- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
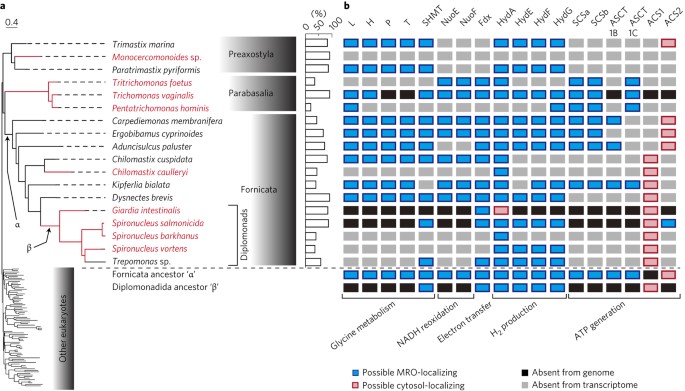
ABSTRACT Many anaerobic microbial parasites possess highly modified mitochondria known as mitochondrion-related organelles (MROs). The best-studied of these are the hydrogenosomes of
_Trichomonas vaginalis_ and _Spironucleus salmonicida_, which produce ATP anaerobically through substrate-level phosphorylation with concomitant hydrogen production; and the mitosomes of
_Giardia intestinalis_, which are functionally reduced and lack any role in ATP production. However, to understand the metabolic specializations that these MROs underwent in adaptation to
parasitism, data from their free-living relatives are needed. Here, we present a large-scale comparative transcriptomic study of MROs across a major eukaryotic group, Metamonada, examining
lineage-specific gain and loss of metabolic functions in the MROs of _Trichomonas_, _Giardia_, _Spironucleus_ and their free-living relatives. Our analyses uncover a complex history of ATP
production machinery in diplomonads such as _Giardia_, and their closest relative, _Dysnectes_; and a correlation between the glycine cleavage machinery and lifestyles. Our data further
suggest the existence of a previously undescribed biochemical class of MRO that generates hydrogen but is incapable of ATP synthesis. Access through your institution Buy or subscribe This is
a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our
best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only
$9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ANAEROBIC ENDOSYMBIONT
GENERATES ENERGY FOR CILIATE HOST BY DENITRIFICATION Article Open access 03 March 2021 THE TERRESTRIAL ISOPOD SYMBIONT ‘_CANDIDATUS_ HEPATINCOLA PORCELLIONUM’ IS A POTENTIAL NUTRIENT
SCAVENGER RELATED TO _HOLOSPORALES_ SYMBIONTS OF PROTISTS Article Open access 08 March 2023 EXTREME MITOCHONDRIAL REDUCTION IN A NOVEL GROUP OF FREE-LIVING METAMONADS Article Open access 09
August 2024 REFERENCES * Stairs, C. W., Leger, M. M. & Roger, A. J. Diversity and origins of anaerobic metabolism in mitochondria and related organelles. _Phil. Trans. R. Soc. Lond. B _
370, 20140326 (2015). Article Google Scholar * Lindmar, k, D. G. & Müller, M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate _Tritrichomonas foetus_, and its role
in pyruvate metabolism. _J. Biol. Chem._ 248, 7724–7728 (1973). Google Scholar * Müller, M. et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. _Microbiol. Mol.
Biol. Rev._ 76, 444–495 (2012). Article PubMed PubMed Central Google Scholar * Jerlstrom-Hultqvist, J. et al. Hydrogenosomes in the diplomonad _Spironucleus salmonicida_. _Nat. Commun._
4, 2493 (2013). Article PubMed Google Scholar * Jedelsky, P. L. et al. The minimal proteome in the reduced mitochondrion of the parasitic protist _Giardia intestinalis_. _PLoS ONE_ 6,
e17285 (2011). Article CAS PubMed PubMed Central Google Scholar * Karnkowska, A. et al. A eukaryote without a mitochondrial organelle. _Curr. Biol._ 26, 1274–1284 (2016). Article CAS
PubMed Google Scholar * Takishita, K. et al. Multigene phylogenies of diverse _Carpediemonas_-like organisms identify the closest relatives of ‘amitochondriate’ diplomonads and
retortamonads. _Protist_ 163, 344–355 (2012). Article PubMed Google Scholar * Simpson, A. G. & Patterson, D. J. On core jakobids and excavate taxa: the ultrastructure of _Jakoba
incarcerata_. _J. Eukaryot. Microbiol_. 48, 480–492 (2001). Article CAS PubMed Google Scholar * Dolezal, P., Likic, V., Tachezy, J. & Lithgow, T. Evolution of the molecular machines
for protein import into mitochondria. _Science_ 313, 314–318 (2006). Article CAS PubMed Google Scholar * Martincova, E. et al. Probing the biology of _Giardia intestinalis_ mitosomes
using _in vivo_ enzymatic tagging. _Mol. Cell. Biol._ 35, 2864–2874 (2015). Article CAS PubMed PubMed Central Google Scholar * Neupert, W. & Herrmann, J. M. Translocation of
proteins into mitochondria. _Annu. Rev. Biochem_. 76, 723–749 (2007). Article CAS PubMed Google Scholar * Yaffe, M. P., Ohta, S. & Schatz, G. A yeast mutant temperature-sensitive for
mitochondrial assembly is deficient in a mitochondrial protease activity that cleaves imported precursor polypeptides. _EMBO J._ 4, 2069–2074 (1985). Article CAS PubMed PubMed Central
Google Scholar * Harris, S. R., Matus, A., Hrdy, I. & Kute _, E._ Reductive evolution of the mitochondrial processing peptidases of the unicellular parasites _Trichomonas vaginalis_ and
_Giardia intestinalis_. _PLoS Pathog._ 4, e1000243 (2008). Google Scholar * Garg, S. et al. Conservation of transit peptide-independent protein import into the mitochondrial and
hydrogenosomal matrix. _Genome Biol. Evol._ 7, 2716–2726 (2015). Article CAS PubMed PubMed Central Google Scholar * Kikuchi, G. The glycine cleavage system: composition, reaction
mechanism, and physiological significance. _Mol. Cell. Biochem._ 1, 169–187 (1973). Article CAS PubMed Google Scholar * Mukherjee, M., Brown, M. T., McArthur, A. G. & Johnson, P. J.
Proteins of the glycine decarboxylase complex in the hydrogenosome of _Trichomonas_ v_aginalis_. _Eukaryot. Cell_ 5, 2062–2071 (2006). Article CAS PubMed PubMed Central Google Scholar *
Morrison, H. G. et al. Genomic minimalism in the early diverging intestinal parasite _Giardia lamblia_. _Science_ 317, 1921–1926 (2007). Article CAS PubMed Google Scholar * Zubacova, Z.
et al. The mitochondrion-like organelle of _Trimastix pyriformis_ contains the complete glycine cleavage system. _PLoS ONE_ 8, e55417 (2013). Article CAS PubMed PubMed Central Google
Scholar * Hampl, V. et al. Genetic evidence for a mitochondriate ancestry in the ‘amitochondriate’ flagellate _Trimastix pyriformis_. _PLoS ONE_ 3, e1383 (2008). Article PubMed PubMed
Central Google Scholar * Nývltová, E., Smutná, T., Tachezy, J. & Hrdý, I. OsmC and incomplete glycine decarboxylase complex mediate reductive detoxification of peroxides in
hydrogenosomes of _Trichomonas vaginalis_. _Mol. Biochem. Parasitol._ 206, 29–38 (2016). Article PubMed Google Scholar * Xu, F. et al. On the reversibility of parasitism: adaptation to a
free-living lifestyle via gene acquisitions in the diplomonad _Trepomonas_ sp. PC1. _BMC Biol._ 14, 62 (2016). Article PubMed PubMed Central Google Scholar * Hampson, R. K., Barron, L.
L. & Olson, M. S. Regulation of the glycine cleavage system in isolated rat liver mitochondria. _J. Biol. Chem._ 258, 2993–2999 (1983). CAS PubMed Google Scholar * Steinbüchel, A.
& Müller, M. Anaerobic pyruvate metabolism of _Tritrichomonas foetus_ and _Trichomonas vaginalis_ hydrogenosomes. _Mol. Biochem. Parasitol._ 20, 57–65 (1986). Article PubMed Google
Scholar * Van Hellemond, J. J., Klockiewicz, M., Gaasenbeek, C. P., Roos, M. H. & Tielens, A. G. Rhodoquinone and complex II of the electron transport chain in anaerobically functioning
eukaryotes. _J. Biol. Chem._ 270, 31065–31070 (1995). Article CAS PubMed Google Scholar * Tielens, A. G. M., van Grinsven, K. W. A., Henze, K., van Hellemond, J. J. & Martin, W.
Acetate formation in the energy metabolism of parasitic helminths and protists. _Int. J. Parasitol_. 40, 387–397 (2010). Article CAS PubMed Google Scholar * Sanchez, L. B. & Müller,
M. Purification and characterization of the acetate forming enzyme, acetyl-CoA synthetase (ADP-forming) from the amitochondriate protist, _Giardia lamblia_. _FEBS Lett_. 378, 240–244 (1996).
Article CAS PubMed Google Scholar * Noguchi, F. et al. Metabolic capacity of mitochondrion-related organelles in the free-living anaerobic stramenopile _Cantina marsupialis_. _Protist_
166, 534–550 (2015). Article CAS PubMed Google Scholar * Field, J., Rosenthal, B. & Samuelson, J. Early lateral transfer of genes encoding malic enzyme, acetyl-CoA synthetase and
alcohol dehydrogenases from anaerobic prokaryotes to _Entamoeba histolytica_. _Mol. Microbiol_. 38, 446–455 (2000). Article CAS PubMed Google Scholar * Nývltová, E. et al. Lateral gene
transfer and gene duplication played a key role in the evolution of _Mastigamoeba balamuthi_ hydrogenosomes. _Mol. Biol. Evol_. 32, 1039–1055 (2015). Article PubMed PubMed Central Google
Scholar * Schneider, R. E. et al. The _Trichomonas vaginalis_ hydrogenosome proteome is highly reduced relative to mitochondria, yet complex compared with mitosomes. _Int. J. Parasitol_.
41, 1421–1434 (2011). Article CAS PubMed PubMed Central Google Scholar * Murcha, M. W., Narsai, R., Devenish, J., Kubiszewski-Jakubiak, S. & Whelan, J. MPIC: a mitochondrial protein
import components database for plant and non-plant species. _Plant Cell Physiol_. 56, e10 (2015). Article PubMed Google Scholar * Zhang, Q. et al. Marine isolates of _Trimastix marina_
form a plesiomorphic deep-branching lineage within Preaxostyla, separate from other known Trimastigids (_Paratrimastix_ n. gen.). _Protist_ 166, 468–491 (2015). Article PubMed Google
Scholar * Kolisko, M. et al. A wide diversity of previously undetected free-living relatives of diplomonads isolated from marine/saline habitats. _Env. Microbiol._ 12, 2700–2710 (2010). CAS
Google Scholar * Chevreux, B. et al. Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. _Genome Res_. 14, 1147–1159
(2004). Article CAS PubMed PubMed Central Google Scholar * Grabherr, M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. _Nat. Biotechnol._ 29,
644–652 (2011). Article CAS PubMed PubMed Central Google Scholar * Gentekaki, E. et al. Large-scale phylogenomic analysis reveals the phylogenetic position of the problematic taxon
_Protocruzia_ and unravels the deep phylogenetic affinities of the ciliate lineages. _Mol. Phylogenet. Evol_. 78, 36–42 (2014). Article CAS PubMed Google Scholar * Katoh, K. &
Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. _Mol. Biol. Evol._ 30, 772–780 (2013). Article CAS PubMed PubMed Central
Google Scholar * Criscuolo, A. & Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence
alignments. _BMC Evol. Biol._ 10, 210 (2010). Article PubMed PubMed Central Google Scholar * Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree: computing large minimum evolution
trees with profiles instead of a distance matrix. _Mol. Biol. Evol._ 26, 1641–1650 (2009). Article CAS PubMed PubMed Central Google Scholar * Stamatakis, A. RAxML version 8: a tool for
phylogenetic analysis and post-analysis of large phylogenies. _Bioinformatics_ 30, 1312–1313 (2014). Article CAS PubMed PubMed Central Google Scholar * Nguyen, L. T., Schmidt, H. A.,
von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. _Mol. Biol. Evol._ 32, 268–274 (2015). Article CAS
PubMed Google Scholar * Minh, B. Q., Nguyen, M. A. & von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. _Mol. Biol. Evol._ 30, 1188–1195 (2013). Article CAS PubMed
PubMed Central Google Scholar * Lartillot, N., Rodrigue, N., Stubbs, D. & Richer, J. PhyloBayes MPI: phylogenetic reconstruction with infinite mixtures of profiles in a parallel
environment. _Syst. Biol._ 62, 611–615 (2013). Article CAS PubMed Google Scholar * Fu, L., Niu, B., Zhu, Z., Wu, S. & Li, W. CD-HIT: accelerated for clustering the next-generation
sequencing data. _Bioinformatics_ 28, 3150–3152 (2012). Article CAS PubMed PubMed Central Google Scholar * Capella-Gutierrez, S., Silla-Martinez, J. M. & Gabaldon, T. trimAl: a tool
for automated alignment trimming in large-scale phylogenetic analyses. _Bioinformatics_ 25, 1972–1973 (2009). Article CAS PubMed PubMed Central Google Scholar * Katoh, K. & Toh, H.
Recent developments in the MAFFT multiple sequence alignment program. _Br. Bioinform._ 9, 286–298 (2008). Article CAS Google Scholar * Noguchi, F., Tanifuji, G., Brown, M. W., Fujikura,
K. & Takishita, K. Complex evolution of two types of cardiolipin synthase in the eukaryotic lineage stramenopiles. _Mol. Phylogenet. Evol_. 101, 133–141 (2016). Article CAS PubMed
Google Scholar * Claros, M. G. MitoProt, a Macintosh application for studying mitochondrial proteins. _Comput. Appl. Biosci._ 11, 441–447 (1995). CAS PubMed Google Scholar * Emanuelsson,
O., Brunak, S., von Heijne, G. & Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. _Nat. Protoc._ 2, 953–971 (2007). Article CAS PubMed Google
Scholar * Fukasawa, Y. et al. MitoFates: improved prediction of mitochondrial targeting sequences and their cleavage sites. _Mol. Cell. Proteomics_ 14, 1113–1126 (2015). Article CAS
PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS M.K., M.M.L. and C.W.S. were supported by a grant (MOP-142349) from the Canadian Institutes of Health Research
awarded to A.J.R. This work was also supported, in part, by a grant from the JSPS Strategic Young Researcher Overseas Visits Program (awarded to R.K.), by NSERC Grant 298366-2009 to
A.G.B.S., by a Czech Science Foundation grant to I.Č. (project GA14-14105S) and by grants from the Japan Society for the Promotion of Science (JSPS; nos 15H05606 and 15K14591 awarded to
R.K., 23117005 and 15H05231 awarded to T.H., and 23117006 awarded to Y.I.). We thank A. A. Heiss for his help with _Trimastix marina_ data generation, and N. Ros for her comments on the
manuscript. AUTHOR INFORMATION Author notes * Michelle M. Leger, Martin Kolisko, Courtney W. Stairs & Laura Eme Present address: ‡ Present addresses: Institute of Evolutionary Biology,
CSIC-Universitat Pompeu Fabra, Passeig Marítim de la Barceloneta, 37–49, 08003 Barcelona, Spain (M.M.L.); Institute of Parasitology, Biology Centre, Czech Academy of Sciences, Branišovská
1160/31, 370 05 České Budĕjovice, Czech Republic (M.K.); Department of Cell and Molecular Biology, Uppsala University, Box 596, 751 24 Uppsala, Sweden (C.W.S. and L.E.), * Michelle M. Leger,
Martin Kolisko and Ryoma Kamikawa: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Biochemistry and Molecular Biology, Dalhousie University, 5850
College Street, PO Box 15000, Halifax, Nova Scotia, B3H 4R2, Canada Michelle M. Leger, Martin Kolisko, Courtney W. Stairs, Laura Eme & Andrew J. Roger * Graduate School of Human and
Environmental Studies, Graduate School of Global Environmental Studies, Kyoto University, Yoshida-Honmachi, Sakyo-ku, Kyoto, 606-8501, Japan Ryoma Kamikawa * Graduate School of Life and
Environmental Sciences, Center for Computational Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, 305-8572, Ibaraki, Japan Keitaro Kume, Yuji Inagaki & Tetsuo Hashimoto *
Department of Zoology, Faculty of Science, Charles University, Vinicna 7, 128 44, Prague 2, Czech Republic Ivan Čepička * Department of Biological Sciences, University of Arkansas,
Fayetteville, 72701, Arkansas, USA Jeffrey D. Silberman * Department of Cell and Molecular Biology, Uppsala University, Box 596, Uppsala, , 751 24, Sweden Jan O. Andersson & Feifei Xu *
Japan Agency for Marine-Earth Science and Technology (JAMSTEC), 2-15, Natsushima-cho, Yokosuka, 237-0061, Kanagawa, Japan Akinori Yabuki & Kiyotaka Takishita * Yantai Institute of
Coastal Zone Research, Chinese Academy of Sciences, 17 Chunhui Road, Yantai, 264003, Shandong, People’s Republic of China Qianqian Zhang * Center for Computational Sciences, University of
Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8577, Japan Yuji Inagaki & Tetsuo Hashimoto * Department of Biology, Dalhousie University, 1355 Oxford Street, PO Box 15000, Halifax, B3H
4R2, Nova Scotia, Canada Alastair G. B. Simpson Authors * Michelle M. Leger View author publications You can also search for this author inPubMed Google Scholar * Martin Kolisko View author
publications You can also search for this author inPubMed Google Scholar * Ryoma Kamikawa View author publications You can also search for this author inPubMed Google Scholar * Courtney W.
Stairs View author publications You can also search for this author inPubMed Google Scholar * Keitaro Kume View author publications You can also search for this author inPubMed Google
Scholar * Ivan Čepička View author publications You can also search for this author inPubMed Google Scholar * Jeffrey D. Silberman View author publications You can also search for this
author inPubMed Google Scholar * Jan O. Andersson View author publications You can also search for this author inPubMed Google Scholar * Feifei Xu View author publications You can also
search for this author inPubMed Google Scholar * Akinori Yabuki View author publications You can also search for this author inPubMed Google Scholar * Laura Eme View author publications You
can also search for this author inPubMed Google Scholar * Qianqian Zhang View author publications You can also search for this author inPubMed Google Scholar * Kiyotaka Takishita View author
publications You can also search for this author inPubMed Google Scholar * Yuji Inagaki View author publications You can also search for this author inPubMed Google Scholar * Alastair G. B.
Simpson View author publications You can also search for this author inPubMed Google Scholar * Tetsuo Hashimoto View author publications You can also search for this author inPubMed Google
Scholar * Andrew J. Roger View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.K., R.K., J.O.A., Y.I., A.G.B.S., T.H. and A.J.R. conceived and
designed the experiments; M.K., K.K., I.Č., J.D.S., F.X., A.Y. and Q.Z. performed the experiments; M.M.L., M.K., R.K., C.W.S., K.K., L.E. and Y.I. analysed the data; R.K., C.W.S., J.D.S.,
K.T., Y.I., A.G.B.S., T.H. and A.J.R. contributed materials and/or analysis tools; and M.M.L., M.K., R.K., A.G.B.S. and A.J.R. wrote the paper. CORRESPONDING AUTHORS Correspondence to Tetsuo
Hashimoto or Andrew J. Roger. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary
Figures 1–20, Supplementary Table 1 (PDF 1444 kb) SUPPLEMENTARY DATA 1 Predicted mitochondrion-related organelle proteins, and selected predicted cytosolic proteins, in metamonads. (XLSX
149 kb) SUPPLEMENTARY DATA 2 File containing the raw phylogenetic trees depicted in Supplementary Figs 4–20, including bootstrap support values, in Newick format. (TXT 539 kb) RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Leger, M., Kolisko, M., Kamikawa, R. _et al._ Organelles that illuminate the origins of _Trichomonas_ hydrogenosomes
and _Giardia_ mitosomes. _Nat Ecol Evol_ 1, 0092 (2017). https://doi.org/10.1038/s41559-017-0092 Download citation * Received: 17 August 2016 * Accepted: 17 January 2017 * Published: 13
March 2017 * DOI: https://doi.org/10.1038/s41559-017-0092 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative