- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
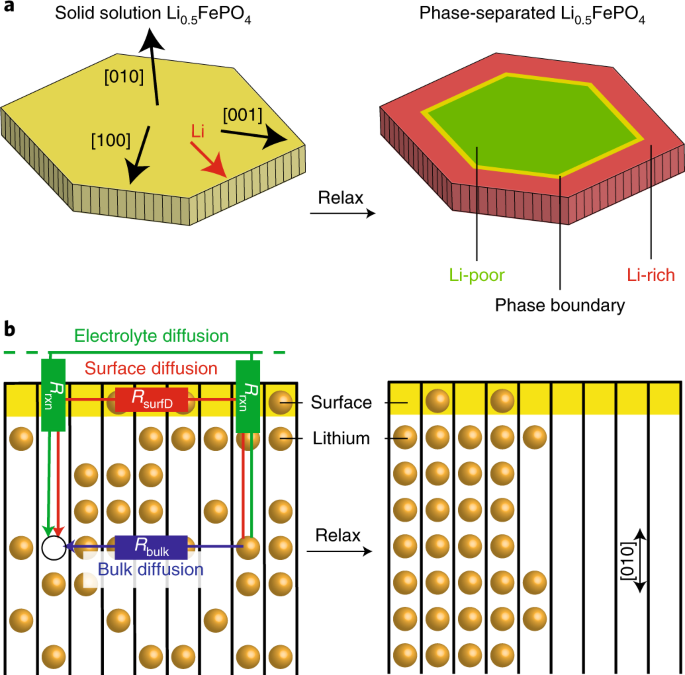
Phase transformations driven by compositional change require mass flux across a phase boundary. In some anisotropic solids, however, the phase boundary moves along a non-conductive
crystallographic direction. One such material is LiXFePO4, an electrode for lithium-ion batteries. With poor bulk ionic transport along the direction of phase separation, it is unclear how
lithium migrates during phase transformations. Here, we show that lithium migrates along the solid/liquid interface without leaving the particle, whereby charge carriers do not cross the
double layer. X-ray diffraction and microscopy experiments as well as ab initio molecular dynamics simulations show that organic solvent and water molecules promote this surface ion
diffusion, effectively rendering LiXFePO4 a three-dimensional lithium-ion conductor. Phase-field simulations capture the effects of surface diffusion on phase transformation. Lowering
surface diffusivity is crucial towards supressing phase separation. This work establishes fluid-enhanced surface diffusion as a key dial for tuning phase transformation in anisotropic
solids.
All experimental data within the article and its Supplementary Information will be made available upon reasonable request to the authors.
This experimental work at Stanford and SLAC was supported by the US Department of Energy (DOE), Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under contract
DE-AC02-76SF00515. Phase-field theoretical work at MIT and Stanford was supported by the Toyota Research Institute through D3BATT: Center for Data-Driven Design of Li-Ion Batteries. The
Advanced Light Source and the Stanford Synchrotron Radiation Lightsource are supported by the DOE Office of Basic Energy Sciences under contracts DE-AC02-05CH11231 and DE-AC02-76SF00515.
M.S.I. and H.C. acknowledge support from the EPSRC (grant EP/K016288) and the Archer HPC facilities through the Materials Chemistry Consortium (EP/L000202). Y.L. and P.M.A. were supported by
the NSF Graduate Research Fellowship under grant DGE-114747. K.L. was supported by the Kwanjeong Education Foundation Fellowship. M.Z.B. was supported by the Global Climate and Energy
Project at Stanford University and the DOE Office of Basic Energy Sciences through the SUNCAT Center for Interface Science and Catalysis. Part of this work was conducted the Stanford Nano
Shared Facilities. We thank W. D. Nix (Stanford) for insightful discussions on metallurgy and mechanical properties and R. B. Smith (MIT) for assistance with the phase-field model. We also
thank A. L. D. Kilcoyne (Berkeley) and D. Shaprio (Berkeley) for assistance with synchrotron measurements.
Present address: Sandia National Laboratories, Livermore, CA, USA
Department of Materials Science & Engineering, Stanford University, Stanford, CA, USA
Yiyang Li, Kipil Lim, Haitao D. Deng, Jongwoo Lim, Peter M. Attia, Sang Chul Lee, Norman Jin, Jihyun Hong, Martin Z. Bazant & William C. Chueh
Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory, Menlo Park, CA, USA
Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, Menlo Park, CA, USA
Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA
Department of Applied Physics, Stanford University, Stanford, CA, USA
Department of Chemistry, Stanford University, Stanford, CA, USA
Advanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA, USA
Faculty of Chemistry and Chemical Technology, University of Ljubljana, Ljubljana, Slovenia
Department of Mathematics, Massachusetts Institute of Technology, Cambridge, MA, USA
SUNCAT Interfacial Science and Catalysis, Stanford University, Stanford, CA, USA
Y.L. conceived and designed the project, analysed the experimental data and performed the phase-field simulations. H.C. and M.S.I. conducted the molecular dynamics simulations. Y.L., K.L.
and J.H. conducted diffraction. Y.L., J.L., P.M.A., N.J., W.E.G. and Y.S.Y. collected the X-ray microscopy images. S.C.L. performed transmission electron microscopy. D.F., Y.L. and M.Z.B.
designed and executed the linear stability analysis. H.D.D., J.M. and M.G. quantified the resistance increase during relaxation. M.S.I. supervised the molecular dynamics simulations. M.Z.B.
supervised the phase-field simulations and linear stability analysis. W.C.C. supervised the experimental components of the work. All authors contributed to writing the text.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Figures 1–17, Supplementary Table 1, Supplementary References
Anyone you share the following link with will be able to read this content:









