- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
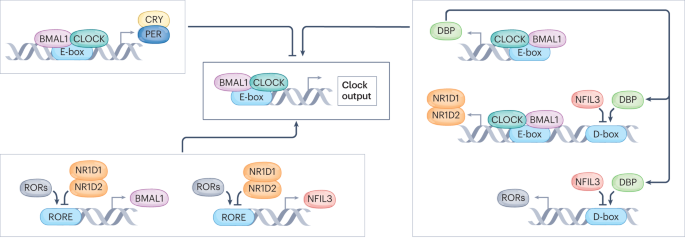
ABSTRACT Circadian rhythms that influence mammalian homeostasis and overall health have received increasing interest over the past two decades. The molecular clock, which is present in
almost every cell, drives circadian rhythms while being a cornerstone of physiological outcomes. The skeletal muscle clock has emerged as a primary contributor to metabolic health, as the
coordinated expression of the core clock factors BMAL1 and CLOCK with the muscle-specific transcription factor MYOD1 facilitates the circadian and metabolic programme that supports skeletal
muscle physiology. The phase of the skeletal muscle clock is sensitive to the time of exercise, which provides a rationale for exploring the interactions between the skeletal muscle clock,
exercise and metabolic health. Here, we review the underlying mechanisms of the skeletal muscle clock that drive muscle physiology, with a particular focus on metabolic health. Additionally,
we highlight the interaction between exercise and the skeletal muscle clock as a means of reinforcing metabolic health and discuss the possible implications of the time of exercise as a
chronotherapeutic approach. KEY POINTS * The BMAL1–CLOCK heterodimeric transcription factor is a key regulator of clock output; partnership with MYOD1 confers muscle specificity. * Skeletal
muscle substrate preference, storage and transport are highly regulated by the skeletal muscle molecular clock, aligning metabolism with physical activity and feeding patterns. * Mice with
knockouts and mutations that affect the circadian clock, and behavioural misalignment in humans, as occurs in metabolic disorders such as type 2 diabetes mellitus, have severe metabolic
consequences that affect insulin sensitivity and glucose handling. * Exercise is a potent Zeitgeber that acts to shift skeletal muscle clocks; exercising at different times of the day
results in divergent transcriptional and metabolic outputs. * Differential time-of-day exercise might prove to be a useful chronotherapeutic strategy for the treatment and management of
metabolic diseases by improving clock alignment and therefore metabolic regulation. Access through your institution Buy or subscribe This is a preview of subscription content, access via
your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days
cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EXERCISE METABOLISM AND ADAPTATION IN SKELETAL MUSCLE Article 24 May 2023 EXERCISE
ADAPTATIONS: MOLECULAR MECHANISMS AND POTENTIAL TARGETS FOR THERAPEUTIC BENEFIT Article 06 July 2020 TIME OF EXERCISE DIFFERENTIALLY IMPACTS BONE GROWTH IN MICE Article 28 May 2024
REFERENCES * Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. _Proc. Natl
Acad. Sci. USA_ 111, 16219–16224 (2014). Article CAS PubMed PubMed Central Google Scholar * Vitaterna, M. H., Takahashi, J. S. & Turek, F. W. Overview of circadian rhythms. _Alcohol
Res. Health_ 25, 85–93 (2001). CAS PubMed PubMed Central Google Scholar * Ko, C. H. & Takahashi, J. S. Molecular components of the mammalian circadian clock. _Hum. Mol. Genet._ 15,
R271–R277 (2006). Article CAS PubMed Google Scholar * Cox, K. H. & Takahashi, J. S. Circadian clock genes and the transcriptional architecture of the clock mechanism. _J. Mol.
Endocrinol._ 63, R93–R102 (2019). Article CAS PubMed PubMed Central Google Scholar * Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals.
_Cell_ 103, 1009–1017 (2000). Article CAS PubMed PubMed Central Google Scholar * Gekakis, N. et al. Role of the CLOCK protein in the mammalian circadian mechanism. _Science_ 280,
1564–1569 (1998). Article CAS PubMed Google Scholar * King, D. P. et al. Positional cloning of the mouse circadian clock gene. _Cell_ 89, 641–653 (1997). Article CAS PubMed PubMed
Central Google Scholar * Akashi, M., Tsuchiya, Y., Yoshino, T. & Nishida, E. Control of intracellular dynamics of mammalian period proteins by casein kinase I ε (CKIε) and CKIδ in
cultured cells. _Mol. Cell. Biol._ 22, 1693–1703 (2002). Article CAS PubMed PubMed Central Google Scholar * Camacho, F. et al. Human casein kinase Iδ phosphorylation of human circadian
clock proteins period 1 and 2. _FEBS Lett._ 489, 159–165 (2001). Article CAS PubMed Google Scholar * Eide, E. J., Vielhaber, E. L., Hinz, W. A. & Virshup, D. M. The circadian
regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iε. _J. Biol. Chem._ 277, 17248–17254 (2002). Article CAS PubMed Google Scholar * Etchegaray, J.-P. et al.
Casein kinase 1 delta regulates the pace of the mammalian circadian clock. _Mol. Cell Biol._ 29, 3853–3866 (2009). Article CAS PubMed PubMed Central Google Scholar * Akashi, M. &
Takumi, T. The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. _Nat. Struct. Mol. Biol._ 12, 441–448 (2005). Article CAS PubMed Google
Scholar * Guillaumond, F., Dardente, H., Giguère, V. & Cermakian, N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. _J. Biol. Rhythms_ 20,
391–403 (2005). Article CAS PubMed Google Scholar * Lee, C., Weaver, D. R. & Reppert, S. M. Direct association between mouse PERIOD and CKIε is critical for a functioning circadian
clock. _Mol. Cell Biol._ 24, 584–594 (2004). Article CAS PubMed PubMed Central Google Scholar * Xu, Y. et al. Modeling of a human circadian mutation yields insights into clock
regulation by PER2. _Cell_ 128, 59–70 (2007). Article CAS PubMed PubMed Central Google Scholar * Reischl, S. et al. β-TrCP1-mediated degradation of PERIOD2 is essential for circadian
dynamics. _J. Biol. Rhythms_ 22, 375–386 (2007). Article CAS PubMed Google Scholar * Wu, G. et al. Structure of a β-TrCP1-Skp1-β-catenin complex: destruction motif binding and lysine
specificity of the SCFβ-TrCP1 ubiquitin ligase. _Mol. Cell_ 11, 1445–1456 (2003). Article CAS PubMed Google Scholar * Ohsaki, K. et al. The role of β-TrCP1 and β-TrCP2 in circadian
rhythm generation by mediating degradation of clock protein PER2. _J. Biochem._ 144, 609–618 (2008). Article CAS PubMed Google Scholar * Meng, Q.-J. et al. Setting clock speed in
mammals: the CK1ɛ tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. _Neuron_ 58, 78–88 (2008). Article CAS PubMed PubMed Central Google
Scholar * Busino, L. et al. SCF Fbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. _Science_ 316, 900–904 (2007). Article CAS
PubMed Google Scholar * Lamia, K. A. et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. _Science_ 326, 437–440 (2009). Article CAS PubMed PubMed
Central Google Scholar * Hirano, A. et al. FBXL21 regulates oscillation of the circadian clock through ubiquitination and stabilization of cryptochromes. _Cell_ 152, 1106–1118 (2013).
Article CAS PubMed Google Scholar * Hirano, A., Fu, Y.-H. & Ptáček, L. J. The intricate dance of post-translational modifications in the rhythm of life. _Nat. Struct. Mol. Biol._ 23,
1053–1060 (2016). Article CAS PubMed Google Scholar * Wheaton, K. L. et al. The phosphorylation of CREB at serine 133 is a key event for circadian clock timing and entrainment in the
suprachiasmatic nucleus. _J. Biol. Rhythms_ 33, 497–514 (2018). Article CAS PubMed PubMed Central Google Scholar * Gau, D. et al. Phosphorylation of CREB Ser142 regulates light-induced
phase shifts of the circadian clock. _Neuron_ 34, 245–253 (2002). Article CAS PubMed Google Scholar * Travnickova-Bendova, Z., Cermakian, N., Reppert, S. M. & Sassone-Corsi, P.
Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. _Proc. Natl Acad. Sci. USA_ 99, 7728–7733 (2002). Article CAS PubMed PubMed Central Google
Scholar * Impey, S. et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. _Cell_ 119, 1041–1054 (2004). CAS PubMed Google Scholar *
Tischkau, S. A., Mitchell, J. W., Tyan, S.-H., Buchanan, G. F. & Gillette, M. U. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for
light-induced signaling in the suprachiasmatic nucleus circadian clock. _J. Biol. Chem._ 278, 718–723 (2003). Article CAS PubMed Google Scholar * Small, L. et al. Contraction influences
Per2 gene expression in skeletal muscle through a calcium‐dependent pathway. _J. Physiol._ 598, 5739–5752 (2020). Article CAS PubMed Google Scholar * Wolff, C. A. & Esser, K. A.
Exercise sets the muscle clock with a calcium assist. _J. Physiol._ 598, 5591–5592 (2020). Article CAS PubMed Google Scholar * Gabriel, B. M. et al. Disrupted circadian oscillations in
type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle. _Sci. Adv._ 7, eabi9654 (2021). Article CAS PubMed PubMed Central Google Scholar * Koike, N.
et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. _Science_ 338, 349–354 (2012). Article CAS PubMed PubMed Central Google Scholar *
Menet, J. S., Rodriguez, J., Abruzzi, K. C. & Rosbash, M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. _eLife_ 1, e00011 (2012). Article PubMed
PubMed Central Google Scholar * Davis, R., Weintraub, H. & Lassar, A. Expression of a single transfected cDNA converts fibroblasts to myoblasts. _Cell_ 51, 987–1000 (1988). Article
Google Scholar * Miller, B. H. et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. _Proc. Natl Acad. Sci. USA_ 104, 3342–3347 (2007). Article
CAS PubMed PubMed Central Google Scholar * Andrews, J. L. et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. _Proc. Natl
Acad. Sci. USA_ 107, 19090–19095 (2010). Article CAS PubMed PubMed Central Google Scholar * Hodge, B. A. et al. MYOD1 functions as a clock amplifier as well as a critical co-factor for
downstream circadian gene expression in muscle. _eLife_ 8, e43017 (2019). Article PubMed PubMed Central Google Scholar * Dyar, K. A. et al. The calcineurin-NFAT pathway controls
activity-dependent circadian gene expression in slow skeletal muscle. _Mol. Metab._ 4, 823–833 (2015). Article CAS PubMed PubMed Central Google Scholar * Pizarro, A., Hayer, K., Lahens,
N. F. & Hogenesch, J. B. CircaDB: a database of mammalian circadian gene expression profiles. _Nucleic Acids Res._ 41, D1009–D1013 (2012). Article PubMed PubMed Central Google
Scholar * Gutierrez‐Monreal, M. A., Harmsen, J., Schrauwen, P. & Esser, K. A. Ticking for metabolic health: the skeletal‐muscle clocks. _Obesity_ 28(Suppl. 1), 46–54 (2020). Google
Scholar * Perrin, L. et al. Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. _eLife_ 7, e34114 (2018). Article PubMed PubMed Central Google
Scholar * Rey, G. et al. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. _PLoS Biol._ 9, e1000595 (2011). Article CAS PubMed
PubMed Central Google Scholar * Dai, Z., Ramesh, V. & Locasale, J. W. The evolving metabolic landscape of chromatin biology and epigenetics. _Nat. Rev. Genet._ 21, 737–753 (2020).
Article CAS PubMed PubMed Central Google Scholar * Grimaldi, B. et al. Chromatin remodeling and circadian control: master regulator CLOCK is an enzyme. _Cold Spring Harb. Symp. Quant.
Biol._ 72, 105–112 (2007). Article CAS PubMed Google Scholar * Katada, S. & Sassone-Corsi, P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression.
_Nat. Struct. Mol. Biol._ 17, 1414–1421 (2010). Article CAS PubMed PubMed Central Google Scholar * Zhu, H., Wang, G. & Qian, J. Transcription factors as readers and effectors of
DNA methylation. _Nat. Rev. Genet._ 17, 551–565 (2016). Article CAS PubMed PubMed Central Google Scholar * Menet, J. S., Pescatore, S. & Rosbash, M. CLOCK:BMAL1 is a pioneer-like
transcription factor. _Genes Dev._ 28, 8–13 (2014). Article CAS PubMed PubMed Central Google Scholar * Petrany, M. J. et al. Single-nucleus RNA-seq identifies transcriptional
heterogeneity in multinucleated skeletal myofibers. _Nat. Commun._ 11, 6374 (2020). Article CAS PubMed PubMed Central Google Scholar * Dos Santos, M. et al. Single-nucleus RNA-seq and
FISH identify coordinated transcriptional activity in mammalian myofibers. _Nat. Commun._ 11, 5102 (2020). Article PubMed PubMed Central Google Scholar * Kim, M. et al. Single-nucleus
transcriptomics reveals functional compartmentalization in syncytial skeletal muscle cells. _Nat. Commun._ 11, 6375 (2020). Article CAS PubMed PubMed Central Google Scholar * Zeng, W.
et al. Single-nucleus RNA-seq of differentiating human myoblasts reveals the extent of fate heterogeneity. _Nucleic Acids Res._ 44, e158 (2016). PubMed PubMed Central Google Scholar *
Zitting, K.-M. et al. Human resting energy expenditure varies with circadian phase. _Curr. Biol._ 28, 3685–3690.e3 (2018). Article CAS PubMed PubMed Central Google Scholar * Harmsen, J.
et al. Circadian misalignment disturbs the skeletal muscle lipidome in healthy young men. _FASEB J._ 35, e21611 (2021). Article CAS PubMed Google Scholar * Wefers, J. et al. Circadian
misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. _Proc. Natl Acad. Sci. USA_ 115, 7789–7794 (2018). Article CAS PubMed
PubMed Central Google Scholar * Morris, C. J. et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. _Proc. Natl Acad.
Sci. USA_ 112, E2225–E2234 (2015). Article CAS PubMed PubMed Central Google Scholar * Morris, J. K. et al. Mild cognitive impairment and donepezil impact mitochondrial respiratory
capacity in skeletal muscle. _Function_ 2, zqab045 (2021). Article PubMed PubMed Central Google Scholar * Hodge, B. A. et al. The endogenous molecular clock orchestrates the temporal
separation of substrate metabolism in skeletal muscle. _Skelet. Muscle_ 5, 17 (2015). Article PubMed PubMed Central Google Scholar * Ezagouri, S. et al. Physiological and molecular
dissection of daily variance in exercise capacity. _Cell Metab._ 30, 78–91.e4 (2019). Article CAS PubMed Google Scholar * Harfmann, B. D. et al. Muscle-specific loss of Bmal1 leads to
disrupted tissue glucose metabolism and systemic glucose homeostasis. _Skelet. Muscle_ 6, 12 (2016). Article PubMed PubMed Central Google Scholar * Yin, H. et al. Metabolic‐sensing of
the skeletal muscle clock coordinates fuel oxidation. _FASEB J._ 34, 6613–6627 (2020). Article CAS PubMed Google Scholar * Dyar, K. A. et al. Muscle insulin sensitivity and glucose
metabolism are controlled by the intrinsic muscle clock. _Mol. Metab._ 3, 29–41 (2014). Article CAS PubMed Google Scholar * McCarthy, J. J. et al. Identification of the circadian
transcriptome in adult mouse skeletal muscle. _Physiol. Genomics_ 31, 86–95 (2007). Article CAS PubMed Google Scholar * van Moorsel, D. et al. Demonstration of a day-night rhythm in
human skeletal muscle oxidative capacity. _Mol. Metab._ 5, 635–645 (2016). Article PubMed PubMed Central Google Scholar * de Goede, P. et al. Time-restricted feeding improves glucose
tolerance in rats, but only when in line with the circadian timing system. _Front. Endocrinol._ 10, 554 (2019). Article Google Scholar * de Goede, P. et al. Differential effects of diet
composition and timing of feeding behavior on rat brown adipose tissue and skeletal muscle peripheral clocks. _Neurobiol. Sleep Circadian Rhythms_ 4, 24–33 (2018). Article PubMed Google
Scholar * Lamia, K. A., Storch, K.-F. & Weitz, C. J. Physiological significance of a peripheral tissue circadian clock. _Proc. Natl Acad. Sci. USA_ 105, 15172–15177 (2008). Article CAS
PubMed PubMed Central Google Scholar * Kondratov, R. V., Kondratova, A. A., Gorbacheva, V. Y., Vykhovanets, O. V. & Antoch, M. P. Early aging and age-related pathologies in mice
deficient in BMAL1, the core component of the circadian clock. _Genes Dev._ 20, 1868–1873 (2006). Article CAS PubMed PubMed Central Google Scholar * Dyar, K. A. et al. Transcriptional
programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. _PLoS Biol._ 16, e2005886 (2018). Article PubMed PubMed Central Google Scholar * Schroder, E. A. et
al. Intrinsic muscle clock is necessary for musculoskeletal health. _J. Physiol._ 593, 5387–5404 (2015). Article CAS PubMed PubMed Central Google Scholar * Lee, S. & Dong, H. H.
FoxO integration of insulin signaling with glucose and lipid metabolism. _J. Endocrinol._ 233, R67–R79 (2017). Article CAS PubMed PubMed Central Google Scholar * Karanth, S. et al.
FOXN3 controls liver glucose metabolism by regulating gluconeogenic substrate selection. _Physiol. Rep._ 7, e14238 (2019). Article CAS PubMed PubMed Central Google Scholar * Bruno, N.
E. et al. Creb coactivators direct anabolic responses and enhance performance of skeletal muscle. _EMBO J._ 33, 1027–1043 (2014). Article CAS PubMed PubMed Central Google Scholar *
Bruno, N. E. et al. Activation of Crtc2/Creb1 in skeletal muscle enhances weight loss during intermittent fasting. _FASEB J._ 35, e21999 (2021). Article CAS PubMed Google Scholar *
Pillon, N. J. et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. _Nat. Commun._ 11, 470 (2020). Article CAS PubMed PubMed Central Google Scholar
* Pastore, S. & Hood, D. A. Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. _J. Appl. Physiol._ 114, 1076–1084 (2013).
Article CAS PubMed Google Scholar * Bae, K. et al. Differential effects of two period genes on the physiology and proteomic profiles of mouse anterior tibialis muscles. _Mol. Cell_ 22,
275–284 (2006). CAS Google Scholar * Woldt, E. et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. _Nat. Med._ 19, 1039–1046
(2013). Article CAS PubMed PubMed Central Google Scholar * Jordan, S. D. et al. CRY1/2 selectively repress PPARδ and limit exercise capacity. _Cell Metab._ 26, 243–255.e6 (2017).
Article CAS PubMed PubMed Central Google Scholar * Fan, W. et al. PPARδ promotes running endurance by preserving glucose. _Cell Metab._ 25, 1186–1193.e4 (2017). Article CAS PubMed
PubMed Central Google Scholar * Yamamoto, H. et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. _Cell_ 147, 827–839 (2011). Article CAS PubMed
PubMed Central Google Scholar * Marcheva, B. et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. _Nature_ 466, 627–631 (2010). Article CAS
PubMed PubMed Central Google Scholar * Loizides-Mangold, U. et al. Lipidomics reveals diurnal lipid oscillations in human skeletal muscle persisting in cellular myotubes cultured in
vitro. _Proc. Natl Acad. Sci. USA_ 114, E8565–E8574 (2017). Article CAS PubMed PubMed Central Google Scholar * Dibner, C. The importance of being rhythmic: living in harmony with your
body clocks. _Acta Physiol._ 228, e13281 (2020). Article CAS Google Scholar * Vetter, C. Circadian disruption: what do we actually mean? _Eur. J. Neurosci._ 51, 531–550 (2020). Article
PubMed Google Scholar * Harmsen, J.-F. et al. The influence of bright and dim light on substrate metabolism, energy expenditure and thermoregulation in insulin-resistant individuals
depends on time of day. _Diabetologia_ 65, 721–732 (2022). Article CAS PubMed PubMed Central Google Scholar * Morris, C. J., Purvis, T. E., Mistretta, J. & Scheer, F. A. J. L.
Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. _J. Clin. Endocrinol. Metab._ 101, 1066–1074 (2016). Article CAS PubMed
PubMed Central Google Scholar * Qian, J. & Scheer, F. A. Circadian system and glucose metabolism: implications for physiology and disease. _Trends Endocrinol. Metab._ 27, 282–293
(2016). Article CAS PubMed PubMed Central Google Scholar * Scheer, F. A. J. L., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of
circadian misalignment. _Proc. Natl Acad. Sci. USA_ 106, 4453–4458 (2009). Article CAS PubMed PubMed Central Google Scholar * Eckel, R. H. et al. Morning circadian misalignment during
short sleep duration impacts insulin sensitivity. _Curr. Biol._ 25, 3004–3010 (2015). Article CAS PubMed Google Scholar * Karthikeyan, R. et al. Should we listen to our clock to prevent
type 2 diabetes mellitus? _Diabetes Res. Clin. Pract._ 106, 182–190 (2014). Article PubMed Google Scholar * Hansen, J. et al. Synchronized human skeletal myotubes of lean, obese and type
2 diabetic patients maintain circadian oscillation of clock genes. _Sci. Rep._ 6, 35047 (2016). Article CAS PubMed PubMed Central Google Scholar * Cardinali, D. P., Brown, G. M. &
Pandi-Perumal, S. R. in _The Human Hypothalamus: Anterior Region_ Handbook of Clinical Neurology series vol. 179 (eds Swaab, D. F., Kreier, F., Lucassen, P. J., Salehe, A. & Buijs, R.
M.) 357–370 (Elsevier, 2021). * Lee, Y., Field, J. M. & Sehgal, A. Circadian rhythms, disease and chronotherapy. _J. Biol. Rhythms_ 36, 503–531 (2021). Article CAS PubMed PubMed
Central Google Scholar * Ruan, W., Yuan, X. & Eltzschig, H. K. Circadian rhythm as a therapeutic target. _Nat. Rev. Drug. Discov._ 20, 287–307 (2021). Article CAS PubMed PubMed
Central Google Scholar * Yoo, S.-H. et al. PERIOD2: LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. _Proc. Natl
Acad. Sci. USA_ 101, 5339–5346 (2004). Article CAS PubMed PubMed Central Google Scholar * Wolff, G. & Esser, K. A. Scheduled exercise phase shifts the circadian clock in skeletal
muscle. _Med. Sci. Sports Exerc._ 44, 1663–1670 (2012). Article PubMed PubMed Central Google Scholar * Kemler, D., Wolff, C. A. & Esser, K. A. Time‐of‐day dependent effects of
contractile activity on the phase of the skeletal muscle clock. _J. Physiol._ 598, 3631–3644 (2020). Article CAS PubMed Google Scholar * Adamovich, Y. et al. Clock proteins and training
modify exercise capacity in a daytime-dependent manner. _Proc. Natl Acad. Sci. USA_ 118, e2101115118 (2021). Article CAS PubMed PubMed Central Google Scholar * Hoffman, N. J. et al.
Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. _Cell Metab._ 22, 922–935 (2015). Article CAS PubMed PubMed
Central Google Scholar * Vieira, E. et al. Relationship between AMPK and the transcriptional balance of clock-related genes in skeletal muscle. _Am. J. Physiol. Endocrinol. Metab._ 295,
E1032–E1037 (2008). Article CAS PubMed Google Scholar * Casanova-Vallve, N. et al. Daily running enhances molecular and physiological circadian rhythms in skeletal muscle. _Mol. Metab._
61, 101504 (2022). Article CAS PubMed PubMed Central Google Scholar * Sato, S. et al. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis.
_Cell Metab._ 30, 92–110.e4 (2019). Article CAS PubMed Google Scholar * Hawley, J. A., Sassone-Corsi, P. & Zierath, J. R. Chrono-nutrition for the prevention and treatment of
obesity and type 2 diabetes: from mice to men. _Diabetologia_ 63, 2253–2259 (2020). Article PubMed Google Scholar * Savikj, M. et al. Afternoon exercise is more efficacious than morning
exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. _Diabetologia_ 62, 233–237 (2019). Article CAS PubMed Google Scholar *
Savikj, M. et al. Exercise timing influences multi-tissue metabolome and skeletal muscle proteome profiles in type 2 diabetic patients — a randomized crossover trial. _Metabolism_ 135,
155268 (2022). Article CAS PubMed Google Scholar * Moholdt, T. et al. The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese
men: a randomised trial. _Diabetologia_ 64, 2061–2076 (2021). Article CAS PubMed PubMed Central Google Scholar * Mancilla, R. et al. Exercise training elicits superior metabolic effects
when performed in the afternoon compared to morning in metabolically compromised humans. _Physiol. Rep._ 8, e14669 (2021). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS The authors acknowledge the support of NIH grants U01AG055137 and R01AR079220 to K.A.E. The authors also thank L. Denes, Institute for Systems Genetics, New York, for kindly
providing the image of the myofibre in Fig. 4b. AUTHOR INFORMATION Author notes * These authors contributed equally: Ryan Martin, Mark Viggars. AUTHORS AND AFFILIATIONS * Department of
Physiology and Aging, University of Florida, Gainesville, FL, USA Ryan A. Martin, Mark R. Viggars & Karyn A. Esser * Myology Institute, University of Florida, Gainesville, FL, USA Ryan
A. Martin, Mark R. Viggars & Karyn A. Esser Authors * Ryan A. Martin View author publications You can also search for this author inPubMed Google Scholar * Mark R. Viggars View author
publications You can also search for this author inPubMed Google Scholar * Karyn A. Esser View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
R.A.M. and M.R.V. researched data for the article. R.A.M, M.R.V and K.A.E. contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript
before submission. CORRESPONDING AUTHOR Correspondence to Karyn A. Esser. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW
INFORMATION _Nature Reviews Endocrinology_ thanks Charna Dibner, Ke Ma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other
rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Martin, R.A., Viggars, M.R. & Esser, K.A. Metabolism and exercise: the skeletal muscle clock takes centre stage. _Nat Rev Endocrinol_ 19,
272–284 (2023). https://doi.org/10.1038/s41574-023-00805-8 Download citation * Accepted: 12 January 2023 * Published: 01 February 2023 * Issue Date: May 2023 * DOI:
https://doi.org/10.1038/s41574-023-00805-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
![[withdrawn] yjb to withdraw from hassockfield stc and hindley yoi](https://assets.publishing.service.gov.uk/media/5a62111ee5274a0a3ad908a5/s960_YJB_logo_Gov.uk.jpg)



