_ (left) and _Csf1_ (right) mRNAs show
differential, region-specific expression in cortex, striatum, CA1, dentate gyrus (DG), CA3, corpus callosum (CC), and cerebellum of wild-type mice (WM: white matter, GM: grey matter, ML:
molecular layer, GCL: granule cell layer, scale bar = 100μm). C, H, The striatal grey matter-specific or white matter-specific microglia depletion was achieved by breeding _Nestin__Cre/+_
mice to _Il34__fl/fl_ mice or _Csf1__fl/fl_ mice, respectively, to generate _Il34__fl/fl__; Nestin__Cre/+_ (purple, C) and _Csf1__fl/fl__; Nestin__Cre/+_ mice (blue, H). D, I, Dot plots
showing relative expression levels of _Il34_ and _Csf1_ mRNA normalized to _Gapdh_ in the striatum of _Il34__fl/fl__; Nestin__Cre/+_ mice (D) or _Csf1__fl/fl_; _Nestin_CRE_/+_ mice (I)
compared with littermate controls (D, _n_ = 4 mice each, _Il34 P <_ 0.0001, _Csf1 P =_ 0.69; I, _n_ = 3 and 5 mice, _Il34 P =_ 0.07, _Csf1 P_ < 0.0001, unpaired two-tailed _t_-test).
E, Dot plots show the average microglia density per mm2 per mouse in cortex, striatum, cerebellum (cortex: _n_ = 9, 12, and 10 mice, _P_ < 0.0001, striatum: _n_ = 9, 13, and 10 mice, _P_
< 0.0001, cerebellum: _n_ = 7, 7, and 8 mice, _P_ = 0.34, One-way ANOVA with Tukey’s post hoc test). F, left, Dot plot shows levels of IL34 protein as determined by western blot analysis
of striatal protein lysate from _Il34__fl/fl__, Il34__fl/+__; Nestin__Cre/+_ or _Il34__fl/fl__; Nestin__Cre/+_ mice normalized to DARPP32 expression (_n_ = 3 mice, _P_ = 0.0077, One-way
ANOVA with Tukey’s post hoc test). G, J, Bar graphs show the average percentage of white matter regions in striatal images (0.5mm × 0.5mm) used to count WM and GM microglia in control and
mutant mice for the data shown in Fig. 2c and e. (G, _P_ = 0.99, _n_ = 4 and 3 mice, unpaired two-tailed _t_-test; J, _n_ = 4 and 2 mice). For gel source data, see Supplementary Fig. 1. Data
shown as mean ± s.e.m. Source data EXTENDED DATA FIG. 4 GENERATION OF MICE WITH STRIATUM-SPECIFIC MICROGLIA DEPLETION. A, B, (left), The striatum-specific microglia depletion was achieved
by breeding _Il34__fl/fl_ mice to _Drd1a__Cre/+_ or _Drd2__Cre/+_ mice to generate _Il34__fl/fl__; Drd1a__Cre/+_ (A, green) and _Il34__fl/fl__; Drd2__Cre/+_ mice (B, grey). Right, dot plots
show relative expression of _Il34_ mRNA in the striatum normalized to _Gapdh_ (A, _n_ = 6 and 7 mice, _P_ < 0.0001; B, _n_ = 4 mice, _P_ = 0.0004, unpaired two-tailed _t_-test). C,
Representative striatal images of sagittal brain slices from _Il34__fl/fl__, Il34__fl/fl__; Drd1a__Cre/+_ and _Il34__fl/fl__; Drd2__Cre/+_ mice following immunofluorescent staining for
P2RY12 (microglia, green) and DAPI (nuclei, blue) (scale bar = 50μm). D, E, Dot plots show the average microglia density per mm2 per mouse per specific region in the hippocampus of
_Il34__fl/fl__; Drd1a__Cre/+_ (D) and _Il34__fl/fl__; Drd2__Cre/+_ mice (E) compared to littermate controls (D, _n_ = 3 mice, DG: _P_ = 0.88, CA3: _P_ = 0.85, CA1: _P_ = 0.1; E, _n_ = 3
mice, DG: _P_ = 0.69, CA3: _P_ = 0.56, CA1: _P_ = 0.72; unpaired two-tailed _t_-test). F, G, Dot plots showing total distance travelled in response to D1 agonist (SKF81297, 3 mg kg–1, i.p.)
in one hour in the open field for _Il34__fl/fl__; Drd1a__Cre/+_ (F) and _Il34__fl/fl__; Drd2__Cre/+_ mice (G) compared with littermate controls (F: _n_ = 8 and 9 mice, _P_ = 0.034 G: _n_ = 8
mice, _P_ = 0.0087, unpaired two-tailed _t_-test). H, Percentage of mice seizing 30 min after administration of picrotoxin (1 mg kg–1, i.p.) shown as a bar graph (_n_ = 21, 9, and 8 mice;
_P_ = 0.80, Chi-squared test). DG: dentate gyrus. I, Microglia-neuron ratio defines the threshold of D1 neuron activation by D1 agonist. Bar graph shows the percentage of mice with stage
IV-V seizures in response to D1 agonist (4 mg kg–1, i.p.) in control, _Il34__fl/fl__; Drd1a__Cre/+_, and microglia deficient mice (_n_ = 11, 13, and 9 mice; right, _P_ = 0.0005, Chi-squared
test). While all mice display an increased seizure response to 5 mg kg–1D1 agonist treatment, only microglia deficient (99% reduction of microglia), but not _Il34__fl/fl__; Drd1a__Cre/+_
(60% reduction of microglia in the striatum) display an increased seizure response at 4 mg kg–1D1 agonist treatment. Data shown as mean ± s.e.m. Source data EXTENDED DATA FIG. 5
STRIATUM-SPECIFIC MICROGLIA REDUCTION HAS NO OVERALL EFFECTS ON STRIATAL CELLULAR COMPOSITION, D1/D2 NEURONAL MORPHOLOGY, D1/D2 MSN CHARACTERISTIC ELECTROPHYSIOLOGICAL AND MOLECULAR
PHENOTYPES, AND GLIAL PHENOTYPES. A, Dot plots show average number of D1 neurons (left, dark green, GFP+, DARPP32+) and D2 neurons (right, light green, GFP-, DARPP32+) per mouse in the
striatum of _Il34__fl/fl__Drd1a__eGFPL10a_ and _Il34__fl/fl__Drd1a__eGFPL10a__Drd1a__Cre/+_ mice. Mice expressing eGFP-tagged ribosomal subunit L10a under the _Drd1a_ promoter were used to
identify GFP+ D1 neurons and GFP- D2 neurons in control _Il34__fl/fl__Drd1a__eGFPL10a_ and mutant _Il34__fl/fl__Drd1a__eGFPL10a__Drd1a__Cre/+_ (_n_ = 2 mice). B, C, D1 or D2 neuron cell
morphology was determined by the number of primary dendrites (B), total dendritic length (C, left), and sholl analysis (C, right) (B, D1 neurons: _n_ = 11 and 15 D1 neurons, _P_ = 0.33; D2
neurons: _n_ = 15 and 11 D2 neurons, _P_ = 0.59; unpaired two-tailed _t_-test; C, D1 neurons, _n_ = 11 and 15 D1 neurons, dendritic length: _P_ = 0.83, unpaired two-tailed _t_-test; sholl,
interaction: _P_ = 0.99; genotype: _P_ = 0.069; distance: _P_ < 0.0001, two-way ANOVA; D2 neurons, _n_ = 15 and 10 D2 neurons, dendritic length: _P_ = 0.80, unpaired two-tailed _t_-test;
sholl: interaction: _P_ = 0.051; genotype: _P_ = 0.67; distance: _P_ < 0.0001, two-way ANOVA). D, Intrinsic excitability of D1 neurons (left) and D2 neurons (right) in ex vivo slices as
measured by current-evoked action potentials (AP, left) and equilibrium potentials as voltage-current (VC) plots (right) (D1: _n_ = 11 and 15 D1 neurons, AP: interaction: _P_ = 1.0;
genotype: _P_ = 0.98; pA: _P_ < 0.0001, subjects: _P_ < 0.0001; VC: interaction: _P_ = 1.0; genotype: _P_ = 0.48; distance: _P_ < 0.0001, subjects: _P_ < 0.0001; D2: _n_ = 16 and
10 D2 neurons; AP: interaction: _P_ = 1.0; genotype: _P_ = 0.5; distance: _P_ < 0.0001, subjects: _P_ < 0.0001; VC: interaction: _P_ = 0.99; genotype: _P_ = 0.7; distance: _P_ <
0.0001, subjects: _P_ < 0.0001; two-way ANOVA). E, Dendritic excitability of D1 neurons (left) and D2 neurons (right) in ex vivo slices as determined by back-propagating action potentials
as measured by Ca2+-sensitive fluorescence (D1: _n_ = 12 and 15 D1 neurons, dendrites: _P_ = 0.90, spines: _P_ = 0.85; D2: _n_ = 16 and 10 D2 neurons, dendrites, _P_ = 0.27, spines, _P_ =
0.61; two-way ANOVA). F, Frequency (Hz) and amplitude (pA) of sEPSPs in D1 neurons from ex vivo slices shown as box and whisker plots (Frequency: _n_ = 19 cells from 5 mice and 16 cells from
5 mice, _P_ = 0.23, unpaired two-tailed _t_-test; amplitude: _n_ = 19 cells from 5 mice and 16 cells from 5 mice, _P_ = 0.796, unpaired two-tailed _t_-test with Welch’s correction). G,
Membrane bound DRD1 protein expression normalized to total DRD1 expression as determined by ex vivo brain slice biotinylation assay shown as a dot plot (_n_ = 6 mice, _P_ = 0.21). H,
Generation of _Il34__fl/fl__Drd1a__Cre/+__Drd1__eGFPL10a_ for D1 neuron specific TRAP sequencing analysis. I, Volcano plot shows lack of any major gene expression changes in D1 neurons in 3
month old _Il34__fl/fl__Drd1a__Cre/+__Drd1__eGFPL10a_ mice and littermate controls as determined by differential expression analysis (DESeq2, _n_ = 3 mice each, _P_ < 0.05, fold change
>1.5, red: upregulated, blue: downregulated). J-K, Total striatal RNA expression analysis from control and _Il34__fl/fl__Drd1a__Cre/+_ mice reveals unperturbed striatum cell-type specific
gene expression pattern except the expected ~50% reduction in the expression of microglia-enriched genes. J, RPKM, normalized to controls, showing pan-medium spiny neuron (MSN), D1 neuron
(D1), D2 neuron (D2), interneuron (IN), astrocyte (astro), oligodendrocyte (oligo), and microglia specific genes in _Il34__fl/fl__Drd1a__eGFPL10a_ and
_Il34__fl/fl__Drd1a__Cre/+__Drd1a__eGFPL10a_ mice (_n_ = 4 mice each, _P2ry12: P =_ 0.003, _Siglech: P =_ 0.001, _Cx3cr1: P =_ 0.01, _Csf1r: P =_ 0.007, _Tmem119: P =_ 0.005, _Fcrls: P =_
0.03, unpaired two-tailed _t_-test). K, RPKM, normalized to controls, showing unperturbed expression of astrocyte-specific activation markers66 (_n_ = 4 mice each, unpaired two-tailed
_t_-test). L, Microglia show wild-type like expression of selected microglia sensome genes67, RPKMs of selected genes have been normalized to _Hexb_ RPKM, (_n_ = 4 mice each, unpaired
two-tailed _t_-test). The experiments shown in H-K have been independently repeated in a second cohort (_n_ = 3 mice) with identical results. For gel source data, see Supplementary Fig. 1.
Box and whisker plots in B, C, E, and F are shown with arithmetic median (middle line), box shows upper and lower quartile, whiskers show min-max range. Data shown as mean ± s.e.m. Source
data EXTENDED DATA FIG. 6 MICROGLIA REGULATE STRIATAL NEURON SYNCHRONY AND RESPONSES TO D1 AGONIST TREATMENT IN AN ADO/A1R DEPENDENT FASHION. A, Representative tile scan of coronal brain
slice showing implantation of GRIN lens and AAV9.hSyn.GCaMP6 s expression in the dorsal striatum. B, Increased synchrony in the dorsal medial striatum of microglia deficient mice (_n_ = 9
mice) at baseline compared with controls (_n_ = 7 mice) (treatment: _P_ < 0.0001, distance: _P_ < 0.0001, interaction: _P_ < 0.0001; Two-way ANOVA with Sidak’s multiple comparisons
test). C, Bar graphs show magnitude of Ca2+ events (ΔF/F) recorded in control (black) and microglia deficient mice (grey) at baseline (left) and in response to D1 agonist (SKF81297, 3 mg
kg–1, right) (baseline: control, _n_ = 824 cells from 7 mice; microglia deficient, _n_ = 775 cells from 9 mice, _P_ = 0.87; D1 agonist: control, _n_ = 995 cells from 7 mice; microglia
deficient, _n_ = 1021 cells from 9 mice; _P_ = 0.89, unpaired two-tailed _t_-test). D, E, Co-administration of A1R agonist (CPA, 0.1 mg kg–1) with D1 agonist (SKF81297, 3 mg kg–1) normalizes
increased neuronal activity in microglia deficient mice. Bar graphs show wild type-like frequency (per mouse, D) and magnitude (ΔF/F, E) of Ca2+ events per neuron per minute in control
(black) and microglia deficient (grey) (D, control, _n_ = 7 mice; microglia deficient, _n_ = 9 mice, _P_ = 0.82, unpaired two-tailed _t_-test; E, control, _n_ = 387 cells from 7 mice;
microglia deficient, _n_ = 305 cells from 9 mice; _P_ = 0.69, unpaired two-tailed _t_-test). F, Spatiotemporal coding of neuronal activity (baseline shown in Fig. 3c) is disrupted by D1
agonist administration (dotted line) and largely normalized by co-administration with an A1R agonist (blue line) in control (top, _n_ = 7 mice) and microglia deficient mice (left, _n_ = 9
mice). For better visualization, the distance axis was logarithmically scaled. (Control, _n_ = 7 mice: interaction: _P_ = 0.0012, distance: _P_ < 0.0001, treatment: _P_ < 0.0001;
Microglia deficient, _n_ = 9 mice: interaction: _P_ = 0.0014, distance: _P_ < 0.0001, treatment: _P_ < 0.0001; Two-way ANOVA with Sidak’s multiple comparisons test). G, Bar graphs show
the frequency of Ca2+ events per neuron per minute in control (left) and microglia deficient (right) mice at baseline, in response to D1 agonist (SKF81297, 3 mg kg–1, i.p.) alone, or in
response to D1 agonist and A1R agonist treatment (CPA, 0.1 mg kg–1, i.p.) treatment (Control: _n_ = 332-995 cells from 7 mice, _P_ < 0.0001; Microglia deficient: _n_ = 243-1021 cells from
9 mice, _P_ < 0.0001; One-way ANOVA with Bonferroni post hoc test). H, Confirmation of CNO-mediated neuronal activation for data shown in Fig. 3h. The neuron-specific expression of
GCaMP6 s and hM3Dq was achieved by injecting the indicated viruses. Virally labelled thalamocortical projection neurons were identified (mCherry expression) and calcium transients were
recorded at baseline, after saline injection, and after CNO injection. I, Representative traces (left) and quantification of the area under the curve (AUC) (right) of calcium transients per
mouse in virally labelled neurons pre-injection, after saline injection, and after CNO injection (_n_ = 3 mice, _P_ = 0.0009, One-way ANOVA with Tukey’s post hoc test). J, Microglia baseline
process velocity (left) and contact with synaptic boutons (right) is not affected by either the expression of the DREADD virus (red bars) or by CNO injection (5 mg kg–1, black bars) alone
(_n_ = 3 mice, left: _P_ = 0.96, right, _P_ = 0.25, unpaired two-tailed _t_-test). The experiments shown in A-G are data combined from two independent imaging cohorts of mice. Box and
whisker plots in C, E, and G are shown with arithmetic median (middle line), box shows upper and lower quartile, whiskers show 1.5x interquartile range. CNO: clozapine-N-oxide; Data shown as
mean ± s.e.m. Source data EXTENDED DATA FIG. 7 MICROGLIAL EXPRESSION OF _ENTPD1_/CD39 AND _NT5E_/CD73 IN VITRO AND _IN VIVO_. A, Dot plots show normalized, ribosome-associated mRNA levels
(RPKM) for _Entpd1_ (left) and _Nt5e_ (right) in astrocytes, neurons, and microglia from distinct brain regions of adult mice using cell-type specific TRAP sequencing (_n_ = 2, 2, 3, 6, 4,
5, 19, and 15 mice). B, CD39 surface protein expression on ex vivo isolated forebrain cells of _Cx3cr1__CreErt2/+(Litt)_ mice (mice express cytosolic YFP in _Cx3cr1+_ microglia).
Percoll-purified cells were incubated with anti-CD39-AlexaFluor700 followed by FACS analysis. The histogram shows expression levels of CD39, which is almost exclusively restricted to YFP+
microglia (red) and is not found on YFP- non-microglia cells (grey) as shown previously73 (data are representative of three independent experiments). C, Scheme shows ex vivo isolation
procedure of CD11b+ microglia following neonatal mouse forebrain tissue dissociation and Percoll enrichment for live cells. D, E, Ex vivo CD11b+ microglia isolation procedure from neonatal
pups yields highly pure microglia population. D, Microglia were positively selected for by using CD11b+ magnetic bead purification and were incubated with anti-CD39-AlexaFluor700 followed by
FACS analysis to assess the purity of the population. The numbers show the percentage of live (DAPI−) cells with distinct pattern of CD39 expression levels (>98% CD39+; data are
representative of two independent experiments). E, Immunofluorescent analysis of purity of CD11b+ microglia isolation. Left, cells were plated on cover slips and stained for cell-type
specific protein expression using antibodies specific for IBA1 (microglia), GFAP (astrocyte), OLIG2 (oligodendrocytes) or NEUN (neurons) to identify and quantify different cells within the
populations in order to assess microglia purity (_n_ = 6 GFAP/IBA1 images and 6 OLIG2/NEUN/IBA1 images). Right, representative image of cover slip containing 99% pure microglia following
CD11b+ isolation procedure is shown (IBA1, green; DAPI, blue). F, left, Cell lysates of increasing numbers of CD11b+ bead-purified microglia cells have been analysed for CD39, CD73, P2RY12,
and IBA1 protein expression by Western Blot analysis as indicated, 5ng of total striatal lysate from control or _Nt5e_−_/_− (CD73-deficient) mice have been used to verify CD73 antibody
specificity, H3 protein expression has been used as a loading control (k = thousand, M = million; SuperSignal ECL substrate was used to visualize CD73 expression, regular ECL was used for
all other proteins) Right, Whole striatal tissue lysates of control and _Nt5e_−_/_− (CD73-deficient) striatal tissue were loaded at low (5ng) and high (30ng) concentrations and analysed for
microglia-specific protein expression (CD39, CD73, P2RY12, and IBA1) by Western Blot analysis as indicated. Whole striatal tissue lysates of control and microglia deficient mice have been
used to verify antibody specificity. H3 protein expression has been used as a loading control. (SuperSignal ECL substrate was used to visualize P2RY12 expression, regular ECL was used for
all other proteins). Blots are representative from two independent experiments. For gel source data, see Supplementary Fig. 1. Data shown as mean ± s.e.m. Source data EXTENDED DATA FIG. 8
MICROGLIA SUPPRESS NEURONAL ACTIVATION VIA AN ATP/AMP/ADO/A1R- DEPENDENT FEEDBACK MECHANISM. A, Scheme for generation of mice with microglia-specific CD39 depletion by breeding _Cd39__fl/fl_
mice to _Cd39__fl/fl__; Cx3cr1__CreErt2/+(Jung)_ mice followed by tamoxifen-mediated Cre induction at 4-6 weeks of age. B, Dot plots show relative expression of _Entpd1_, _Il34_, and _Csf1_
mRNA in the striatum of _Cd39__fl/fl__; Cx3cr1__CreErt2/+_ mice and littermate controls normalized to _Gapdh_ (_n_ = 5 and 6 mice, _Entpd1: P =_ 0.0012, _Il34: P =_ 0.38, _Csf1: P =_ 0.22,
unpaired two-tailed _t_-test). C, left, Representative images of striatal sections from _Cd39__fl/fl_ and _Cd39__fl/fl__; Cx3cr1__CreErt2/+_ mice stained for IBA1 (microglia, green) and DAPI
(nuclei, blue) (scale bar:100μm); right, dot plots show the average number of microglia per mm2 per mouse in the striatum of _Cd39__fl/fl_ and _Cd39__fl/fl__; Cx3cr1__CreErt2/+_ mice (_n_ =
4 mice, _P_ = 0.33, unpaired two-tailed _t_-test with Welch’s correction for variance). D, Microglia-specific CD39 ablation leads to increased levels of neuronal PKA activity in the
striatum as measured by phosphorylation levels of GLUR1 at Ser845 in striatal protein lysate from _Cd39__fl/fl__; Cx3cr1__CreErt2/+_ and littermate controls, pGLUR1 levels have been
normalized to total GLUR1 in each sample, (_n_ = 8 and 6 mice, _P_ = 0.029, two-tailed Mann–Whitney Test). E, F, Increased seizure response in _Cd39__fl/fl__; Cx3cr1__CreErt2/+__:_ E, Dot
plot shows number of stage IV-V seizures recorded within one hour in response to D1 agonist (SKF81297, 5 mg kg–1) (_n_ = 11 mice each, _P_ = 0.0004; unpaired two-tailed _t_-test). F, Bar
graph showing percentage of mice (left) and dot plot showing number (right) of stage IV-V seizures in response to kainic acid (15 mg kg–1) in _Cd39__fl/fl__; Cx3cr1__CreErt2/+_ mice as
compared to littermate controls (_n_ = 5 and 8 mice; left, _P_ = 0.17, Fisher’s exact test with Yates correction, right, _P_ = 0.032, unpaired two-tailed _t_-test). G, left, Scheme for the
generation of mice with a D1 neuron-specific _Adora1_ depletion by breeding _Adora1__fl/fl_ mice to _Drd1a__Cre/+_ mice; right, dot plots show relative expression of _Adora1_ mRNA in the
striatum of _Adora1__fl/fl__; Drd1a__Cre/+_ mice and littermate controls normalized to _Gapdh_ (_n_ = 5 and 4 mice, _P_ = 0.002, unpaired two-tailed _t_-test). H, Co-administration of A1R
agonist (CPA, 0.1 mg kg–1) and D1 agonist (SKF81297, 5 mg kg–1) does not prevent the increased seizure susceptibility in _Adora1__fl/fl__; Drd1a__Cre/+_ mice (_n_ = 12 and 6 mice, _P_ =
0.009, Fisher’s exact test with Yates correction). I, Bar graph shows percentage of microglia deficient mice with seizures in response to D1 agonist alone (SKF81297, 5 mg kg–1, i.p.) or
co-administered with an A2AR agonist (CGS21680, 0.1 mg kg–1, i.p.) or an A1R agonist (CPA, 0.1 mg kg–1, i.p.) (_n_ = 9-10 mice, _P_ = 0.005, Chi-squared test with Bonferroni post hoc
adjustment). J, A1R agonist administration (CPA, 0.1 mg kg–1) normalizes increased PKA activity in _Il34__fl/fl__; Drd1a__Cre/+_ mice but does not affect PKA activity in control
_Il34__fl/fl_ mice as measure by phosphorylation levels of GLUR1 at Ser845 in striatal protein lysate, pGLUR1 levels have been normalized to total GLUR1 expression in each sample
(_Il34__fl/fl_ mice, _n_ = 5 mice, _P_ = 0.62, _Il34__fl/fl__; Drd1a__Cre/+_ mice, _n_ = 5 mice, _P_ = 0.06, unpaired two-tailed _t_-test). All statistical tests are two-tailed; Data shown
as mean ± s.e.m. Source data EXTENDED DATA FIG. 9 MICROGLIA CAN SUPPRESS GLUTAMATE-INDUCED CORTICAL NEURON ACTIVATION IN A CD39/ADO/A1R-DEPENDENT FASHION IN VITRO. A-D, Experimental
approaches for the assessment of adenosine-mediated regulation of cortical neuron activity in vitro. Embryonic cortical neurons were cultured on Axion microelectrode array (MEA) plates which
allow for continuous electrical field recordings. A, A1Rs modulate cortical neuronal activity at baseline and in response to glutamate. On day in vitro (DIV) 14, neuronal cultures were
treated with vehicle, glutamate (10μM), A1R agonist (CPA, 100nM), A1R antagonist (DCPCX, 100nM), glutamate and A1R agonist, or glutamate and A1R antagonist. Dot plot shows the percentage
change in mean firing rate of neurons 1 h after treatment compared to their baseline before drug treatment. (_n_ = 7 wells, _P_ < 0.0001, One-way ANOVA with Tukey’s post hoc test). B,
Adenosine suppresses neuronal activity via A1R activation. On DIV14, cultures were treated with vehicle, adenosine (10μM), A1R antagonist (DCPCX, 100nM), or co-treated with adenosine and A1R
antagonist. Dot plot shows percentage change in mean firing rate of neurons 1 h after treatment compared to their baseline before drug treatment. (_n_ = 8 wells, _P_ < 0.0001, One-way
ANOVA with Tukey’s post hoc test). C, Microglia suppress neuronal activity in response to glutamate-induced activation in an A1R-dependent manner. Microglia were isolated from neonatal pups,
plated onto the neuronal culture on DIV 14, and allowed to settle for 48 h. Mixed cultures were treated with vehicle and/or glutamate (10μM) and/or A1R antagonist (100nM) on DIV 16. Dot
plot shows percentage change in mean firing rate of neurons 1 h after treatment compared to their baseline before drug treatment. (left, _n_ = 12 wells, _P_ < 0.0001, right, _n_ = 4, 6,
9, and 7 wells, _P_ = 0.001, One-way ANOVA with Tukey’s post hoc test). D, Microglia suppress neuronal activity in a CD39-dependent manner in response to glutamate-induced activation.
Microglia were isolated from neonatal pups, plated onto the neuronal culture on DIV 14, and allowed to settle for 48 h. Mixed cultures were pretreated with CD39 inhibitor (ARL67156, 200μM)
or vehicle (30 min) and then treated with glutamate (10μM). Dot plot shows percentage change in mean firing rate of neurons 1 h after treatment compared to the corresponding baseline
neuronal activity levels before their baseline before drug treatment. (_n_ = 12, 12, 11, and 11 wells, _P_ = 0.0045, One-way ANOVA with Tukey’s post hoc test). Data shown as mean ± s.e.m.
and representative of 2-3 independent experiments. Source data EXTENDED DATA FIG. 10 REACTIVE MICROGLIA IN DIFFERENT NEUROINFLAMMATORY AND NEURODEGENERATIVE CONDITIONS SHOW A REDUCTION IN
_ENTPD1_ AND _P2RY12_ EXPRESSION THAT IS ASSOCIATED WITH AN A1R-DEPENDENT INCREASE IN D1 NEURON RESPONSES. A-G, Changes in _Entpd1_ and _P2ry12_ gene expression are shown in: A, RNA
extracted from whole striatum of 6-month old control mice and Q175 (Huntington’s disease) mice94 (_Entpd1: P =_ 0.0001; _P2ry12: P =_ 0.004; _n_ = 8 mice, fold change and _P_-value provided
in publication). B, RNA from FACS-sorted CD11b+/F4/80+ cortical and hippocampal microglia from 8.5-month old control and 5xfAD mouse model of Alzheimer’s Disease95 (_Entpd1: P =_ 0.009;
_P2ry12: P =_ 0.0035; _n_ = 5 mice, fold change and _P_-value provided in publication). C, RNA from FACS-sorted forebrain microglia from 10-month old control and APP/PS1 Alzheimer’s disease
mouse model39 (_n_ = 3 mice, _Entpd1: P =_ 0.038; _P2ry12: P =_ 0.023, unpaired two-tailed _t_-test). D, RNA from FACS-sorted FCRLS+ phagocytic and non-phagocytic microglia isolated after
stereotaxic injection of apoptotic neurons39 (_n_ = 4 mice, _Entpd1: P <_ 0.0001; _P2ry12: P <_ 0.0001, unpaired two-tailed _t_-test). E, FACS-sorted FCRLS+ microglia in 24-month old
control mice or APP/PS1 Alzheimer’s disease mouse model. Plaque associated microglia were identified and sorted based on CLEC7A expression39 (_n_ = 6 mice, _Entpd1: P =_ 0.01; _P2ry12: P
<_ 0.0001, One-way ANOVA with Tukey’s post hoc test). F, Massively parallel single-cell RNA-seq (MARS-seq) from isolated homeostatic microglia and disease associated microglia (DAM) in
_5xfAD_ mice96 (_Entpd1: P <_ 0.0001; _P2ry12: P <_ 0.0001; _n_ = 893 single microglia, fold change and _P_-value provided in publication). G, FACS-sorted CD11b+CD45int single
microglia in control and LPS-injected mice (4 mg kg–1)97 (_Entpd1: P <_ 0.0001; _P2ry12: P <_ 0.0001; _n_ = 477 microglia from saline injected mice and 770 microglia from LPS injected
mice, fold change and _P_-value provided in publication). H, I, Bar graphs show increased seizure susceptibility to D1 agonist administration (SKF81297, 5 mg kg–1, i.p.) in LPS-injected
(indicated doses, i.p.) (H) and 6-8-month old _5xfAD_ Alzheimer’s mice (I) that is prevented by co-administration of an A1R agonist (CPA, 0.1 mg kg–1, i.p.) (H, _n_ = 10-22 male mice, _P_ =
0.032, Chi-squared test; I, _n_ = 5-10 mice per genotype, left, _P_ = 0.031, Fisher’s exact test with Yates correction; right, _P_ = 0.49, Fisher’s exact test with Yates correction). J,
Scheme illustrating the model of microglia-mediated adenosine-controlled regulation of D1 neuron responses in the healthy striatum (left) and its potential dysfunction upon microglia
activation during inflammatory and/or neurodegenerative diseases (right). All statistical tests are two-tailed; Data shown as mean ± s.e.m. Source data SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION This file contains Supplementary Figs 1-5 and legends for Supplementary Tables 1-3 and Supplementary Videos 1-2. REPORTING SUMMARY SUPPLEMENTARY TABLE 1 Genes
enriched in striatal microglia upon neuronal activation (DESeq2, n=3 mice per group; _P_ value < 0.05, fold change> 1.2) over unbound fraction (DESeq2, n=3/TRAP and unbound; _P_ value
< 0.05, fold > 2). SUPPLEMENTARY TABLE 2 Genes enriched in striatal microglia upon neuronal inhibition (DESeq2, n=2 mice per group; _P_ value < 0.05, fold change> 1.2) over
unbound fraction (DESeq2, n=2/TRAP and unbound; _P_ value < 0.05, fold > 2). SUPPLEMENTARY TABLE 3 Genes enriched in D1 neurons in _Il34fl/flDrd1Cre/+Drd1aTRAP_ mice over cre-negative
littermate controls (DESeq2, n=3 mice per group; p value < 0.05, fold > 1.5) over unbound fraction (DESeq2, n=3 TRAP and 4 unbound; p value < 0.05, fold > 2). VIDEO 1
Representative field of view for live imaging of calcium transients in striatal neurons for data shown in Figure 3a-f and Extended Data Figure 6a-g. VIDEO 2 Representative field of view for
live imaging of microglia (green) contact with neuronal terminals (red) for data shown in Figure 3g-h and Extended Data Figure 6h-j. Scale bar =20μM. SOURCE DATA SOURCE DATA FIG. 1 SOURCE
DATA FIG. 2 SOURCE DATA FIG. 3 SOURCE DATA FIG. 4 SOURCE DATA EXTENDED DATA FIG. 1 SOURCE DATA EXTENDED DATA FIG. 2 SOURCE DATA EXTENDED DATA FIG. 3 SOURCE DATA EXTENDED DATA FIG. 4 SOURCE
DATA EXTENDED DATA FIG. 5 SOURCE DATA EXTENDED DATA FIG. 6 SOURCE DATA EXTENDED DATA FIG. 7 SOURCE DATA EXTENDED DATA FIG. 8 SOURCE DATA EXTENDED DATA FIG. 9 SOURCE DATA EXTENDED DATA FIG.
10 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Badimon, A., Strasburger, H.J., Ayata, P. _et al._ Negative feedback control of neuronal activity by
microglia. _Nature_ 586, 417–423 (2020). https://doi.org/10.1038/s41586-020-2777-8 Download citation * Received: 26 November 2019 * Accepted: 28 August 2020 * Published: 30 September 2020 *
Issue Date: 15 October 2020 * DOI: https://doi.org/10.1038/s41586-020-2777-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




