- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
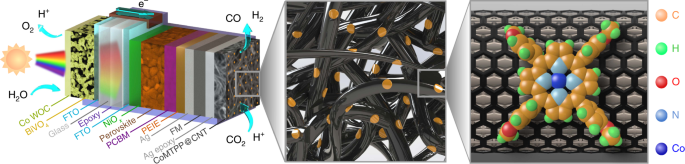
ABSTRACT The photoelectrochemical (PEC) production of syngas from water and CO2 represents an attractive technology towards a circular carbon economy. However, the high overpotential, low
selectivity and cost of commonly employed catalysts pose challenges for this sustainable energy-conversion process. Here we demonstrate highly tunable PEC syngas production by integrating a
cobalt porphyrin catalyst immobilized on carbon nanotubes with triple-cation mixed halide perovskite and BiVO4 photoabsorbers. Empirical data analysis is used to clarify the optimal
electrode selectivity at low catalyst loadings. The perovskite photocathodes maintain selective aqueous CO2 reduction for one day at light intensities as low as 0.1 sun, which provides
pathways to maximize daylight utilization by operating even under low solar irradiance. Under 1 sun irradiation, the perovskite–BiVO4 PEC tandems sustain bias-free syngas production coupled
to water oxidation for three days. The devices present solar-to-H2 and solar-to-CO conversion efficiencies of 0.06 and 0.02%, respectively, and are able to operate as standalone artificial
leaves in neutral pH solution. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal
Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS LONG-TERM SOLAR WATER AND CO2 SPLITTING WITH PHOTOELECTROCHEMICAL BIOI–BIVO4 TANDEMS Article 26 May 2022 FLOATING PEROVSKITE-BIVO4 DEVICES FOR SCALABLE
SOLAR FUEL PRODUCTION Article 17 August 2022 SOLAR-DRIVEN LIQUID MULTI-CARBON FUEL PRODUCTION USING A STANDALONE PEROVSKITE–BIVO4 ARTIFICIAL LEAF Article 18 May 2023 DATA AVAILABILITY The
raw data that support the findings of this study are available from the University of Cambridge data repository53: https://doi.org/10.17863/CAM.44164. REFERENCES * Behrens, M. et al. The
active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. _Science_ 336, 893–897 (2012). CAS Google Scholar * Khodakov, A. Y., Chu, W. & Fongarland, P. Advances in the
development of novel cobalt Fischer–Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. _Chem. Rev._ 107, 1692–1744 (2007). CAS Google Scholar * Bharadwaj, S. S.
& Schmidt, L. D. Catalytic partial oxidation of natural gas to syngas. _Fuel Process. Technol._ 42, 109–127 (1995). CAS Google Scholar * Abdoulmoumine, N., Adhikari, S., Kulkarni, A.
& Chattanathan, S. A review on biomass gasification syngas cleanup. _Appl. Energy_ 155, 294–307 (2015). CAS Google Scholar * Graves, C., Ebbesen, S. D., Mogensen, M. & Lackner, K.
S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. _Renew. Sustain. Energy Rev._ 15, 1–23 (2011). CAS Google Scholar * Shaner, M. R. et al.
Photoelectrochemistry of core–shell tandem junction n–p+-Si/n-WO3 microwire array photoelectrodes. _Energy Environ. Sci._ 7, 779–790 (2014). CAS Google Scholar * Brillet, J. et al. Highly
efficient water splitting by a dual-absorber tandem cell. _Nat. Photon._ 6, 824 (2012). CAS Google Scholar * Pan, L. et al. Boosting the performance of Cu2O photocathodes for unassisted
solar water splitting devices. _Nat. Catal._ 1, 412–420 (2018). CAS Google Scholar * Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen
energy conversion efficiency exceeding 1%. _Nat. Mater._ 15, 611 (2016). CAS Google Scholar * Lu, H. et al. Single-source bismuth (transition metal) polyoxovanadate precursors for the
scalable synthesis of doped BiVO4 photoanodes. _Adv. Mater._ 30, 1804033 (2018). Google Scholar * Luo, J. et al. Water photolysis at 12.3% efficiency via perovskite photovoltaics and
earth-abundant catalysts. _Science_ 345, 1593–1596 (2014). CAS Google Scholar * Andrei, V. et al. Scalable triple cation mixed halide perovskite–BiVO4 tandems for bias-free water
splitting. _Adv. Energy Mater._ 8, 1801403 (2018). Google Scholar * Schreier, M. et al. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. _Nat. Commun._
6, 7326 (2015). CAS Google Scholar * Jang, Y. J. et al. Unbiased sunlight-driven artificial photosynthesis of carbon monoxide from CO2 using a ZnTe-based photocathode and a perovskite
solar cell in tandem. _ACS Nano_ 10, 6980–6987 (2016). CAS Google Scholar * Sokol, K. P. et al. Photoreduction of CO2 with a formate dehydrogenase driven by photosystem II using a
semi-artificial Z-scheme architecture. _J. Am. Chem. Soc._ 140, 16418–16422 (2018). CAS Google Scholar * Li, C. et al. Photoelectrochemical CO2 reduction to adjustable syngas on
grain-boundary-mediated a-Si/TiO2/Au photocathodes with low onset potentials. _Energy Environ. Sci._ 12, 923–928 (2019). CAS Google Scholar * Sahara, G. et al. Photoelectrochemical
reduction of CO2 coupled to water oxidation using a photocathode with a Ru(ii)–Re(i) complex photocatalyst and a CoO_x_/TaON photoanode. _J. Am. Chem. Soc._ 138, 14152–14158 (2016). CAS
Google Scholar * Urbain, F. et al. A prototype reactor for highly selective solar-driven CO2 reduction to synthesis gas using nanosized earth-abundant catalysts and silicon photovoltaics.
_Energy Environ. Sci._ 10, 2256–2266 (2017). CAS Google Scholar * Arai, T., Sato, S., Sekizawa, K., Suzuki, T. M. & Morikawa, T. Solar-driven CO2 to CO reduction utilizing H2O as an
electron donor by earth-abundant Mn–bipyridine complex and Ni-modified Fe-oxyhydroxide catalysts activated in a single-compartment reactor. _Chem. Commun._ 55, 237–240 (2019). CAS Google
Scholar * Voiry, D., Shin, H. S., Loh, K. P. & Chhowalla, M. Low-dimensional catalysts for hydrogen evolution and CO2 reduction. _Nat. Rev. Chem._ 2, 0105 (2018). CAS Google Scholar *
Francke, R., Schille, B. & Roemelt, M. Homogeneously catalyzed electroreduction of carbon dioxide—methods, mechanisms, and catalysts. _Chem. Rev._ 118, 4631–4701 (2018). CAS Google
Scholar * Dalle, K. E. et al. Electro- and solar-driven fuel synthesis with first row transition metal complexes. _Chem. Rev._ 119, 2752–2875 (2019). CAS Google Scholar * Costentin, C.,
Drouet, S., Robert, M. & Savéant, J.-M. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. _Science_ 338, 90–94 (2012). CAS Google Scholar * Lin, S.
et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. _Science_ 349, 1208–1213 (2015). CAS Google Scholar * Zhu, M., Ye, R., Jin, K.,
Lazouski, N. & Manthiram, K. Elucidating the reactivity and mechanism of CO2 electroreduction at highly dispersed cobalt phthalocyanine. _ACS Energy Lett._ 3, 1381–1386 (2018). CAS
Google Scholar * Hu, X.-M., Rønne, M. H., Pedersen, S. U., Skrydstrup, T. & Daasbjerg, K. Enhanced catalytic activity of cobalt porphyrin in CO2 electroreduction upon immobilization on
carbon materials. _Angew. Chem. Int. Ed._ 56, 6468–6472 (2017). CAS Google Scholar * Zhang, X. et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt
phthalocyanine/carbon nanotube hybrid structures. _Nat. Commun._ 8, 14675 (2017). Google Scholar * Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid
fuel on oxide-derived nanocrystalline copper. _Nature_ 508, 504–507 (2014). CAS Google Scholar * Hall, A. S., Yoon, Y., Wuttig, A. & Surendranath, Y. Mesostructure-induced selectivity
in CO2 reduction catalysis. _J. Am. Chem. Soc._ 137, 14834–14837 (2015). CAS Google Scholar * Ma, M., Trześniewski, B. J., Xie, J. & Smith, W. A. Selective and efficient reduction of
carbon dioxide to carbon monoxide on oxide-derived nanostructured silver electrocatalysts. _Angew. Chem. Int. Ed._ 55, 9748–9752 (2016). CAS Google Scholar * de Luna, P. et al. Catalyst
electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. _Nat. Catal._ 1, 103–110 (2018). Google Scholar * Suter, S. & Haussener, S.
Optimizing mesostructured silver catalysts for selective carbon dioxide conversion into fuels. _Energy Environ. Sci._ 12, 1668–1678 (2019). CAS Google Scholar * Sharma, A. & Kakkar, A.
Forecasting daily global solar irradiance generation using machine learning. _Renew. Sustain. Energy Rev._ 82, 2254–2269 (2018). Google Scholar * Stoffel, T. & Andreas, A. _NREL Solar
Radiation Research Laboratory (SRRL): Baseline Measurement System (BMS); Golden, Colorado (Data)_ NREL Report no. DA-5500-56488(NREL, 1981). * Hernández-Pagán, E. A. et al. Resistance and
polarization losses in aqueous buffer–membrane electrolytes for water-splitting photoelectrochemical cells. _Energy Environ. Sci._ 5, 7582–7589 (2012). Google Scholar * Vermaas, D. A. &
Smith, W. A. Synergistic electrochemical CO2 reduction and water oxidation with a bipolar membrane. _ACS Energy Lett._ 1, 1143–1148 (2016). CAS Google Scholar * Singh, M. R., Xiang, C.
& Lewis, N. S. Evaluation of flow schemes for near-neutral pH electrolytes in solar-fuel generators. _Sustain. Energy Fuels_ 1, 458–466 (2017). CAS Google Scholar * McKone, J. R.,
Lewis, N. S. & Gray, H. B. Will solar-driven water-splitting devices see the light of day? _Chem. Mater._ 26, 407–414 (2014). CAS Google Scholar * Lee, Y. W. et al. Unbiased
biocatalytic solar-to-chemical conversion by FeOOH/BiVO4/perovskite tandem structure. _Nat. Commun._ 9, 4208 (2018). Google Scholar * Zhang, H. et al. A sandwich-like organolead halide
perovskite photocathode for efficient and durable photoelectrochemical hydrogen evolution in water. _Adv. Energy Mater._ 8, 1800795 (2018). Google Scholar * Joya, K. S., Joya, Y. F.,
Ocakoglu, K. & van de Krol, R. Water-splitting catalysis and solar fuel devices: artificial leaves on the move. _Angew. Chem. Int. Ed._ 52, 10426–10437 (2013). CAS Google Scholar * Hu,
S., Xiang, C., Haussener, S., Berger, A. D. & Lewis, N. S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems.
_Energy Environ. Sci._ 6, 2984–2993 (2013). CAS Google Scholar * Wang, Q., Dong, Q., Li, T., Gruverman, A. & Huang, J. Thin insulating tunneling contacts for efficient and
water-resistant perovskite solar cells. _Adv. Mater._ 28, 6734–6739 (2016). CAS Google Scholar * Qiu, Y. et al. Efficient solar-driven water splitting by nanocone BiVO4–perovskite tandem
cells. _Sci. Adv._ 2, e1501764 (2016). Google Scholar * Azcarate, I., Costentin, C., Robert, M. & Savéant, J.-M. Through-space charge interaction substituent effects in molecular
catalysis leading to the design of the most efficient catalyst of CO2-to-CO electrochemical conversion. _J. Am. Chem. Soc._ 138, 16639–16644 (2016). CAS Google Scholar * Sato, S., Saita,
K., Sekizawa, K., Maeda, S. & Morikawa, T. Low-energy electrocatalytic CO2 reduction in water over Mn–complex catalyst electrode aided by a nanocarbon support and K+ cations. _ACS
Catal._ 8, 4452–4458 (2018). CAS Google Scholar * Zhou, X. et al. Solar-driven reduction of 1 atm of CO2 to formate at 10% energy-conversion efficiency by use of a TiO2-protected III–V
tandem photoanode in conjunction with a bipolar membrane and a Pd/C cathode. _ACS Energy Lett._ 1, 764–770 (2016). CAS Google Scholar * Reece, S. Y. et al. Wireless solar water splitting
using silicon-based semiconductors and earth-abundant catalysts. _Science_ 334, 645–648 (2011). CAS Google Scholar * Rao, H., Schmidt, L. C., Bonin, J. & Robert, M.
Visible-light-driven methane formation from CO2 with a molecular iron catalyst. _Nature_ 548, 74 (2017). CAS Google Scholar * Reuillard, B., Warnan, J., Leung, J. J., Wakerley, D. W. &
Reisner, E. A poly(cobaloxime)/carbon nanotube electrode: freestanding buckypaper with polymer-enhanced H2-evolution performance. _Angew. Chem. Int. Ed._ 55, 3952–3957 (2016). CAS Google
Scholar * Hoye, R. L. Z. et al. Strongly enhanced photovoltaic performance and defect physics of air-stable bismuth oxyiodide (BiOI). _Adv. Mater._ 29, 1702176 (2017). Google Scholar *
Harris, D. C. _Quantitative Chemical Analysis_ 7th edn. (W.H. Freeman and Co., New York, NY, 2007). * Andrei, V., Reuillard, B. & Reisner, E. _Raw Data Supporting Article: Bias-free
Solar Syngas Production by Integrating a Molecular Cobalt Catalyst with Perovskite–BiVO_ _4_ _T__andems_ (2019); https://doi.org/10.17863/CAM.44164 Download references ACKNOWLEDGEMENTS This
work was supported by the Christian Doppler Research Association (Austrian Federal Ministry for Digital and Economic Affairs and the National Foundation for Research, Technology and
Development) and the OMV Group (E.R.). V.A. is grateful for the financial support from the Cambridge Trusts (Vice-Chancellor’s Award) and the Winton Programme for the Physics of
Sustainability. B.R. was supported by the BBSRC (grant no. BB/K010220/1). XPS data collection was performed at the EPSRC National Facility for Photoelectron spectroscopy (‘HarwellXPS’),
operated by Cardiff University and UCL under contract no. PR16195. We acknowledge D. S. Wright (University of Cambridge) for providing us the Co WOC precursor. We thank A. Dickerson
(University of Cambridge) for the ICP-OES measurements. We are grateful to D. Achilleos (University of Cambridge) for help with XPS sample preparation and data analysis. We thank K. P. Sokol
(University of Cambridge) for helpful advice on the O2 measurements, and K. P. Sokol and A. Wagner (University of Cambridge) for useful feedback on the manuscript. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Christian Doppler Laboratory for Sustainable SynGas Chemistry, Department of Chemistry, University of Cambridge, Cambridge, UK Virgil Andrei, Bertrand Reuillard
& Erwin Reisner Authors * Virgil Andrei View author publications You can also search for this author inPubMed Google Scholar * Bertrand Reuillard View author publications You can also
search for this author inPubMed Google Scholar * Erwin Reisner View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS V.A., B.R. and E.R. designed
the project. V.A. prepared the photoelectrodes, performed the experiments and drafted the manuscript. V.A., B.R. and E.R. analysed the data. B.R. and E.R. contributed to the discussion and
completion of the manuscript. E.R. supervised the work. CORRESPONDING AUTHOR Correspondence to Erwin Reisner. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION Supplementary Information REPORTING SUMMARY SUPPLEMENTARY INFORMATION Supplementary Video RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Andrei, V., Reuillard, B. & Reisner, E. Bias-free solar syngas production by integrating a molecular cobalt catalyst with perovskite–BiVO4 tandems. _Nat.
Mater._ 19, 189–194 (2020). https://doi.org/10.1038/s41563-019-0501-6 Download citation * Received: 29 March 2019 * Accepted: 05 September 2019 * Published: 21 October 2019 * Issue Date:
February 2020 * DOI: https://doi.org/10.1038/s41563-019-0501-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative










