- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
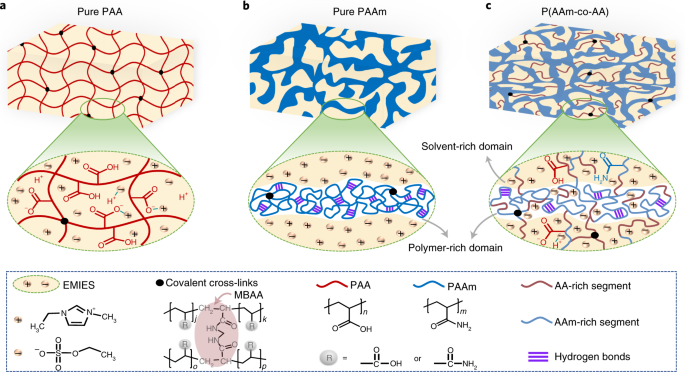
ABSTRACT Ionogels are compelling materials for technological devices due to their excellent ionic conductivity, thermal and electrochemical stability, and non-volatility. However, most
existing ionogels suffer from low strength and toughness. Here, we report a simple one-step method to achieve ultra-tough and stretchable ionogels by randomly copolymerizing two common
monomers with distinct solubility of the corresponding polymers in an ionic liquid. Copolymerization of acrylamide and acrylic acid in 1-ethyl-3-methylimidazolium ethyl sulfate results in a
macroscopically homogeneous covalent network with in situ phase separation: a polymer-rich phase with hydrogen bonds that dissipate energy and toughen the ionogel; and an elastic
solvent-rich phase that enables for large strain. These ionogels have high fracture strength (12.6 MPa), fracture energy (~24 kJ m−2) and Young’s modulus (46.5 MPa), while being highly
stretchable (~600% strain) and having self-healing and shape-memory properties. This concept can be applied to other monomers and ionic liquids, offering a promising way to tune ionogel
microstructure and properties in situ during one-step polymerization. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time
Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EMERGING APPLICATIONS OF TOUGH IONOGELS Article Open access 15 December 2023 HEALABLE SOFT MATERIALS BASED ON IONIC
LIQUIDS AND BLOCK COPOLYMER SELF-ASSEMBLY Article 01 April 2021 NANOCONFINED POLYMERIZATION LIMITS CRACK PROPAGATION IN HYSTERESIS-FREE GELS Article 26 October 2023 DATA AVAILABILITY Data
generated or analysed during this study are provided as Source Data or included in the Supplementary Information. Further data are available from the corresponding authors on request.
Further details on the methods are available in the Supplementary Information. REFERENCE * Lodge, T. P. & Ueki, T. Mechanically tunable, readily processable ion gels by self-assembly of
block copolymers in ionic liquids. _Acc. Chem. Res._ 49, 2107–2114 (2016). Article CAS Google Scholar * Ding, Y. et al. Preparation of high‐performance ionogels with excellent
transparency, good mechanical strength, and high conductivity. _Adv. Mater._ 29, 1704253 (2017). Article Google Scholar * Zhou, B. et al. Flexible, self-healing, and fire-resistant polymer
electrolytes fabricated via photopolymerization for all-solid-state lithium metal batteries. _ACS Macro Lett._ 9, 525–532 (2020). Article CAS Google Scholar * Shi, L. et al. Highly
stretchable and transparent ionic conductor with novel hydrophobicity and extreme-temperature tolerance. _Research_ 2020, 2505619 (2020). Article CAS Google Scholar * Tamate, R. et al.
Self‐healing micellar ion gels based on multiple hydrogen bonding. _Adv. Mater._ 30, 1802792 (2018). Article Google Scholar * Kim, Y. M. & Moon, H. C. Ionoskins: nonvolatile, highly
transparent, ultrastretchable ionic sensory platforms for wearable electronics. _Adv. Funct. Mater._ 30, 1907290 (2020). Article CAS Google Scholar * Ren, Y. et al. Ionic liquid–based
click-ionogels. _Sci. Adv._ 5, eaax0648 (2019). Article CAS Google Scholar * Lu, B. et al. Pure PEDOT: PSS hydrogels. _Nat. Commun._ 10, 1043 (2019). Article Google Scholar * Xu, J. et
al. Multi-scale ordering in highly stretchable polymer semiconducting films. _Nat. Mater._ 18, 594–601 (2019). Article CAS Google Scholar * Liu, Y. et al. Soft and elastic hydrogel-based
microelectronics for localized low-voltage neuromodulation. _Nat. Biomed. Eng._ 3, 58–68 (2019). Article CAS Google Scholar * Ma, Y. et al. Flexible hybrid electronics for digital
healthcare. _Adv. Mater._ 32, 1902062 (2020). Article CAS Google Scholar * Kim, H. J., Chen, B., Suo, Z. & Hayward, R. C. Ionoelastomer junctions between polymer networks of fixed
anions and cations. _Science_ 367, 773–776 (2020). Article CAS Google Scholar * Osada, I., de Vries, H., Scrosati, B. & Passerini, S. Ionic‐liquid‐based polymer electrolytes for
battery applications. _Angew. Chem. Int. Ed. Engl._ 55, 500–513 (2016). Article CAS Google Scholar * Chen, N., Zhang, H., Li, L., Chen, R. & Guo, S. Ionogel electrolytes for
high‐performance lithium batteries: a review. _Adv. Energy Mater._ 8, 1702675 (2018). Article Google Scholar * Chen, N. et al. Biomimetic ant-nest ionogel electrolyte boosts the
performance of dendrite-free lithium batteries. _Energy Environ. Sci._ 10, 1660–1667 (2017). Article CAS Google Scholar * Hyun, W. J., Thomas, C. M. & Hersam, M. C. Nanocomposite
ionogel electrolytes for solid‐state rechargeable batteries. _Adv. Energy Mater._ 10, 2002135 (2020). Article CAS Google Scholar * Shi, L. et al. Dielectric gels with ultra-high
dielectric constant, low elastic modulus, and excellent transparency. _NPG Asia Mater._ 10, 821–826 (2018). Article CAS Google Scholar * Liu, Y., He, K., Chen, G., Leow, W. R. & Chen,
X. Nature-inspired structural materials for flexible electronic devices. _Chem. Rev._ 117, 12893–12941 (2017). Article CAS Google Scholar * Shi, L. et al. Highly stretchable and
transparent ionic conducting elastomers. _Nat. Commun._ 9, 2630 (2018). Article Google Scholar * Lei, Z. & Wu, P. A highly transparent and ultra-stretchable conductor with stable
conductivity during large deformation. _Nat. Commun._ 10, 3429 (2019). Article Google Scholar * Cao, Z., Liu, H. & Jiang, L. Transparent, mechanically robust, and ultrastable ionogels
enabled by hydrogen bonding between elastomers and ionic liquids. _Mater. Horiz._ 7, 912–918 (2020). Article CAS Google Scholar * Zhang, L. M. et al. Self‐healing, adhesive, and highly
stretchable ionogel as a strain sensor for extremely large deformation. _Small_ 15, 1804651 (2019). Article Google Scholar * Li, T., Wang, Y., Li, S., Liu, X. & Sun, J. Mechanically
robust, elastic, and healable ionogels for highly sensitive ultra‐durable ionic skins. _Adv. Mater._ 32, 2002706 (2020). Article Google Scholar * Correia, D. M. et al. Ionic liquid–polymer
composites: a new platform for multifunctional applications. _Adv. Funct. Mater._ 30, 1909736 (2020). Article CAS Google Scholar * Le Bideau, J., Viau, L. & Vioux, A. Ionogels, ionic
liquid based hybrid materials. _Chem. Soc. Rev._ 40, 907–925 (2011). Article Google Scholar * Yu, L. et al. Highly tough, Li‐metal compatible organic–inorganic double‐network solvate
ionogel. _Adv. Energy Mater._ 9, 1900257 (2019). Article Google Scholar * Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. _Soft
Matter_ 10, 672–687 (2014). Article CAS Google Scholar * Gong, J. P., Katsuyama, Y., Kurokawa, T. & Osada, Y. Double‐network hydrogels with extremely high mechanical strength. _Adv.
Mater._ 15, 1155–1158 (2003). Article CAS Google Scholar * Sun, J.-Y. et al. Highly stretchable and tough hydrogels. _Nature_ 489, 133–136 (2012). Article CAS Google Scholar * Zhang,
H. J. et al. Tough physical double‐network hydrogels based on amphiphilic triblock copolymers. _Adv. Mater._ 28, 4884–4890 (2016). Article CAS Google Scholar * Sato, K. et al.
Phase‐separation‐induced anomalous stiffening, toughening, and self‐healing of polyacrylamide gels. _Adv. Mater._ 27, 6990–6998 (2015). Article CAS Google Scholar * Gong, J. P. Why are
double network hydrogels so tough? _Soft Matter_ 6, 2583–2590 (2010). Article CAS Google Scholar * Corkhill, P., Trevett, A. & Tighe, B. The potential of hydrogels as synthetic
articular cartilage. _Proc. Inst. Mech. Eng. H_ 204, 147–155 (1990). Article CAS Google Scholar * Zhang, H. et al. Polyelectrolyte microcapsules as ionic liquid reservoirs within ionomer
membrane to confer high anhydrous proton conductivity. _J. Power Sources_ 279, 667–677 (2015). Article CAS Google Scholar * Ueki, T. & Watanabe, M. Polymers in ionic liquids: dawn of
neoteric solvents and innovative materials. _Bull. Chem. Soc. Jpn_ 85, 33–50 (2012). Article CAS Google Scholar * Ueki, T., Watanabe, M. & Lodge, T. P. Doubly thermosensitive
self-assembly of diblock copolymers in ionic liquids. _Macromolecules_ 42, 1315–1320 (2009). Article CAS Google Scholar * Weng, D. et al. Polymeric complex-based transparent and healable
ionogels with high mechanical strength and ionic conductivity as reliable strain sensors. _ACS Appl. Mater. Interfaces_ 12, 57477–57485 (2020). Article CAS Google Scholar * Sun, T. L. et
al. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. _Nat. Mater._ 12, 932–937 (2013). Article CAS Google Scholar * Hu, X.,
Vatankhah‐Varnoosfaderani, M., Zhou, J., Li, Q. & Sheiko, S. S. Weak hydrogen bonding enables hard, strong, tough, and elastic hydrogels. _Adv. Mater._ 27, 6899–6905 (2015). Article CAS
Google Scholar * Kong, W. et al. Muscle‐inspired highly anisotropic, strong, ion‐conductive hydrogels. _Adv. Mater._ 30, 1801934 (2018). Article Google Scholar * Mredha, M. T. I. et al.
A facile method to fabricate anisotropic hydrogels with perfectly aligned hierarchical fibrous structures. _Adv. Mater._ 30, 1704937 (2018). Article Google Scholar * Asaletha, R.,
Kumaran, M. & Thomas, S. Thermoplastic elastomers from blends of polystyrene and natural rubber: morphology and mechanical properties. _Eur. Polym. J._ 35, 253–271 (1999). Article CAS
Google Scholar * Bai, R., Yang, J. & Suo, Z. Fatigue of hydrogels. _Eur. J. Mech. A_ 74, 337–370 (2019). Article Google Scholar Download references ACKNOWLEDGEMENTS M.D. acknowledges
support from the Coastal Studies Institute. J.H. acknowledges the support of the National Natural Science Foundation of China (11702207). We thank Prof. L. Cai for helpful discussion. We
thank Mr M. Yang and Mr X. Chen for help with 3D printing. Nano-IR analysis was performed by W.Q. at the NanoEngineering Research Core Facility (NERCF), which is partially funded by the
Nebraska Research Initiative. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory for Strength and Vibration of Mechanical Structures, International Center for Applied
Mechanics, Department of Engineering Mechanics, Xi’an Jiaotong University, Xi’an, China Meixiang Wang, Pengyao Zhang & Jian Hu * Department of Chemical and Biomolecular Engineering,
North Carolina State University, Raleigh, NC, USA Meixiang Wang, Mohammad Shamsi, Jacob L. Thelen, Vi Khanh Truong, Jinwoo Ma & Michael D. Dickey * Department of Mechanical &
Materials Engineering, University of Nebraska-Lincoln, Lincoln, NE, USA Wen Qian * School of Science, STEM College, RMIT University, Melbourne, Victoria, Australia Vi Khanh Truong Authors *
Meixiang Wang View author publications You can also search for this author inPubMed Google Scholar * Pengyao Zhang View author publications You can also search for this author inPubMed
Google Scholar * Mohammad Shamsi View author publications You can also search for this author inPubMed Google Scholar * Jacob L. Thelen View author publications You can also search for this
author inPubMed Google Scholar * Wen Qian View author publications You can also search for this author inPubMed Google Scholar * Vi Khanh Truong View author publications You can also search
for this author inPubMed Google Scholar * Jinwoo Ma View author publications You can also search for this author inPubMed Google Scholar * Jian Hu View author publications You can also
search for this author inPubMed Google Scholar * Michael D. Dickey View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.W., J.H. and M.D.D.
conceived the idea. J.H. and M.D.D. supervised the project. M.W. carried out most of the experiments. P.Z. participated in the fracture energy measurements. M.S. and V.K.T. participated in
the SEM measurements. M.S. participated in the SAXS measurements. J.L.T. contributed to the SAXS data processing and analysis. W.Q. conducted the nano-IR measurements. J.M. contributed to
the demonstration of the falling metal ball. M.W., J.H., and M.D.D. wrote the paper, and all authors reviewed the manuscript. CORRESPONDING AUTHORS Correspondence to Jian Hu or Michael D.
Dickey. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Materials_ thanks Xuanhe Zhao and the other,
anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–17, Notes 1–4, Tables 1 and 2, Videos 1–5 and refs. 1–7.
SUPPLEMENTARY VIDEO 1 This movie shows that the P(AAm0.8125-co-AA0.1875) ionogel is strong enough to lift a 1 kg weight, while the pure PAA and PAAm ionogel and P(AAm0.8125-co-AA0.1875)
hydrogel break. _C_m = 6 M, _C_MBAA = 0.1 mol%. SUPPLEMENTARY VIDEO 2 This movie demonstrates the ultra-tough properties of the gel when a metal ball drops on a membrane of
P(AAm0.8125-co-AA0.1875) ionogel stretched across a rigid frame. The membrane (thickness = 0.5 mm) was glued to two polyacrylate clamps with a circular opening (diameter = 7 cm). A stainless
steel ball with a diameter of 2.54 cm and mass of 64 g was dropped from a height of 2 m. Upon hitting the membrane, the ball bounced back and the membrane remained intact with small
deformation, while the P(AAm0.8125-co-AA0.1875) hydrogel was stretched to rupture after large deformation. _C_m = 6 M, _C_MBAA = 0.1 mol%. SUPPLEMENTARY VIDEO 3 This movie shows the
excellent self-healing property of the P(AAm0.8125-co-AA0.1875) ionogel. The dog-bone samples were cut into half pieces and then the pieces from two different samples were put together to
heal. After storing the sample at 80 °C for 1 h, the self-healed sample could lift the 1 kg weight. The copolymer ionogel samples were stained with methylene blue and rhodamine B. _C_m = 6
M, _C_MBAA = 0.1 mol%. SUPPLEMENTARY VIDEO 4 This movie shows the excellent shape-memory properties of P(AAm0.8125-co-AA0.1875) ionogel by demonstrating the fast programming and recovery
process. The ionogel sample was stained with rhodamine B for visualization. _C_m = 6 M, _C_MBAA = 0.1 mol%. SUPPLEMENTARY VIDEO 5 This movie exhibits superb shape-memory behaviour of
P(AAm0.8125-co-AA0.1875) ionogel using a more complicated six-layer structure of a ‘blooming flower’. Six layers of the copolymer ionogel samples were glued together to mimic the flower bud
blooming process and the ionogels could fully recover within 25 s. The ionogel samples were stained with methylene blue. _C_m = 6 M, _C_MBAA = 0.1 mol%. SOURCE DATA SOURCE DATA FIG. 3 Source
data. SOURCE DATA FIG. 4 Source data. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, M., Zhang, P., Shamsi, M. _et al._ Tough and stretchable
ionogels by in situ phase separation. _Nat. Mater._ 21, 359–365 (2022). https://doi.org/10.1038/s41563-022-01195-4 Download citation * Received: 12 January 2021 * Accepted: 03 January 2022 *
Published: 21 February 2022 * Issue Date: March 2022 * DOI: https://doi.org/10.1038/s41563-022-01195-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






