- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Despite its position as the first-line drug for treatment of type 2 diabetes mellitus, the mechanisms underlying the plasma glucose level-lowering effects of metformin
(1,1-dimethylbiguanide) still remain incompletely understood. Metformin is thought to exert its primary antidiabetic action through the suppression of hepatic glucose production. In
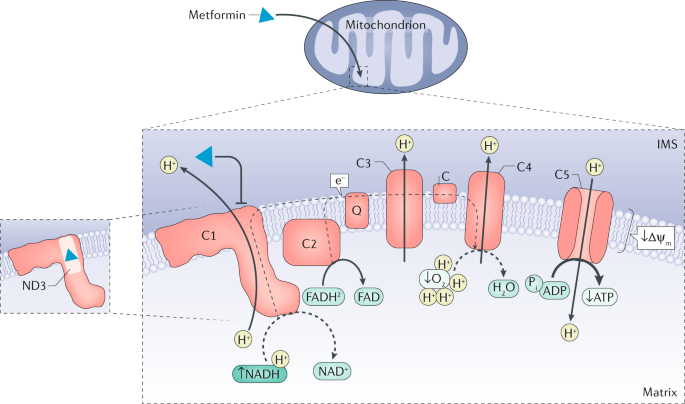
addition, the discovery that metformin inhibits the mitochondrial respiratory chain complex 1 has placed energy metabolism and activation of AMP-activated protein kinase (AMPK) at the centre
of its proposed mechanism of action. However, the role of AMPK has been challenged and might only account for indirect changes in hepatic insulin sensitivity. Various mechanisms involving
alterations in cellular energy charge, AMP-mediated inhibition of adenylate cyclase or fructose-1,6-bisphosphatase 1 and modulation of the cellular redox state through direct inhibition of
mitochondrial glycerol-3-phosphate dehydrogenase have been proposed for the acute inhibition of gluconeogenesis by metformin. Emerging evidence suggests that metformin could improve
obesity-induced meta-inflammation via direct and indirect effects on tissue-resident immune cells in metabolic organs (that is, adipose tissue, the gastrointestinal tract and the liver).
Furthermore, the gastrointestinal tract also has a major role in metformin action through modulation of glucose-lowering hormone glucagon-like peptide 1 and the intestinal bile acid pool and
alterations in gut microbiota composition. KEY POINTS * Metformin is the first-line drug for treatment of type 2 diabetes mellitus, with an excellent safety profile, high efficacy in
glycaemic control and clear but incompletely understood cardioprotective benefits. * The pleiotropic properties of metformin suggest that the drug acts on multiple tissues through various
underlying mechanisms rather than on a single organ via a unifying mode of action. * Mitochondrial respiratory chain complex 1 is targeted by metformin and its inhibition is involved in
AMP-activated protein kinase-independent regulation of hepatic gluconeogenesis by triggering alterations in cellular energy charge and redox state. * Metformin might contribute to
improvements in obesity-associated meta-inflammation and tissue-specific insulin sensitivity through direct and indirect effects on various resident immune cells in metabolic organs. * The
gastrointestinal tract has an important role in the action of metformin, which modulates bile acid recirculation and enhances the secretion of the glucose-lowering gut incretin hormone
glucagon-like peptide 1. * The gut microbiota is a novel target in the mechanisms of metformin action and is involved in both the therapeutic and adverse effects of the drug. Access through
your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature
Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access
$209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are
calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
METFORMIN: UPDATE ON MECHANISMS OF ACTION AND REPURPOSING POTENTIAL Article 02 May 2023 THE GASTROINTESTINAL TRACT IS A MAJOR SOURCE OF THE ACUTE METFORMIN-STIMULATED RISE IN GDF15 Article
Open access 22 January 2024 THE IMPORTANCE OF THE AMPK GAMMA 1 SUBUNIT IN METFORMIN SUPPRESSION OF LIVER GLUCOSE PRODUCTION Article Open access 26 June 2020 REFERENCES * Davies, M. J. et al.
Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). _Diabetes
Care_ 41, 2669–2701 (2018). PubMed Google Scholar * Davies, M. J. et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association
(ADA) and the European Association for the Study of Diabetes (EASD). _Diabetologia_ 61, 2461–2498 (2018). PubMed Google Scholar * United Kingdom Prospective Diabetes Study (UKPDS) Group.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. _Lancet_ 352,
854–865 (1998). Google Scholar * United Kingdom Prospective Diabetes Study (UKPDS) Group. United Kingdom prospective diabetes study 24: a 6-year, randomized, controlled trial comparing
sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group.
_Ann. Intern. Med._ 128, 165–175 (1998). Google Scholar * Maruthur, N. M. et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a
systematic review and meta-analysis. _Ann. Intern. Med._ 164, 740–751 (2016). PubMed Google Scholar * Palmer, S. C. et al. Comparison of clinical outcomes and adverse events associated
with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. _JAMA_ 316, 313–324 (2016). CAS PubMed Google Scholar * Sanchez-Rangel, E. & Inzucchi, S. E. Metformin:
clinical use in type 2 diabetes. _Diabetologia_ 60, 1586–1593 (2017). CAS PubMed Google Scholar * Howlett, H. C. & Bailey, C. J. A risk-benefit assessment of metformin in type 2
diabetes mellitus. _Drug Saf._ 20, 489–503 (1999). CAS PubMed Google Scholar * Bailey, C. J. Metformin: historical overview. _Diabetologia_ 60, 1566–1576 (2017). CAS PubMed Google
Scholar * Werner, E. & Bell, J. The preparation of methylguanidine, and of ββ-dimethylguanidine by interaction of dicyanodiamide, and methylammonium and dimethylammonium chlorides
respectively. _J. Am. Chem. Soc._ 121, 1790–1794 (1922). CAS Google Scholar * Nattrass, M. et al. Hyperlactatemia in diabetics with retinopathy during combined sulphonylurea and phenformin
therapy. _Diabete Metab._ 4, 1–4 (1978). CAS PubMed Google Scholar * Foretz, M., Guigas, B., Bertrand, L., Pollak, M. & Viollet, B. Metformin: from mechanisms of action to therapies.
_Cell Metab._ 20, 953–966 (2014). CAS PubMed Google Scholar * Hundal, R. S. et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. _Diabetes_ 49, 2063–2069
(2000). CAS PubMed PubMed Central Google Scholar * Cusi, K., Consoli, A. & DeFronzo, R. A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent
diabetes mellitus. _J. Clin. Endocrinol. Metab._ 81, 4059–4067 (1996). CAS PubMed Google Scholar * Hother-Nielsen, O., Schmitz, O., Andersen, P. H., Beck-Nielsen, H. & Pedersen, O.
Metformin improves peripheral but not hepatic insulin action in obese patients with type II diabetes. _Acta Endocrinol._ 120, 257–265 (1989). CAS PubMed Google Scholar * Stumvoll, M.,
Nurjhan, N., Perriello, G., Dailey, G. & Gerich, J. E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. _N. Engl. J. Med._ 333, 550–554 (1995). CAS PubMed
Google Scholar * DeFronzo, R. A., Barzilai, N. & Simonson, D. C. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. _J. Clin. Endocrinol. Metab._
73, 1294–1301 (1991). CAS PubMed Google Scholar * Inzucchi, S. E. et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. _N. Engl. J. Med._ 338,
867–872 (1998). CAS PubMed Google Scholar * Bailey, C. J., Mynett, K. J. & Page, T. Importance of the intestine as a site of metformin-stimulated glucose utilization. _Br. J.
Pharmacol._ 112, 671–675 (1994). CAS PubMed PubMed Central Google Scholar * Buse, J. B. et al. The primary glucose-lowering effect of metformin resides in the gut, not the circulation:
results from short-term pharmacokinetic and 12-week dose-ranging studies. _Diabetes Care_ 39, 198–205 (2016). THIS HUMAN STUDY PROVIDES STRONG EVIDENCE FOR A GUT-MEDIATED MECHANISM IN THE
BLOOD GLUCOSE-LOWERING ACTION OF METFORMIN. CAS PubMed Google Scholar * McCreight, L. J., Bailey, C. J. & Pearson, E. R. Metformin and the gastrointestinal tract. _Diabetologia_ 59,
426–435 (2016). CAS PubMed PubMed Central Google Scholar * Forslund, K. et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. _Nature_ 528,
262–266 (2015). CAS PubMed PubMed Central Google Scholar * Hardie, D. G. AMPK-sensing energy while talking to other signaling pathways. _Cell Metab._ 20, 939–952 (2014). CAS PubMed
PubMed Central Google Scholar * Cao, J. et al. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). _J. Biol. Chem._ 289,
20435–20446 (2014). CAS PubMed PubMed Central Google Scholar * Graham, G. G. et al. Clinical pharmacokinetics of metformin. _Clin. Pharmacokinet._ 50, 81–98 (2011). CAS PubMed Google
Scholar * Tucker, G. T. et al. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. _Br. J. Clin. Pharmacol._ 12, 235–246 (1981). CAS PubMed PubMed Central
Google Scholar * Wilcock, C. & Bailey, C. J. Accumulation of metformin by tissues of the normal and diabetic mouse. _Xenobiotica_ 24, 49–57 (1994). CAS PubMed Google Scholar *
Jensen, J. B. et al. [11C]-Labeled metformin distribution in the liver and small intestine using dynamic positron emission tomography in mice demonstrates tissue-specific transporter
dependency. _Diabetes_ 65, 1724–1730 (2016). CAS PubMed Google Scholar * Gormsen, L. C. et al. In vivo imaging of human 11C-metformin in peripheral organs: dosimetry, biodistribution, and
kinetic analyses. _J. Nucl. Med._ 57, 1920–1926 (2016). CAS PubMed Google Scholar * Liang, X. & Giacomini, K. M. Transporters involved in metformin pharmacokinetics and treatment
response. _J. Pharm. Sci._ 106, 2245–2250 (2017). CAS PubMed Google Scholar * Todd, J. N. & Florez, J. C. An update on the pharmacogenomics of metformin: progress, problems and
potential. _Pharmacogenomics_ 15, 529–539 (2014). CAS PubMed PubMed Central Google Scholar * Dujic, T. et al. Variants in pharmacokinetic transporters and glycemic response to metformin:
a Metgen meta-analysis. _Clin. Pharmacol. Ther._ 101, 763–772 (2017). CAS PubMed PubMed Central Google Scholar * Zhou, K. et al. Variation in the glucose transporter gene SLC2A2 is
associated with glycemic response to metformin. _Nat. Genet._ 48, 1055–1059 (2016). CAS PubMed PubMed Central Google Scholar * Ait-Omar, A. et al. GLUT2 accumulation in enterocyte apical
and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice. _Diabetes_ 60, 2598–2607 (2011). CAS PubMed PubMed Central Google Scholar *
Jablonski, K. A. et al. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. _Diabetes_ 59,
2672–2681 (2010). CAS PubMed PubMed Central Google Scholar * Hollunger, G. Guanidines and oxidative phosphorylations. _Acta Pharmacol. Toxicol._ 11, 1–84 (1955). CAS Google Scholar *
Schafer, G. Site-specific uncoupling and inhibition of oxidative phosphorylation by biguanides. II. _Biochim. Biophys. Acta_ 172, 334–337 (1969). CAS PubMed Google Scholar * El-Mir, M. Y.
et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. _J. Biol. Chem._ 275, 223–228 (2000). CAS PubMed Google Scholar *
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. _Biochem. J._
348, 607–614 (2000). THIS STUDY TOGETHER WITH EL-MIR ET AL. (2000) WERE THE FIRST TO REPORT SPECIFIC INHIBITION OF METFORMIN ON THE MITOCHONDRIAL RESPIRATORY CHAIN COMPLEX 1. CAS PubMed
PubMed Central Google Scholar * Degli Esposti, M. Inhibitors of NADH-ubiquinone reductase: an overview. _Biochim. Biophys. Acta_ 1364, 222–235 (1998). CAS PubMed Google Scholar *
Fontaine, E. Metformin-induced mitochondrial complex I inhibition: facts, uncertainties, and consequences. _Front. Endocrinol._ 9, 753 (2018). Google Scholar * Vial, G., Detaille, D. &
Guigas, B. Role of mitochondria in the mechanism(s) of action of metformin. _Front. Endocrinol._ 10, 294 (2019). Google Scholar * Stephenne, X. et al. Metformin activates AMP-activated
protein kinase in primary human hepatocytes by decreasing cellular energy status. _Diabetologia_ 54, 3101–3110 (2011). THE IS THE FIRST STUDY TO SHOW THAT METFORMIN INHIBITS MITOCHONDRIAL
RESPIRATORY CHAIN COMPLEX 1 AND ACTIVATES AMPK IN PRIMARY HUMAN HEPATOCYTES. CAS PubMed PubMed Central Google Scholar * Andrzejewski, S., Gravel, S. P., Pollak, M. & St-Pierre, J.
Metformin directly acts on mitochondria to alter cellular bioenergetics. _Cancer Metab._ 2, 12 (2014). PubMed PubMed Central Google Scholar * Bridges, H. R., Jones, A. J., Pollak, M. N.
& Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. _Biochem. J._ 462, 475–487 (2014). THIS ELEGANT STUDY DISSECTS THE MECHANISM BY WHICH
METFORMIN INHIBITS THE MITOCHONDRIAL RESPIRATORY CHAIN COMPLEX 1. CAS PubMed PubMed Central Google Scholar * Guigas, B. et al. Metformin inhibits mitochondrial permeability transition
and cell death: a pharmacological in vitro study. _Biochem. J._ 382, 877–884 (2004). CAS PubMed PubMed Central Google Scholar * Wheaton, W. W. et al. Metformin inhibits mitochondrial
complex I of cancer cells to reduce tumorigenesis. _eLife_ 3, e02242 (2014). PubMed PubMed Central Google Scholar * Zannella, V. E. et al. Reprogramming metabolism with metformin improves
tumor oxygenation and radiotherapy response. _Clin. Cancer Res._ 19, 6741–6750 (2013). CAS PubMed Google Scholar * Gui, D. Y. et al. Environment dictates dependence on mitochondrial
complex I for NAD+ and aspartate production and determines cancer cell sensitivity to metformin. _Cell Metab._ 24, 716–727 (2016). CAS PubMed PubMed Central Google Scholar * Cheng, G. et
al. Mitochondria-targeted analogues of metformin exhibit enhanced antiproliferative and radiosensitizing effects in pancreatic cancer cells. _Cancer Res._ 76, 3904–3915 (2016). CAS PubMed
PubMed Central Google Scholar * Boukalova, S. et al. Mitochondrial targeting of metformin enhances its activity against pancreatic cancer. _Mol. Cancer Ther._ 15, 2875–2886 (2016). CAS
PubMed Google Scholar * Guo, Z. et al. Heme binding biguanides target cytochrome P450-dependent cancer cell mitochondria. _Cell Chem. Biol._ 24, 1314–1275.e6 (2017). CAS PubMed Google
Scholar * Thakur, S. et al. Metformin targets mitochondrial glycerophosphate dehydrogenase to control rate of oxidative phosphorylation and growth of thyroid cancer in vitro and in vivo.
_Clin. Cancer Res._ 24, 4030–4043 (2018). CAS PubMed PubMed Central Google Scholar * Fontaine, E. Metformin and respiratory chain complex I: the last piece of the puzzle? _Biochem. J._
463, e3–e5 (2014). CAS PubMed Google Scholar * Wilcock, C., Wyre, N. D. & Bailey, C. J. Subcellular distribution of metformin in rat liver. _J. Pharm. Pharmacol._ 43, 442–444 (1991).
CAS PubMed Google Scholar * Detaille, D. et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process.
_Diabetes_ 54, 2179–2187 (2005). THIS IS THE FIRST STUDY DEMONSTRATING THAT METFORMIN CAN REDUCE OXIDATIVE STRESS THROUGH ITS MITOCHONDRIAL ACTION. CAS PubMed Google Scholar * Detaille,
D., Guigas, B., Leverve, X., Wiernsperger, N. & Devos, P. Obligatory role of membrane events in the regulatory effect of metformin on the respiratory chain function. _Biochem.
Pharmacol._ 63, 1259–1272 (2002). CAS PubMed Google Scholar * El-Mir, M. Y. et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical
neurons. _J. Mol. Neurosci._ 34, 77–87 (2008). CAS PubMed Google Scholar * Bridges, H. R., Sirvio, V. A., Agip, A. N. & Hirst, J. Molecular features of biguanides required for
targeting of mitochondrial respiratory complex I and activation of AMP-kinase. _BMC Biol._ 14, 65 (2016). PubMed PubMed Central Google Scholar * Logie, L. et al. Cellular responses to the
metal-binding properties of metformin. _Diabetes_ 61, 1423–1433 (2012). CAS PubMed PubMed Central Google Scholar * Hirst, J. Mitochondrial complex I. _Annu. Rev. Biochem._ 82, 551–575
(2013). CAS PubMed Google Scholar * Zickermann, V. et al. Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. _Science_ 347, 44–49 (2015). CAS
PubMed Google Scholar * Saheki, T. et al. Citrin/mitochondrial glycerol-3-phosphate dehydrogenase double knock-out mice recapitulate features of human citrin deficiency. _J. Biol. Chem._
282, 25041–25052 (2007). CAS PubMed Google Scholar * Shu, Y. et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. _J. Clin. Invest._ 117,
1422–1431 (2007). CAS PubMed PubMed Central Google Scholar * He, L. & Wondisford, F. E. Metformin action: concentrations matter. _Cell Metab._ 21, 159–162 (2015). CAS PubMed Google
Scholar * Christensen, M. M. et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. _Pharmacogenet. Genomics_ 21,
837–850 (2011). CAS PubMed Google Scholar * Lalau, J. D., Lemaire-Hurtel, A. S. & Lacroix, C. Establishment of a database of metformin plasma concentrations and erythrocyte levels in
normal and emergency situations. _Clin. Drug Investig._ 31, 435–438 (2011). CAS PubMed Google Scholar * Foretz, M. et al. Metformin inhibits hepatic gluconeogenesis in mice independently
of the LKB1/AMPK pathway via a decrease in hepatic energy state. _J. Clin. Invest._ 120, 2355–2369 (2010). THIS PAPER PROVIDES GENETIC EVIDENCE OF AMPK-INDEPENDENT ACTION OF METFORMIN IN THE
INHIBITION OF HEPATIC GLUCOSE PRODUCTION. CAS PubMed PubMed Central Google Scholar * Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and
the insulin-sensitizing effects of metformin. _Nat. Med._ 19, 1649–1654 (2013). THIS PAPER PROVIDES GENETIC EVIDENCE OF AMPK-MEDIATED PHOSPHORYLATION OF BOTH ACC1 AND ACC2 IN METFORMIN
REGULATION OF LIPID METABOLISM AND INSULIN SENSITIVITY. CAS PubMed PubMed Central Google Scholar * Hunter, R. W. et al. Metformin reduces liver glucose production by inhibition of
fructose-1-6-bisphosphatase. _Nat. Med._ 24, 1395–1406 (2018). THIS STUDY PROVIDES GENETIC EVIDENCE OF METFORMIN INHIBITION OF HEPATIC GLUCOSE PRODUCTION THROUGH ALLOSTERIC INHIBITION OF
FRUCTOSE-1-6-BISPHOSPHATASE VIA METFORMIN-INDUCED INCREASES OF INTRACELLULAR AMP LEVELS. CAS PubMed PubMed Central Google Scholar * Miller, R. A. et al. Biguanides suppress hepatic
glucagon signalling by decreasing production of cyclic AMP. _Nature_ 494, 256–260 (2013). THIS PAPER DESCRIBES THE SUPPRESSION OF HEPATIC GLUCAGON SIGNALLING IN RESPONSE TO METFORMIN BY
INHIBITION OF ADENYLATE CYCLASE VIA METFORMIN-INDUCED INCREASES OF INTRACELLULAR AMP LEVELS. CAS PubMed PubMed Central Google Scholar * Clarke, J. D. et al. Mechanism of altered
metformin distribution in nonalcoholic steatohepatitis. _Diabetes_ 64, 3305–3313 (2015). CAS PubMed PubMed Central Google Scholar * Scheen, A. J. Clinical pharmacokinetics of metformin.
_Clin. Pharmacokinet._ 30, 359–371 (1996). CAS PubMed Google Scholar * Alshawi, A. & Agius, L. Low metformin causes a more oxidized mitochondrial NADH/NAD redox state in hepatocytes
and inhibits gluconeogenesis by a redox-independent mechanism. _J. Biol. Chem._ 294, 2839–2853 (2019). CAS PubMed Google Scholar * Madiraju, A. K. et al. Metformin suppresses
gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. _Nature_ 510, 542–546 (2014). THIS PAPER DESCRIBES A REDOX-DEPENDENT MECHANISM TO ACCOUNT FOR THE INHIBITION OF
HEPATIC GLUCONEOGENESIS BY METFORMIN. CAS PubMed PubMed Central Google Scholar * Al-Oanzi, Z. H. et al. Opposite effects of a glucokinase activator and metformin on glucose-regulated
gene expression in hepatocytes. _Diabetes Obes. Metab._ 19, 1078–1087 (2017). CAS PubMed Google Scholar * Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin
action. _J. Clin. Invest._ 108, 1167–1174 (2001). THIS IS THE FIRST PAPER SHOWING AMPK ACTIVATION BY METFORMIN. CAS PubMed PubMed Central Google Scholar * Hawley, S. A. et al. Use of
cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. _Cell Metab._ 11, 554–565 (2010). CAS PubMed PubMed Central Google Scholar * Zhang, C. S. et
al. The lysosomal v-ATPase-ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. _Cell Metab._ 20, 526–540 (2014). THIS STUDY
REPORTS AN AMP-INDEPENDENT MECHANISM FOR GLUCOSE SENSING BY AMPK THROUGH THE LYSOSOMAL PATHWAY. CAS PubMed Google Scholar * Li, M. et al. Transient receptor potential V channels are
essential for glucose sensing by aldolase and AMPK. _Cell Metab._ https://doi.org/10.1016/j.cmet.2019.05.018 (2019). PubMed PubMed Central Google Scholar * Zhang, C. S. et al. Metformin
activates AMPK through the lysosomal pathway. _Cell Metab._ 24, 521–522 (2016). PubMed Google Scholar * Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and
therapeutic effects of metformin. _Science_ 310, 1642–1646 (2005). CAS PubMed PubMed Central Google Scholar * He, L. et al. Metformin and insulin suppress hepatic gluconeogenesis through
phosphorylation of CREB binding protein. _Cell_ 137, 635–646 (2009). CAS PubMed PubMed Central Google Scholar * Lee, J. M. et al. AMPK-dependent repression of hepatic gluconeogenesis
via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner. _J. Biol. Chem._ 285, 32182–32191 (2010). CAS PubMed PubMed Central Google Scholar * Caton, P.
W. et al. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. _J. Endocrinol._ 205, 97–106 (2010). CAS PubMed Google Scholar * Boudaba, N. et al. AMPK
re-activation suppresses hepatic steatosis but its downregulation does not promote fatty liver development. _EBioMedicine_ 28, 194–209 (2018). THIS PAPER PROVIDES GENETIC EVIDENCE OF
AMPK-DEPENDENT EFFECTS OF METFORMIN ON LIPOGENESIS INHIBITION AND FATTY ACID OXIDATION STIMULATION. PubMed PubMed Central Google Scholar * Cokorinos, E. C. et al. Activation of skeletal
muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. _Cell Metab._ 25, 1147–1159.e10 (2017). THIS STUDY PROVIDES PHARMACOLOGICAL AND GENETIC EVIDENCE OF
THE BLOOD GLUCOSE-LOWERING EFFECT OF AMPK ACTIVATION IN SKELETAL MUSCLE. CAS PubMed Google Scholar * Madiraju, A. K. et al. Metformin inhibits gluconeogenesis via a redox-dependent
mechanism in vivo. _Nat. Med._ 24, 1384–1394 (2018). CAS PubMed PubMed Central Google Scholar * Ringler, R. L. & Singer, T. P. Studies on the mitochondrial alpha-glycerophosphate
dehydrogenase. I. Reaction of the dehydrogenase with electron acceptors and the respiratory chain. _J. Biol. Chem._ 234, 2211–2217 (1959). CAS PubMed Google Scholar * Lee, Y. P. &
Lardy, H. A. Influence of thyroid hormones on L-α-glycerophosphate dehydrogenases and other dehydrogenases in various organs of the rat. _J. Biol. Chem._ 240, 1427–1436 (1965). CAS PubMed
Google Scholar * Lin, E. C. Glycerol utilization and its regulation in mammals. _Annu. Rev. Biochem._ 46, 765–795 (1977). CAS PubMed Google Scholar * Brisson, D., Vohl, M. C., St-Pierre,
J., Hudson, T. J. & Gaudet, D. Glycerol: a neglected variable in metabolic processes? _Bioessays_ 23, 534–542 (2001). CAS PubMed Google Scholar * Baur, J. A. & Birnbaum, M. J.
Control of gluconeogenesis by metformin: does redox trump energy charge? _Cell Metab._ 20, 197–199 (2014). CAS PubMed PubMed Central Google Scholar * Cool, B. et al. Identification and
characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. _Cell Metab._ 3, 403–416 (2006). THIS IS THE FIRST PAPER
DESCRIBING A SMALL-MOLECULE DIRECT AMPK ACTIVATOR. CAS PubMed Google Scholar * Esquejo, R. M. et al. Activation of liver AMPK with PF-06409577 corrects NAFLD and lowers cholesterol in
rodent and primate preclinical models. _EBioMedicine_ 31, 122–132 (2018). PubMed PubMed Central Google Scholar * Mazza, A. et al. The role of metformin in the management of NAFLD. _Exp.
Diabetes Res._ 2012, 716404 (2012). PubMed Google Scholar * Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. _Nature_ 542, 177–185 (2017). CAS PubMed
Google Scholar * Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. _Cell_ 161, 146–160 (2015). CAS PubMed PubMed Central Google Scholar *
Lackey, D. E. & Olefsky, J. M. Regulation of metabolism by the innate immune system. _Nat. Rev. Endocrinol._ 12, 15–28 (2016). CAS PubMed Google Scholar * McNelis, J. C. &
Olefsky, J. M. Macrophages, immunity, and metabolic disease. _Immunity_ 41, 36–48 (2014). CAS PubMed Google Scholar * Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a
phenotypic switch in adipose tissue macrophage polarization. _J. Clin. Invest._ 117, 175–184 (2007). CAS PubMed PubMed Central Google Scholar * Huang, W. et al. Depletion of liver
Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. _Diabetes_ 59, 347–357 (2010). CAS PubMed Google Scholar * Lanthier, N. et al. Kupffer
cell activation is a causal factor for hepatic insulin resistance. _Am. J. Physiol. Gastrointest. Liver Physiol._ 298, G107–G116 (2010). CAS PubMed Google Scholar * Talukdar, S. et al.
Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. _Nat. Med._ 18, 1407–1412 (2012). CAS PubMed PubMed Central Google Scholar * Cai, D. et al.
Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. _Nat. Med._ 11, 183–190 (2005). CAS PubMed PubMed Central Google Scholar * Kang, K. et al.
Adipocyte-derived Th2 cytokines and myeloid PPARδ regulate macrophage polarization and insulin sensitivity. _Cell Metab._ 7, 485–495 (2008). CAS PubMed PubMed Central Google Scholar *
Odegaard, J. I. et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. _Cell Metab._ 7, 496–507 (2008). CAS PubMed PubMed Central
Google Scholar * Ricardo-Gonzalez, R. R. et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. _Proc. Natl Acad. Sci. USA_ 107, 22617–22622 (2010).
CAS PubMed Google Scholar * Stanya, K. J. et al. Direct control of hepatic glucose production by interleukin-13 in mice. _J. Clin. Invest._ 123, 261–271 (2013). CAS PubMed Google
Scholar * Odegaard, J. I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. _Nature_ 447, 1116–1120 (2007). CAS PubMed PubMed Central
Google Scholar * Ilan, Y. et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. _Proc. Natl Acad. Sci. USA_ 107, 9765–9770
(2010). CAS PubMed Google Scholar * Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. _Nat. Med._ 15,
930–939 (2009). CAS PubMed PubMed Central Google Scholar * Evia-Viscarra, M. L. et al. The effects of metformin on inflammatory mediators in obese adolescents with insulin resistance:
controlled randomized clinical trial. _J. Pediatr. Endocrinol. Metab._ 25, 41–49 (2012). CAS PubMed Google Scholar * Fidan, E. et al. The effects of rosiglitazone and metformin on
inflammation and endothelial dysfunction in patients with type 2 diabetes mellitus. _Acta Diabetol._ 48, 297–302 (2011). CAS PubMed Google Scholar * Cameron, A. R. et al.
Anti-inflammatory effects of metformin irrespective of diabetes status. _Circ. Res._ 119, 652–665 (2016). THIS STUDY SHOWS SOME BENEFICIAL ANTI-INFLAMMATORY EFFECTS OF METFORMIN IN HUMANS,
IRRESPECTIVE OF THEIR DIABETIC STATUS. CAS PubMed PubMed Central Google Scholar * Lu, C. H., Hung, Y. J. & Hsieh, P. S. Additional effect of metformin and celecoxib against lipid
dysregulation and adipose tissue inflammation in high-fat fed rats with insulin resistance and fatty liver. _Eur. J. Pharmacol._ 789, 60–67 (2016). CAS PubMed Google Scholar * Xue, W. et
al. Alkannin inhibited hepatic inflammation in diabetic Db/Db mice. _Cell. Physiol. Biochem._ 45, 2461–2470 (2018). CAS PubMed Google Scholar * Shen, C. L. et al. Annatto-extracted
tocotrienols improve glucose homeostasis and bone properties in high-fat diet-induced type 2 diabetic mice by decreasing the inflammatory response. _Sci. Rep._ 8, 11377 (2018). PubMed
PubMed Central Google Scholar * Jing, Y. et al. Metformin improves obesity-associated inflammation by altering macrophages polarization. _Mol. Cell. Endocrinol._ 461, 256–264 (2018). CAS
PubMed Google Scholar * de Oliveira, S. _et al_. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish.
_J. Hepatol_. 70, 710-721(2018). * Kim, J. et al. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3
(ATF-3) induction. _J. Biol. Chem._ 289, 23246–23255 (2014). CAS PubMed PubMed Central Google Scholar * Kelly, B., Tannahill, G. M., Murphy, M. P. & O’Neill, L. A. Metformin inhibits
the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide
(LPS)-activated macrophages. _J. Biol. Chem._ 290, 20348–20359 (2015). CAS PubMed PubMed Central Google Scholar * Moiseeva, O. et al. Metformin inhibits the senescence-associated
secretory phenotype by interfering with IKK/NF-κB activation. _Aging Cell_ 12, 489–498 (2013). CAS PubMed Google Scholar * Vasamsetti, S. B. et al. Metformin inhibits
monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. _Diabetes_ 64, 2028–2041 (2015). THIS STUDY SHOWS THE INHIBITION
OF MONOCYTE-TO-MACROPHAGE DIFFERENTIATION AND ATHEROMATOUS PLAQUE FORMATION BY METFORMIN IN _APOE_ −/− MICE THROUGH AN AMPK–STAT3-DEPENDENT MECHANISM. CAS PubMed Google Scholar * Yan, Z.
et al. Metformin suppresses UHMWPE particle-induced osteolysis in the mouse calvaria by promoting polarization of macrophages to an anti-inflammatory phenotype. _Mol. Med._ 24, 20 (2018).
CAS PubMed PubMed Central Google Scholar * Buldak, L. et al. Metformin affects macrophages’ phenotype and improves the activity of glutathione peroxidase, superoxide dismutase, catalase
and decreases malondialdehyde concentration in a partially AMPK-independent manner in LPS-stimulated human monocytes/macrophages. _Pharmacol. Rep._ 66, 418–429 (2014). CAS PubMed Google
Scholar * Arai, M. et al. Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in
human monocytes in vitro. _J. Pharmacol. Exp. Ther._ 334, 206–213 (2010). CAS PubMed Google Scholar * Stienstra, R., Netea-Maier, R. T., Riksen, N. P., Joosten, L. A. B. & Netea, M.
G. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. _Cell Metab._ 26, 142–156 (2017). CAS PubMed Google Scholar * Geltink, R. I.
K., Kyle, R. L. & Pearce, E. L. Unraveling the complex interplay between T cell metabolism and function. _Annu. Rev. Immunol._ 36, 461–488 (2018). CAS PubMed Google Scholar * Buck, M.
D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. _Cell_ 169, 570–586 (2017). CAS PubMed PubMed Central Google Scholar * Negrotto, L., Farez, M. F.
& Correale, J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. _JAMA Neurol._ 73, 520–528 (2016). PubMed Google Scholar * Lee,
S. Y. et al. Metformin suppresses systemic autoimmunity in Roquin(san/san) mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3. _J. Immunol._
198, 2661–2670 (2017). CAS PubMed PubMed Central Google Scholar * Son, H. J. et al. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg
balance and osteoclastogenesis. _Mediators Inflamm._ 2014, 973986 (2014). PubMed PubMed Central Google Scholar * Lee, S. Y. et al. Metformin ameliorates inflammatory bowel disease by
suppression of the STAT3 signaling pathway and regulation of the between Th17/treg balance. _PLOS ONE_ 10, e0135858 (2015). PubMed PubMed Central Google Scholar * Eikawa, S. et al.
Immune-mediated antitumor effect by type 2 diabetes drug, metformin. _Proc. Natl Acad. Sci. USA_ 112, 1809–1814 (2015). CAS PubMed Google Scholar * Kunisada, Y. et al. Attenuation of
CD4+CD25+ regulatory T cells in the tumor microenvironment by metformin, a type 2 diabetes drug. _EBioMedicine_ 25, 154–164 (2017). PubMed PubMed Central Google Scholar * Li, L. et al.
Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. _Cancer Res._ 78, 1779–1791 (2018). CAS PubMed PubMed Central
Google Scholar * Pereira, F. V. et al. Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response.
_Oncotarget_ 9, 25808–25825 (2018). PubMed PubMed Central Google Scholar * Natali, A. & Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose
production and stimulation of glucose uptake in type 2 diabetes: a systematic review. _Diabetologia_ 49, 434–441 (2006). CAS PubMed Google Scholar * Wu, T., Horowitz, M. & Rayner, C.
K. New insights into the anti-diabetic actions of metformin: from the liver to the gut. _Expert Rev. Gastroenterol. Hepatol._ 11, 157–166 (2017). CAS PubMed Google Scholar * Bonora, E. et
al. Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects. _Curr. Med. Res. Opin._ 9, 47–51
(1984). CAS PubMed Google Scholar * Stepensky, D., Friedman, M., Raz, I. & Hoffman, A. Pharmacokinetic-pharmacodynamic analysis of the glucose-lowering effect of metformin in diabetic
rats reveals first-pass pharmacodynamic effect. _Drug Metab. Dispos._ 30, 861–868 (2002). CAS PubMed Google Scholar * Sum, C. F. et al. The effect of intravenous metformin on glucose
metabolism during hyperglycaemia in type 2 diabetes. _Diabet. Med._ 9, 61–65 (1992). CAS PubMed Google Scholar * Koffert, J. P. et al. Metformin treatment significantly enhances
intestinal glucose uptake in patients with type 2 diabetes: results from a randomized clinical trial. _Diabetes Res. Clin. Pract._ 131, 208–216 (2017). CAS PubMed Google Scholar * Wu, T.
et al. Metformin reduces the rate of small intestinal glucose absorption in type 2 diabetes. _Diabetes Obes. Metab._ 19, 290–293 (2017). CAS PubMed Google Scholar * Bailey, C. J.,
Wilcock, C. & Day, C. Effect of metformin on glucose metabolism in the splanchnic bed. _Br. J. Pharmacol._ 105, 1009–1013 (1992). CAS PubMed PubMed Central Google Scholar * Penicaud,
L., Hitier, Y., Ferre, P. & Girard, J. Hypoglycaemic effect of metformin in genetically obese (fa/fa) rats results from an increased utilization of blood glucose by intestine. _Biochem.
J._ 262, 881–885 (1989). CAS PubMed PubMed Central Google Scholar * Gontier, E. et al. High and typical 18F-FDG bowel uptake in patients treated with metformin. _Eur. J. Nucl. Med. Mol.
Imaging_ 35, 95–99 (2008). CAS PubMed Google Scholar * Ikeda, T., Iwata, K. & Murakami, H. Inhibitory effect of metformin on intestinal glucose absorption in the perfused rat
intestine. _Biochem. Pharmacol._ 59, 887–890 (2000). CAS PubMed Google Scholar * Sakar, Y. et al. Metformin-induced regulation of the intestinal D-glucose transporters. _J. Physiol.
Pharmacol._ 61, 301–307 (2010). CAS PubMed Google Scholar * Walker, J. et al. 5-Aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a
possible role for AMPK. _Biochem. J._ 385, 485–491 (2005). CAS PubMed PubMed Central Google Scholar * Lenzen, S., Lortz, S. & Tiedge, M. Effect of metformin on SGLT1, GLUT2, and
GLUT5 hexose transporter gene expression in small intestine from rats. _Biochem. Pharmacol._ 51, 893–896 (1996). CAS PubMed Google Scholar * Bauer, P. V. et al. Metformin alters upper
small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. _Cell Metab._ 27, 101–117.e5 (2018). THIS STUDY IN RODENTS SHOWS THE EFFECT OF METFORMIN ON UPPER
SMALL INTESTINAL MICROBIOTA AND GLUCOSE SENSING. CAS PubMed Google Scholar * Bailey, C. J., Wilcock, C. & Scarpello, J. H. Metformin and the intestine. _Diabetologia_ 51, 1552–1553
(2008). CAS PubMed Google Scholar * Duca, F. A. et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. _Nat. Med._ 21, 506–511 (2015).
THIS PAPER PRESENTS STUDIES IN RODENT MODELS THAT SHOW THAT METFORMIN LOWERS BLOOD LEVELS OF GLUCOSE BY INHIBITING HEPATIC GLUCOSE PRODUCTION THROUGH A NEURON-MEDIATED GUT–BRAIN–LIVER AXIS.
CAS PubMed PubMed Central Google Scholar * Borg, M. J. et al. Comparative effects of proximal and distal small intestinal administration of metformin on plasma glucose and glucagon-like
peptide-1, and gastric emptying after oral glucose, in type 2 diabetes. _Diabetes Obes. Metab._ 21, 640–647 (2019). CAS PubMed Google Scholar * Henry, R. R. et al. Improved glycemic
control with minimal systemic metformin exposure: effects of metformin delayed-release (metformin DR) targeting the lower bowel over 16 weeks in a randomized trial in subjects with type 2
diabetes. _PLOS ONE_ 13, e0203946 (2018). PubMed PubMed Central Google Scholar * Oh, J. et al. Inhibition of the multidrug and toxin extrusion (MATE) transporter by pyrimethamine
increases the plasma concentration of metformin but does not increase antihyperglycaemic activity in humans. _Diabetes Obes. Metab._ 18, 104–108 (2016). CAS PubMed Google Scholar *
Mannucci, E. et al. Effects of metformin on glucagon-like peptide-1 levels in obese patients with and without type 2 diabetes. _Diabetes Nutr. Metab._ 17, 336–342 (2004). CAS PubMed Google
Scholar * Napolitano, A. et al. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. _PLOS ONE_ 9, e100778 (2014). PubMed PubMed Central Google Scholar *
Maida, A., Lamont, B. J., Cao, X. & Drucker, D. J. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice.
_Diabetologia_ 54, 339–349 (2011). CAS PubMed Google Scholar * DeFronzo, R. A. et al. Once-daily delayed-release metformin lowers plasma glucose and enhances fasting and postprandial
GLP-1 and PYY: results from two randomised trials. _Diabetologia_ 59, 1645–1654 (2016). CAS PubMed PubMed Central Google Scholar * Bahne, E. et al. Metformin-induced glucagon-like
peptide-1 secretion contributes to the actions of metformin in type 2 diabetes. _JCI Insight_ 3, 93936 (2018). THIS PAPER PRESENTS HUMAN STUDIES THAT ANALYSE THE ROLE OF GLP1 RELEASE IN THE
GLUCOSE-LOWERING EFFECT OF METFORMIN. PubMed Google Scholar * Li, M. et al. Efficacy and safety of liraglutide versus sitagliptin both in combination with metformin in patients with type 2
diabetes: a systematic review and meta-analysis. _Medicine_ 96, e8161 (2017). CAS PubMed PubMed Central Google Scholar * Bahne, E. et al. Involvement of glucagon-like peptide-1 in the
glucose-lowering effect of metformin. _Diabetes Obes. Metab._ 18, 955–961 (2016). CAS PubMed Google Scholar * Mulherin, A. J. et al. Mechanisms underlying metformin-induced secretion of
glucagon-like peptide-1 from the intestinal L cell. _Endocrinology_ 152, 4610–4619 (2011). CAS PubMed Google Scholar * Kim, M. H. et al. Metformin enhances glucagon-like peptide 1 via
cooperation between insulin and Wnt signaling. _J. Endocrinol._ 220, 117–128 (2014). CAS PubMed Google Scholar * Kappe, C., Patrone, C., Holst, J. J., Zhang, Q. & Sjoholm, A.
Metformin protects against lipoapoptosis and enhances GLP-1 secretion from GLP-1-producing cells. _J. Gastroenterol._ 48, 322–332 (2013). CAS PubMed Google Scholar * Bronden, A. et al.
Single-dose metformin enhances bile acid-induced glucagon-like peptide-1 secretion in patients with type 2 diabetes. _J. Clin. Endocrinol. Metab._ 102, 4153–4162 (2017). PubMed Google
Scholar * Carter, D., Howlett, H. C., Wiernsperger, N. F. & Bailey, C. J. Differential effects of metformin on bile salt absorption from the jejunum and ileum. _Diabetes Obes. Metab._
5, 120–125 (2003). CAS PubMed Google Scholar * Lien, F. et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. _J. Clin. Invest._ 124, 1037–1051 (2014). CAS
PubMed PubMed Central Google Scholar * Hansen, M. et al. Effect of chenodeoxycholic acid and the bile acid sequestrant colesevelam on glucagon-like peptide-1 secretion. _Diabetes Obes.
Metab._ 18, 571–580 (2016). CAS PubMed Google Scholar * Wu, T. et al. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy
humans. _Diabetes Obes. Metab._ 15, 474–477 (2013). CAS PubMed Google Scholar * Thomas, C. et al. TGR5-mediated bile acid sensing controls glucose homeostasis. _Cell Metab._ 10, 167–177
(2009). CAS PubMed PubMed Central Google Scholar * Trabelsi, M. S. et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. _Nat. Commun._ 6,
7629 (2015). PubMed PubMed Central Google Scholar * Duca, F. A., Bauer, P. V., Hamr, S. C. & Lam, T. K. Glucoregulatory relevance of small intestinal nutrient sensing in physiology,
bariatric surgery, and pharmacology. _Cell Metab._ 22, 367–380 (2015). CAS PubMed Google Scholar * Waise, T. M. Z., Dranse, H. J. & Lam, T. K. T. The metabolic role of vagal afferent
innervation. _Nat. Rev. Gastroenterol. Hepatol._ 15, 625–636 (2018). PubMed Google Scholar * Kuhre, R. E., Frost, C. R., Svendsen, B. & Holst, J. J. Molecular mechanisms of
glucose-stimulated GLP-1 secretion from perfused rat small intestine. _Diabetes_ 64, 370–382 (2015). CAS PubMed Google Scholar * Parker, H. E. et al. Predominant role of active versus
facilitative glucose transport for glucagon-like peptide-1 secretion. _Diabetologia_ 55, 2445–2455 (2012). CAS PubMed PubMed Central Google Scholar * Sun, L. et al. Gut microbiota and
intestinal FXR mediate the clinical benefits of metformin. _Nat. Med._ 24, 1919–1929 (2018). CAS PubMed PubMed Central Google Scholar * Cote, C. D. et al. Resveratrol activates duodenal
Sirt1 to reverse insulin resistance in rats through a neuronal network. _Nat. Med._ 21, 498–505 (2015). CAS PubMed Google Scholar * Waise, T. M. Z. et al. Inhibition of upper small
intestinal mTOR lowers plasma glucose levels by inhibiting glucose production. _Nat. Commun._ 10, 714 (2019). PubMed PubMed Central Google Scholar * Karlsson, F. H. et al. Gut metagenome
in European women with normal, impaired and diabetic glucose control. _Nature_ 498, 99–103 (2013). CAS PubMed Google Scholar * Qin, J. et al. A metagenome-wide association study of gut
microbiota in type 2 diabetes. _Nature_ 490, 55–60 (2012). CAS PubMed Google Scholar * Lee, H. & Ko, G. Effect of metformin on metabolic improvement and gut microbiota. _Appl.
Environ. Microbiol._ 80, 5935–5943 (2014). PubMed PubMed Central Google Scholar * Shin, N. R. et al. An increase in the _Akkermansia_ spp. population induced by metformin treatment
improves glucose homeostasis in diet-induced obese mice. _Gut_ 63, 727–735 (2014). THESE RODENT STUDIES SHOW THAT METFORMIN INDUCES A PROFOUND SHIFT IN THE FAECAL MICROBIAL COMMUNITY PROFILE
IN HFD-FED MICE, WITH HIGHER ABUNDANCE OF THE MUCIN-DEGRADING BACTERIUM _AKKERMANSIA_ ACCOMPANIED BY AN INCREASE IN THE NUMBER OF MUCIN-PRODUCING GOBLET CELLS AND ADIPOSE TISSUE-RESIDENT
REGULATORY T CELLS. CAS PubMed Google Scholar * Zhang, X. et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats.
_Sci. Rep._ 5, 14405 (2015). CAS PubMed PubMed Central Google Scholar * de la Cuesta-Zuluaga, J. et al. Metformin is associated with higher relative abundance of mucin-degrading
_Akkermansia muciniphila_ and several short-chain fatty acid-producing microbiota in the gut. _Diabetes Care_ 40, 54–62 (2017). THESE STUDIES IN HUMANS SHOW THAT METFORMIN SHIFTS GUT
MICROBIOTA COMPOSITION THROUGH ENRICHMENT OF SCFA-PRODUCING AND MUCIN-DEGRADING BACTERIA. PubMed Google Scholar * Wu, H. et al. Metformin alters the gut microbiome of individuals with
treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. _Nat. Med._ 23, 850–858 (2017). THIS STUDY IN HUMANS SHOWS THAT ALTERED GUT MICROBIOTA MEDIATES SOME OF
METFORMIN’S ANTIDIABETIC EFFECTS IN TREATMENT-NAIVE PATIENTS WITH TYPE 2 DIABETES MELLITUS. CAS PubMed Google Scholar * Tong, X. et al. Structural alteration of gut microbiota during the
amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional chinese herbal formula: a multicenter, randomized, open label clinical trial. _mBio._ 9, e02392-17
(2018). PubMed PubMed Central Google Scholar * Rosario, D. et al. Understanding the representative gut microbiota dysbiosis in metformin-treated type 2 diabetes patients using
genome-scale metabolic modeling. _Front. Physiol._ 9, 775 (2018). PubMed PubMed Central Google Scholar * De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits
via gut-brain neural circuits. _Cell_ 156, 84–96 (2014). PubMed Google Scholar * Gibson, G. R. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: introducing the
concept of prebiotics. _J. Nutr._ 125, 1401–1412 (1995). CAS PubMed Google Scholar * Cani, P. D. et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut
peptide production with consequences for appetite sensation and glucose response after a meal. _Am. J. Clin. Nutr._ 90, 1236–1243 (2009). CAS PubMed Google Scholar * Chambers, E. S. et
al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. _Gut_ 64, 1744–1754 (2015). CAS PubMed
Google Scholar * Ridlon, J. M., Kang, D. J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. _J. Lipid Res._ 47, 241–259 (2006). CAS PubMed Google Scholar
* Everard, A. et al. Cross-talk between _Akkermansia muciniphila_ and intestinal epithelium controls diet-induced obesity. _Proc. Natl Acad. Sci. USA_ 110, 9066–9071 (2013). CAS PubMed
Google Scholar * Plovier, H. et al. A purified membrane protein from _Akkermansia muciniphila_ or the pasteurized bacterium improves metabolism in obese and diabetic mice. _Nat. Med._ 23,
107–113 (2017). CAS PubMed Google Scholar * Depommier, C. et al. Supplementation with _Akkermansia muciniphila_ in overweight and obese human volunteers: a proof-of-concept exploratory
study. _Nat. Med._ 25, 1096–1103 (2019). CAS PubMed Google Scholar * Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. _Nature_ 555, 623–628 (2018). CAS
PubMed PubMed Central Google Scholar * Vashisht, R. & Brahmachari, S. K. Metformin as a potential combination therapy with existing front-line antibiotics for tuberculosis. _J.
Transl. Med._ 13, 83 (2015). PubMed PubMed Central Google Scholar * Cabreiro, F. et al. Metformin retards aging in _C. elegans_ by altering microbial folate and methionine metabolism.
_Cell_ 153, 228–239 (2013). CAS PubMed PubMed Central Google Scholar * Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. _Immunity_ 46, 562–576 (2017). CAS
PubMed PubMed Central Google Scholar * Postler, T. S. & Ghosh, S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. _Cell Metab._
26, 110–130 (2017). CAS PubMed PubMed Central Google Scholar * McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. _Nature_ 483,
345–349 (2012). CAS PubMed PubMed Central Google Scholar * Weisman, A., Bai, J. W., Cardinez, M., Kramer, C. K. & Perkins, B. A. Effect of artificial pancreas systems on glycaemic
control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. _Lancet Diabetes Endocrinol._ 5, 501–512 (2017). CAS PubMed
Google Scholar * Wood, J. R. et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and
Adolescent Diabetes clinical guidelines. _Diabetes Care_ 36, 2035–2037 (2013). PubMed PubMed Central Google Scholar * Petrie, J. R. et al. Cardiovascular and metabolic effects of
metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. _Lancet Diabetes Endocrinol._ 5, 597–609 (2017). CAS PubMed PubMed Central
Google Scholar * Meng, H. et al. Effect of metformin on glycaemic control in patients with type 1 diabetes: a meta-analysis of randomized controlled trials. _Diabetes Metab. Res. Rev._ 34,
e2983 (2018). PubMed Google Scholar * Lund, S. S. et al. Effect of adjunct metformin treatment in patients with type-1 diabetes and persistent inadequate glycaemic control. A randomized
study. _PLOS ONE_ 3, e3363 (2008). PubMed PubMed Central Google Scholar * Scheen, A. J. Will delayed release metformin provide better management of diabetes type 2? _Expert Opin.
Pharmacother._ 17, 627–630 (2016). PubMed Google Scholar * Fujita, Y. & Inagaki, N. Metformin: new preparations and nonglycemic benefits. _Curr. Diab. Rep._ 17, 5 (2017). PubMed
Google Scholar * Berstein, L. M. Metformin: not only per os. _Expert Rev. Endocrinol. Metab._ 13, 63–65 (2018). CAS PubMed Google Scholar * Cetin, M. & Sahin, S. Microparticulate and
nanoparticulate drug delivery systems for metformin hydrochloride. _Drug Deliv._ 23, 2796–2805 (2016). CAS PubMed Google Scholar * Zhao, Y. et al. Polymetformin combines carrier and
anticancer activities for in vivo siRNA delivery. _Nat. Commun._ 7, 11822 (2016). PubMed PubMed Central Google Scholar * Bouchoucha, M., Uzzan, B. & Cohen, R. Metformin and digestive
disorders. _Diabetes Metab._ 37, 90–96 (2011). CAS PubMed Google Scholar * Bonnet, F. & Scheen, A. Understanding and overcoming metformin gastrointestinal intolerance. _Diabetes Obes.
Metab._ 19, 473–481 (2017). CAS PubMed Google Scholar * McCreight, L. J. et al. Pharmacokinetics of metformin in patients with gastrointestinal intolerance. _Diabetes Obes. Metab._ 20,
1593–1601 (2018). CAS PubMed PubMed Central Google Scholar * Dujic, T. et al. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: a GoDARTS
study. _Diabetes_ 64, 1786–1793 (2015). CAS PubMed Google Scholar * Dujic, T., Zhou, K., Tavendale, R., Palmer, C. N. & Pearson, E. R. Effect of serotonin transporter 5-HTTLPR
polymorphism on gastrointestinal intolerance to metformin: a GoDARTS study. _Diabetes Care_ 39, 1896–1901 (2016). CAS PubMed PubMed Central Google Scholar * Hoffmann, I. S., Roa, M.,
Torrico, F. & Cubeddu, L. X. Ondansetron and metformin-induced gastrointestinal side effects. _Am. J. Ther._ 10, 447–451 (2003). PubMed Google Scholar * Scarpello, J. H., Hodgson, E.
& Howlett, H. C. Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. _Diabet. Med._ 15, 651–656 (1998). CAS PubMed Google Scholar *
Elbere, I. et al. Association of metformin administration with gut microbiome dysbiosis in healthy volunteers. _PLOS ONE_ 13, e0204317 (2018). PubMed PubMed Central Google Scholar *
Greenway, F., Wang, S. & Heiman, M. A novel cobiotic containing a prebiotic and an antioxidant augments the glucose control and gastrointestinal tolerability of metformin: a case report.
_Benef. Microbes_ 5, 29–32 (2014). CAS PubMed Google Scholar * Burton, J. H. et al. Addition of a gastrointestinal microbiome modulator to metformin improves metformin tolerance and
fasting glucose levels. _J. Diabetes Sci. Technol._ 9, 808–814 (2015). CAS PubMed PubMed Central Google Scholar * Escobar-Morreale, H. F. Polycystic ovary syndrome: definition,
aetiology, diagnosis and treatment. _Nat. Rev. Endocrinol._ 14, 270–284 (2018). PubMed Google Scholar * Burt Solorzano, C. M. et al. Neuroendocrine dysfunction in polycystic ovary
syndrome. _Steroids_ 77, 332–337 (2012). CAS PubMed Google Scholar * Ovalle, F. & Azziz, R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. _Fertil.
Steril._ 77, 1095–1105 (2002). PubMed Google Scholar * Carmina, E. & Lobo, R. A. Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary
syndrome. _Fertil. Steril._ 82, 661–665 (2004). PubMed Google Scholar * Adashi, E. Y., Hsueh, A. J. & Yen, S. S. Insulin enhancement of luteinizing hormone and follicle-stimulating
hormone release by cultured pituitary cells. _Endocrinology_ 108, 1441–1449 (1981). CAS PubMed Google Scholar * Nestler, J. E. et al. Insulin stimulates testosterone biosynthesis by human
thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. _J. Clin. Endocrinol. Metab._ 83,
2001–2005 (1998). CAS PubMed Google Scholar * Carmina, E. et al. The contributions of oestrogen and growth factors to increased adrenal androgen secretion in polycystic ovary syndrome.
_Hum. Reprod._ 14, 307–311 (1999). CAS PubMed Google Scholar * Tosi, F. et al. Insulin enhances ACTH-stimulated androgen and glucocorticoid metabolism in hyperandrogenic women. _Eur. J.
Endocrinol._ 164, 197–203 (2011). CAS PubMed Google Scholar * Wild, R. A. et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic
ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. _J. Clin. Endocrinol. Metab._ 95, 2038–2049 (2010). CAS PubMed Google Scholar
* Morin-Papunen, L. et al. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. _J. Clin. Endocrinol. Metab._ 88, 4649–4654 (2003). CAS PubMed
Google Scholar * Tang, T. et al. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre
study. _Hum. Reprod._ 21, 80–89 (2006). PubMed Google Scholar * Naderpoor, N. et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis.
_Hum. Reprod. Update_ 21, 560–574 (2015). CAS PubMed Google Scholar * Orio, F. Jr. et al. Improvement in endothelial structure and function after metformin treatment in young
normal-weight women with polycystic ovary syndrome: results of a 6-month study. _J. Clin. Endocrinol. Metab._ 90, 6072–6076 (2005). CAS PubMed Google Scholar * Nestler, J. E., Jakubowicz,
D. J., Evans, W. S. & Pasquali, R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. _N. Engl. J. Med._ 338, 1876–1880 (1998). CAS
PubMed Google Scholar * Lord, J. M., Flight, I. H. & Norman, R. J. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic
ovary syndrome. _Cochrane Database Syst. Rev_. 3, CD003053 (2003). * Kashyap, S., Wells, G. A. & Rosenwaks, Z. Insulin-sensitizing agents as primary therapy for patients with polycystic
ovarian syndrome. _Hum. Reprod._ 19, 2474–2483 (2004). CAS PubMed Google Scholar * Legro, R. S. et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. _N.
Engl. J. Med._ 356, 551–566 (2007). CAS PubMed Google Scholar * Morley, L. C., Tang, T., Yasmin, E., Norman, R. J. & Balen, A. H. Insulin-sensitising drugs (metformin, rosiglitazone,
pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. _Cochrane Database Syst. Rev._ 11, CD003053 (2017). PubMed Google Scholar *
Balen, A. H. et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. _Hum.
Reprod. Update_ 22, 687–708 (2016). PubMed Google Scholar * Heckman-Stoddard, B. M., DeCensi, A., Sahasrabuddhe, V. V. & Ford, L. G. Repurposing metformin for the prevention of cancer
and cancer recurrence. _Diabetologia_ 60, 1639–1647 (2017). CAS PubMed PubMed Central Google Scholar * Kurelac, I. et al. Inducing cancer indolence by targeting mitochondrial complex I
is potentiated by blocking macrophage-mediated adaptive responses. _Nat. Commun._ 10, 903 (2019). PubMed PubMed Central Google Scholar * Rotermund, C., Machetanz, G. & Fitzgerald, J.
C. The therapeutic potential of metformin in neurodegenerative diseases. _Front. Endocrinol._ 9, 400 (2018). Google Scholar * Anisimov, V. N. et al. Metformin slows down aging and extends
life span of female SHR mice. _Cell Cycle_ 7, 2769–2773 (2008). CAS PubMed Google Scholar * Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to
target aging. _Cell Metab._ 23, 1060–1065 (2016). CAS PubMed PubMed Central Google Scholar * Justice, J. N. et al. A framework for selection of blood-based biomarkers for
geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. _Geroscience_ 40, 419–436 (2018). CAS PubMed PubMed Central Google Scholar * Degner, N. R., Wang, J. Y.,
Golub, J. E. & Karakousis, P. C. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. _Clin. Infect. Dis._ 66, 198–205 (2018).
CAS PubMed Google Scholar * Singhal, A. et al. Metformin as adjunct antituberculosis therapy. _Sci. Transl. Med._ 6, 263ra159 (2014). THIS STUDY PROVIDES EVIDENCE FOR THE BENEFITS OF
METFORMIN TREATMENT AS HOST-ADJUNCTIVE THERAPY FOR IMPROVING THE EFFECTIVE TREATMENT OF TUBERCULOSIS. PubMed Google Scholar * Rangarajan, S. et al. Metformin reverses established lung
fibrosis in a bleomycin model. _Nat. Med._ 24, 1121–1127 (2018). CAS PubMed PubMed Central Google Scholar * Sato, N. et al. Metformin attenuates lung fibrosis development via NOX4
suppression. _Respir. Res._ 17, 107 (2016). PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The authors acknowledge the support of grants from Inserm, CNRS,
Université Paris Descartes, Agence Nationale de la Recherche (ANR), Société Francophone du Diabète (SFD), Fondation pour la Recherche Médicale (FRM), the Dutch Organization for Scientific
Research (ZonMW) and DiabetesFonds. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * INSERM, U1016, Institut Cochin, Paris, France Marc Foretz & Benoit Viollet * CNRS, UMR8104, Paris,
France Marc Foretz & Benoit Viollet * Université Paris Descartes, Sorbonne Paris Cité, Paris, France Marc Foretz & Benoit Viollet * Department of Parasitology, Leiden University
Medical Centre, Leiden, Netherlands Bruno Guigas Authors * Marc Foretz View author publications You can also search for this author inPubMed Google Scholar * Bruno Guigas View author
publications You can also search for this author inPubMed Google Scholar * Benoit Viollet View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
The authors contributed equally to all aspects of the article. CORRESPONDING AUTHOR Correspondence to Benoit Viollet. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. GLOSSARY * Lactic
acidosis A medical condition characterized by excessively low pH in the bloodstream due to excess lactate production by glycolytic tissues, inadequate lactate utilization by gluconeogenic
tissues, or varying combinations of these two processes. * Pharmacokinetics The study of the transit of a dosed drug in body fluids and tissues over time, as defined by its rate of
absorption, distribution, metabolism and excretion. * Pharmacodynamics The study of the action of a drug in the body, and its biochemical and physiological effects. * Half-maximal inhibitory
concentration (IC50). The concentration of an inhibitor required to decrease the response of the target by 50%. * Pyruvate tolerance A measure of glycaemic excursion in response to an
intraperitoneal or intravenous injection of pyruvate, used to assess hepatic gluconeogenesis. * Cytosolic redox potential Cytoplasmic oxidation state of the cell, which is assessed by the
ratio of reduced to oxidized intracellular metabolite redox couples (for example, lactate/pyruvate ratio). * Type 2 immune cells Cells involved in type 2 immune responses, such as type 2
innate lymphoid cells, eosinophils, T helper 2 cells, mast cells, basophils and alternatively-activated macrophages. * Reverse electron transport (RET). The transport of electrons from
ubiquinol back to respiratory complex 1, generating a substantial amount of reactive oxygen species. * Incretins Incretins are gut hormones that are secreted after nutrient intake and
stimulate glucose-stimulated insulin secretion. * Lipoapoptosis A non-canonical form of programmed cell death, which is the result of fatty acid over-accumulation that occurs in diseases
associated with over-nutrition and ageing. * Short-chain fatty acid (SCFA). A fatty acid with fewer than six carbon atoms (for example, acetate, propionate and butyrate) that is the
end-product of fermentation of dietary fibres by the anaerobic intestinal microbiota and acts as a signal molecule in the control of mammalian energy metabolism. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Foretz, M., Guigas, B. & Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus.
_Nat Rev Endocrinol_ 15, 569–589 (2019). https://doi.org/10.1038/s41574-019-0242-2 Download citation * Accepted: 11 July 2019 * Published: 22 August 2019 * Issue Date: October 2019 * DOI:
https://doi.org/10.1038/s41574-019-0242-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative