- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Organic light-emitting diodes (OLEDs)1,2,3,4,5, quantum-dot-based LEDs6,7,8,9,10, perovskite-based LEDs11,12,13 and micro-LEDs14,15 have been championed to fabricate lightweight and
flexible units for next-generation displays and active lighting. Although there are already some high-end commercial products based on OLEDs, costs must decrease whilst maintaining high
operational efficiencies for the technology to realise wider impact. Here we demonstrate efficient action of radical-based OLEDs16, whose emission originates from a spin doublet, rather
than a singlet or triplet exciton. While the emission process is still spin-allowed in these OLEDs, the efficiency limitations imposed by triplet excitons are circumvented for doublets.
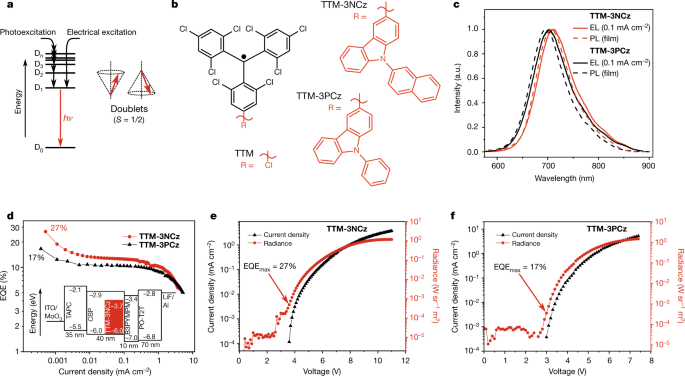
Using a luminescent radical emitter, we demonstrate an OLED with maximum external quantum efficiency of 27 per cent at a wavelength of 710 nanometres—the highest reported value for deep-red
and infrared LEDs. For a standard closed-shell organic semiconductor, holes and electrons occupy the highest occupied and lowest unoccupied molecular orbitals (HOMOs and LUMOs),
respectively, and recombine to form singlet or triplet excitons. Radical emitters have a singly occupied molecular orbital (SOMO) in the ground state, giving an overall spin-1/2 doublet.
If—as expected on energetic grounds—both electrons and holes occupy this SOMO level, recombination returns the system to the ground state, giving no light emission. However, in our very
efficient OLEDs, we achieve selective hole injection into the HOMO and electron injection to the SOMO to form the fluorescent doublet excited state with near-unity internal quantum
efficiency. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access
Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 51 print
issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS EFFICIENT AND STABLE HYBRID PEROVSKITE-ORGANIC LIGHT-EMITTING DIODES WITH EXTERNAL QUANTUM EFFICIENCY EXCEEDING 40 PER CENT Article Open access 12 June 2024 SINGLET
AND TRIPLET TO DOUBLET ENERGY TRANSFER: IMPROVING ORGANIC LIGHT-EMITTING DIODES WITH RADICALS Article Open access 18 May 2022 EFFICIENT AND STABLE ONE-MICROMETRE-THICK ORGANIC LIGHT-EMITTING
DIODES Article 24 October 2022 DATA AVAILABILITY The datasets collected and analysed in this work are available at https://doi.org/10.17863/CAM.31543. REFERENCES * Tang, C. W. &
VanSlyke, S. A. Organic electroluminescent diodes. _Appl. Phys. Lett_. 51, 913–915 (1987). Article CAS ADS Google Scholar * Burroughes, J. H. et al. Light-emitting diodes based on
conjugated polymers. _Nature_ 347, 539–541 (1990); correction 348, 352 (1990). Article CAS ADS Google Scholar * Baldo, M. A. et al. Highly efficient phosphorescent emission from organic
electroluminescent devices. _Nature_ 395, 151–154 (1998). Article CAS ADS Google Scholar * Ma, Y., Zhang, H., Shen, J. & Che, C. Electroluminescence from triplet metal-ligand
charge-transfer excited state of transition metal complexes. _Synth. Met_. 94, 245–248 (1998). Article CAS Google Scholar * Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C.
Highly efficient organic light-emitting diodes from delayed fluorescence. _Nature_ 492, 234–238 (2012). Article CAS ADS Google Scholar * Tessler, N., Medvedev, V., Kazes, M., Kan, S.
& Banin, U. Efficient near-infrared polymer nanocrystal light-emitting diodes. _Science_ 295, 1506–1508 (2002). Article ADS Google Scholar * Sun, Q. et al. Bright, multicoloured
light-emitting diodes based on quantum dots. _Nat. Photon_. 1, 717–722 (2007). Article CAS ADS Google Scholar * Dai, X. et al. Solution-processed, high-performance light-emitting diodes
based on quantum dots. _Nature_ 515, 96–99 (2014). Article CAS ADS Google Scholar * Yang, Y. et al. High-efficiency light-emitting devices based on quantum dots with tailored
nanostructures. _Nat. Photon_. 9, 259–266 (2015). Article CAS ADS Google Scholar * Dai, X., Deng, Y., Peng, X. & Jin, Y. Quantum-dot light-emitting diodes for large-area displays:
towards the dawn of commercialization. _Adv. Mater_. 29, 1607022 (2017). Article Google Scholar * Tan, Z.-K. et al. Bright light-emitting diodes based on organometal halide perovskite.
_Nat. Nanotechnol_. 9, 687–692 (2014). Article CAS ADS Google Scholar * Cho, H. et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes.
_Science_ 350, 1222–1225 (2015). Article CAS ADS Google Scholar * Wang, N. et al. Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells.
_Nat. Photon_. 10, 699–704 (2016). Article CAS ADS Google Scholar * Jin, S. X., Li, J., Li, J. Z., Lin, J. Y. & Jiang, H. X. GaN microdisk light emitting diodes. _Appl. Phys. Lett_.
76, 631–633 (2000). Article CAS ADS Google Scholar * Zhang, K., Peng, D., Lau, K. M. & Liu, Z. Fully-integrated active matrix programmable UV and blue micro-LED display
system-on-panel (SoP). _J. Soc. Inf. Disp_. 25, 240–248 (2017). Article CAS Google Scholar * Peng, Q., Obolda, A., Zhang, M. & Li, F. Organic light-emitting diodes using a neutral π
radical as emitter: the emission from a doublet. _Angew. Chem. Int. Ed_. 54, 7091–7095 (2015). Article CAS Google Scholar * Ballester, M., Molinet, C. & Castañer, J. Preparation of
highly strained aromatic chlorocarbons. I. A powerful nuclear chlorinating agent. Relevant reactivity phenomena traceable to molecular strain. _J. Am. Chem. Soc_. 82, 4254–4258 (1960).
Article CAS Google Scholar * Armet, O. et al. Inert carbon free radicals. 8. Polychlorotriphenylmethyl radicals: synthesis, structure, and spin-density distribution. _J. Phys. Chem_. 91,
5608–5616 (1987). Article CAS Google Scholar * Heckmann, A., Lambert, C., Goebel, M. & Wortmann, R. Synthesis and photophysics of a neutral organic mixed-valence compound. _Angew.
Chem. Int. Ed_. 43, 5851–5856 (2004). Article CAS Google Scholar * Velasco, D. et al. Red organic light-emitting radical adducts of carbazole and tris(2,4,6-trichlorotriphenyl)methyl
radical that exhibit high thermal stability and electrochemical amphotericity. _J. Org. Chem_. 72, 7523–7532 (2007). Article CAS Google Scholar * Castellanos, S., Velasco, D.,
López-Calahorra, F., Brillas, E. & Julia, L. Taking advantage of the radical character of tris(2,4,6-trichlorophenyl)methyl to synthesize new paramagnetic glassy molecular materials. _J.
Org. Chem_. 73, 3759–3767 (2008). Article CAS Google Scholar * Hattori, Y., Kusamoto, T. & Nishihara, H. Luminescence, stability, and proton response of an open-shell
(3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical. _Angew. Chem. Int. Ed_. 53, 11845–11848 (2014). Article CAS Google Scholar * Ai, X., Chen, Y., Feng, Y. & Li, F. A
stable room-temperature luminescent biphenylmethyl radical. _Angew. Chem. Int. Ed_. 57, 2869–2873 (2018). Article CAS Google Scholar * Hung, W. Y. et al. The first tandem,
all-exciplex-based WOLED. _Sci. Rep_. 4, 5161 (2014). Article CAS Google Scholar * Sasabe, H. et al. 2-Phenylpyrimidine skeleton-based electron-transport materials for extremely efficient
green organic light-emitting devices. _Chem. Commun_. 5821–5823 (2008). * Li, C. et al. Deep-red to near-infrared thermally activated delayed fluorescence in organic solid films and
electroluminescent devices. _Angew. Chem. Int. Ed_. 56, 11525–11529 (2017). Article CAS Google Scholar * Kim, D.-H. et al. High-efficiency electroluminescence and amplified spontaneous
emission from a thermally activated delayed fluorescent near-infrared emitter. _Nat. Photon_. 12, 98–104 (2018). Article CAS ADS Google Scholar * Xue, J. et al. High-efficiency
near-infrared fluorescent organic light-emitting diodes with small efficiency roll-off: a combined design from emitters to devices. _Adv. Funct. Mater_. 27, 1703283 (2017). Article Google
Scholar * Ipatov, A. et al. Excited-state spin-contamination in time-dependent density-functional theory for molecules with open-shell ground states. _J. Mol. Struct. Theochem_ 914, 60–73
(2009). Article CAS Google Scholar * Neier, E. et al. Solution-processed organic light-emitting diodes with emission from a doublet exciton; using (2,4,6-trichlorophenyl)methyl as
emitter. _Org. Electron_. 44, 126–131 (2017). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS X.A., S.D., H.G., Y.C. and F.L. are grateful for the financial support
received from the National Key R&D Program of China (grant number 2016YFB0401001), the National Natural Science Foundation of China (grant numbers 51673080 and 91233113) and the National
Key Basic Research and Development Program of China (973 programme, grant number 2015CB655003). E.W.E., A.J.G. and R.H.F. thank the EPSRC for funding (EP/M01083X/1, EP/M005143/1). T.J.H.H.
thanks Jesus College, Cambridge for a Research Fellowship. F.L. is an academic visitor at the Cavendish Laboratory, Cambridge and is supported by the China Scholarship Council (CSC) and the
Talents Cultivation Program (Jilin University, China). REVIEWER INFORMATION _Nature_ thanks T. Kusamoto and the other anonymous reviewer(s) for their contribution to the peer review of this
work. AUTHOR INFORMATION Author notes * These authors contributed equally: Xin Ai, Emrys W. Evans, Shengzhi Dong AUTHORS AND AFFILIATIONS * State Key Laboratory of Supramolecular Structure
and Materials, College of Chemistry, Jilin University, Changchun, China Xin Ai, Shengzhi Dong, Haoqing Guo, Yingxin Chen & Feng Li * Cavendish Laboratory, University of Cambridge,
Cambridge, UK Emrys W. Evans, Alexander J. Gillett, Timothy J. H. Hele, Richard H. Friend & Feng Li Authors * Xin Ai View author publications You can also search for this author inPubMed
Google Scholar * Emrys W. Evans View author publications You can also search for this author inPubMed Google Scholar * Shengzhi Dong View author publications You can also search for this
author inPubMed Google Scholar * Alexander J. Gillett View author publications You can also search for this author inPubMed Google Scholar * Haoqing Guo View author publications You can also
search for this author inPubMed Google Scholar * Yingxin Chen View author publications You can also search for this author inPubMed Google Scholar * Timothy J. H. Hele View author
publications You can also search for this author inPubMed Google Scholar * Richard H. Friend View author publications You can also search for this author inPubMed Google Scholar * Feng Li
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.A., S.D. and H.G. designed and synthesized the luminescent radicals and performed the
steady-state spectroscopy. E.W.E. performed the transient-photoluminescence measurements and the quantum chemical calculations. T.J.H.H devised the group theory treatment. A.J.G. conducted
the transient-absorption spectroscopy measurements. X.A., Y.C. and F.L. optimized the devices. E.W.E., R.H.F. and F.L. initiated, designed and supervised the work. E.W.E., R.H.F. and F.L.
wrote the manuscript, with input from all authors. CORRESPONDING AUTHORS Correspondence to Richard H. Friend or Feng Li. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED
DATA FIGURES AND TABLES EXTENDED DATA FIG. 1 THERMAL STABILITY AND ELECTRON PARAMAGNETIC RESONANCE MEASUREMENTS OF TTM-3NCZ AND TTM-3PCZ. A, Thermogravimetric analysis measurements show
thermal decomposition temperatures of 362 °C (TTM-3NCz) and 367 °C (TTM-3PCz). B, EPR spectra for solid samples at room temperature. ESR, electron spin resonance; _B_, magnetic field.
EXTENDED DATA FIG. 2 ELECTROCHEMICAL PROPERTIES AND STABILITY OF TTM-3NCZ AND TTM-3PCZ. A, C, Cyclic voltammograms of TTM-3NCz (A) and TTM-3PCz (C) in CH2Cl2. For both TTM-3NCz and TTM-3PCz,
the average of the cathodic and anodic potentials gives a reduction potential of −1.1 V, first oxidation potential of +0.4 V and second oxidation potential of +0.9 V. B, D, Multi-cycle (20
cycles) cyclic voltammetry measurements of TTM-3NCz (B) and TTM-3PCz in CH2Cl2 (D). A ferrocence cation/ferrocence (Fc+/Fc) reference redox couple was used for the measurements. EXTENDED
DATA FIG. 3 PHOTOSTABILITY OF TTM AND TTM-3NCZ. Luminescence intensity (_l_) of TTM-3NCz and TTM solutions (10 µM, cyclohexane) as a function of time. A pulsed laser of 355 nm with an energy
density of 315 kW cm−2 (pulse width, 8 ns; frequency, 10 Hz) was used under ambient conditions. EXTENDED DATA FIG. 4 DEVICE REPRODUCIBILITY FOR TTM-3NCZ. A, EQE–current density plots for
five TTM-3NCz devices. The peak EQEmax values are: 25% (device 1), 27% (device 2), 20% (device 3), 24% (device 4) and 16% (device 5). The EQE at 1 mA cm−2 is: 8% (device 1), 10% (device 2),
7% (device 3), 9% (device 4) and 7% (device 5). B, Radiance–voltage plots for the same five TTM-3NCz devices. The radiance levels for EQEmax are indicated, which can be distinguished from
the noise. C, Current density–voltage plots for the same five TTM-3NCz devices. EXTENDED DATA FIG. 5 DEVICE STABILITY FOR TTM-3NCZ. Electroluminescence spectra for TTM-3NCz devices operated
at current densities of 0.006–1.6 mA cm−2. There is no current dependence. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains further experimental details; Supplementary
Text: including synthesis and MO diagram derivation; Supplementary Figures: 1–2) TA data, 3–4) MO diagrams and basis set, 5–6) DFT orbitals and difference density plots; and Supplementary
Tables: 1) TDDFT orbital contributions, 2) red LED literature. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ai, X., Evans, E.W., Dong, S. _et al._
Efficient radical-based light-emitting diodes with doublet emission. _Nature_ 563, 536–540 (2018). https://doi.org/10.1038/s41586-018-0695-9 Download citation * Received: 12 June 2018 *
Accepted: 02 October 2018 * Published: 21 November 2018 * Issue Date: 22 November 2018 * DOI: https://doi.org/10.1038/s41586-018-0695-9 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * Singly Occupied Molecular Orbital (SOMO) * Organic Light-emitting Diodes (OLEDs) * Excited Doublet State * Luminescent Groups * Triplet Excitons







