- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
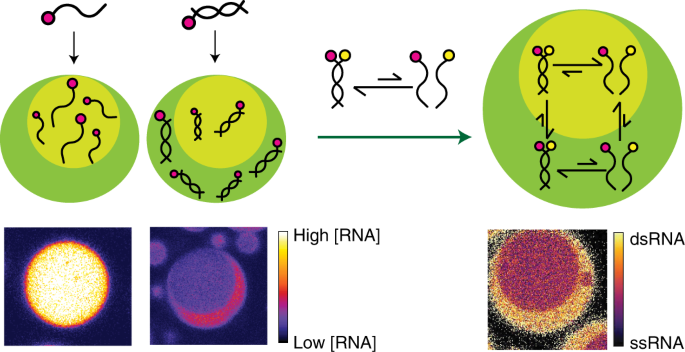
ABSTRACT Liquid–liquid phase separation has emerged as an important means of intracellular RNA compartmentalization. Some membraneless organelles host two or more compartments serving
different putative biochemical roles. The mechanisms for, and functional consequences of, this subcompartmentalization are not yet well understood. Here we show that adjacent phases of
decapeptide-based multiphase model membraneless organelles differ markedly in their interactions with RNA. Single- and double-stranded RNAs preferentially accumulate in different phases
within the same droplet, and one phase is more destabilizing for RNA duplexes than the other. Single-phase peptide droplets did not capture this behaviour. Phase coexistence introduces new
thermodynamic equilibria that alter RNA duplex stability and RNA sorting by hybridization state. These effects require neither biospecific RNA-binding sites nor full-length proteins. As
such, they are more general and point to primitive versions of mechanisms operating in extant biology that could aid understanding and enable the design of functional artificial membraneless
organelles. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access
Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print
issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS DETERMINANTS THAT ENABLE DISORDERED PROTEIN ASSEMBLY INTO DISCRETE CONDENSED PHASES Article 05 February 2024 ACTIVE COACERVATE DROPLETS AS A MODEL FOR MEMBRANELESS
ORGANELLES AND PROTOCELLS Article Open access 14 October 2020 ANXA11 BIOMOLECULAR CONDENSATES FACILITATE PROTEIN-LIPID PHASE COUPLING ON LYSOSOMAL MEMBRANES Article Open access 21 March 2025
DATA AVAILABILITY All data supporting the findings of this study are available within the Article and its Supplementary Information, and also from the corresponding authors upon request.
Source data are provided with this Paper. REFERENCES * Yewdall, N. A., André, A. A. M., Lu, T. & Spruijt, E. Coacervates as models of membraneless organelles. _Curr. Opin. Colloid
Interface Sci._ 52, 101416 (2021). Article CAS Google Scholar * Roden, C. & Gladfelter, A. S. RNA contributions to the form and function of biomolecular condensates. _Nat. Rev. Mol.
Cell Biol._ 22, 183–195 (2021). Article CAS PubMed Google Scholar * Zhang, H. et al. RNA controls polyQ protein phase transitions. _Mol. Cell_ 60, 220–230 (2015). Article CAS PubMed
PubMed Central Google Scholar * Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. _Science_ 357, eaaf4382 (2017). Article PubMed Google Scholar
* Cakmak, F. P., Choi, S., Meyer, M. O., Bevilacqua, P. C. & Keating, C. D. Prebiotically-relevant low polyion multivalency can improve functionality of membraneless compartments. _Nat.
Commun._ 11, 5949 (2020). Article CAS PubMed PubMed Central Google Scholar * Poudyal, R. R. et al. Template-directed RNA polymerization and enhanced ribozyme catalysis inside
membraneless compartments formed by coacervates. _Nat. Commun_. https://doi.org/10.1038/s41467-019-08353-4 (2019). * Poudyal, R. R., Keating, C. D. & Bevilacqua, P. C. Polyanion-assisted
ribozyme catalysis inside complex coacervates. _ACS Chem. Biol._ 14, 1243–1248 (2019). Article CAS PubMed PubMed Central Google Scholar * Poudyal, R. R., Pir Cakmak, F., Keating, C. D.
& Bevilacqua, P. C. Physical principles and extant biology reveal roles for RNA-containing membraneless compartments in origins of life chemistry. _Biochemistry_ 57, 2509–2519 (2018).
Article CAS PubMed Google Scholar * Drobot, B. et al. Compartmentalised RNA catalysis in membrane-free coacervate protocells. _Nat. Commun._ 9, 3643 (2018). Article PubMed PubMed
Central Google Scholar * Lafontaine, D. L., Riback, J. A., Bascetin, R. & Brangwynne, C. P. The nucleolus as a multiphase liquid condensate. _Nat. Rev. Mol. Cell Biol._ 22, 165–182
(2020). Article PubMed Google Scholar * Nott, T. J., Craggs, T. D. & Baldwin, A. J. Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. _Nat.
Chem._ 8, 569–575 (2016). Article CAS PubMed Google Scholar * Nott, T. J. et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless
organelles. _Mol. Cell_ 57, 936–947 (2015). Article CAS PubMed PubMed Central Google Scholar * Sun, L. et al. RNA structure maps across mammalian cellular compartments. _Nat. Struct.
Mol. Biol._ 26, 322–330 (2019). Article CAS PubMed PubMed Central Google Scholar * Rouskin, S., Zubradt, M., Washietl, S., Kellis, M. & Weissman, J. S. Genome-wide probing of RNA
structure reveals active unfolding of mRNA structures in vivo. _Nature_ 505, 701–705 (2014). Article CAS PubMed Google Scholar * Guillén-Boixet, J. et al. RNA-induced conformational
switching and clustering of G3BP drive stress granule assembly by condensation. _Cell_ 181, 346–361 (2020). Article PubMed PubMed Central Google Scholar * Saldi, T. K. et al. TDP‐1, the
_Caenorhabditis elegans_ ortholog of TDP‐43, limits the accumulation of double‐stranded RNA. _EMBO J._ 33, 2947–2966 (2014). Article CAS PubMed PubMed Central Google Scholar * Feric, M.
et al. Coexisting liquid phases underlie nucleolar subcompartments. _Cell_ 165, 1686–1697 (2016). Article CAS PubMed PubMed Central Google Scholar * Yao, R.-W. et al. Nascent pre-rRNA
sorting via phase separation drives the assembly of dense fibrillar components in the human nucleolus. _Mol. Cell_ 76, 767–783 (2019). Article CAS PubMed Google Scholar * Jain, S. et al.
ATPase-modulated stress granules contain a diverse proteome and substructure. _Cell_ 164, 487–498 (2016). Article CAS PubMed PubMed Central Google Scholar * Sanders, D. W. et al.
Competing protein-RNA interaction networks control multiphase intracellular organization. _Cell_ 181, 306–324 (2020). Article CAS PubMed PubMed Central Google Scholar * Youn, J.-Y. et
al. Properties of stress granule and P-body proteomes. _Mol. Cell_ 76, 286–294 (2019). Article CAS PubMed Google Scholar * Riback, J. A. et al. Composition-dependent thermodynamics of
intracellular phase separation. _Nature_ 581, 209–214 (2020). Article CAS PubMed PubMed Central Google Scholar * Mitrea, D. M. et al. Self-interaction of NPM1 modulates multiple
mechanisms of liquid–liquid phase separation. _Nat. Commun._ 9, 842 (2018). Article PubMed PubMed Central Google Scholar * Fisher, R. S. & Elbaum-Garfinkle, S. Tunable multiphase
dynamics of arginine and lysine liquid condensates. _Nat. Commun._ 11, 4628 (2020). Article CAS PubMed PubMed Central Google Scholar * Mountain, G. A. & Keating, C. D. Formation of
multiphase complex coacervates and partitioning of biomolecules within them. _Biomacromolecules_ 21, 630–640 (2020). Article CAS PubMed Google Scholar * Lu, T. & Spruijt, E.
Multiphase complex coacervate droplets. _J. Am. Chem. Soc._ 142, 2905–2914 (2020). Article CAS PubMed PubMed Central Google Scholar * Simon, J. R., Carroll, N. J., Rubinstein, M.,
Chilkoti, A. & López, G. P. Programming molecular self-assembly of intrinsically disordered proteins containing sequences of low complexity. _Nat. Chem._ 9, 509–515 (2017). Article CAS
PubMed PubMed Central Google Scholar * Moreau, N. G., Martin, N., Gobbo, P., Tang, T. Y. D. & Mann, S. Spontaneous membrane-less multi-compartmentalization via aqueous two-phase
separation in complex coacervate micro-droplets. _Chem. Commun._ 56, 12717–12720 (2020). Article CAS Google Scholar * Boeynaems, S. et al. Spontaneous driving forces give rise to
protein-RNA condensates with coexisting phases and complex material properties. _Proc. Natl Acad. Sci. USA_ 116, 7889–7898 (2019). Article CAS PubMed PubMed Central Google Scholar *
Kaur, T. et al. Sequence-encoded and composition-dependent protein-RNA interactions control multiphasic condensate morphologies. _Nat. Commun._ 12, 872 (2021). Article CAS PubMed PubMed
Central Google Scholar * Schuster, B. S. et al. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. _Nat. Commun._ 9, 2985 (2018).
Article PubMed PubMed Central Google Scholar * Alshareedah, I. et al. Interplay between short-range attraction and long-range repulsion controls reentrant liquid condensation of
ribonucleoprotein-RNA complexes. _J. Am. Chem. Soc._ 141, 14593–14602 (2019). Article CAS PubMed PubMed Central Google Scholar * Chong, P. A., Vernon, R. M. & Forman-Kay, J. D.
RGG/RG motif regions in RNA binding and phase separation. _J. Mol. Biol._ 430, 4650–4665 (2018). Article CAS PubMed Google Scholar * Torza, S. & Mason, S. G. Three-phase interactions
in shear and electrical fields. _J. Colloid Interface Sci._ 33, 67–83 (1970). Article CAS Google Scholar * Li, L. et al. Phase behavior and salt partitioning in polyelectrolyte complex
coacervates. _Macromolecules_ 51, 2988–2995 (2018). Article CAS Google Scholar * Chen, Y., Yang, M., Shaheen, S. A. & Schlenoff, J. B. Influence of nonstoichiometry on the
viscoelastic properties of a polyelectrolyte complex. _Macromolecules_ 54, 7890–7899 (2021). Article CAS Google Scholar * Friedowitz, S. et al. Looping-in complexation and ion
partitioning in nonstoichiometric polyelectrolyte mixtures. _Sci. Adv._ 7, eabg8654 (2021). Article CAS PubMed PubMed Central Google Scholar * Schneider, C. P., Shukla, D. & Trout,
B. L. Arginine and the Hofmeister series: the role of ion-ion interactions in protein aggregation suppression. _J. Phys. Chem. B_ 115, 7447–7458 (2011). Article CAS PubMed PubMed Central
Google Scholar * Luscombe, N. M., Laskowski, R. A. & Thornton, J. M. Amino acid-base interactions: a three-dimensional analysis of protein-DNA interactions at an atomic level.
_Nucleic Acids Res._ 29, 2860–2874 (2001). Article CAS PubMed PubMed Central Google Scholar * Chin, K., Sharp, K. A., Honig, B. & Pyle, A. M. Calculating the electrostatic
properties of RNA provides new insights into molecular interactions and function. _Nat. Struct. Biol._ 6, 1055–1061 (1999). Article CAS PubMed Google Scholar * Frenkel-Pinter, M. et al.
Mutually stabilizing interactions between proto-peptides and RNA. _Nat. Commun._ 11, 3137 (2020). Article CAS PubMed PubMed Central Google Scholar * Raman, B. et al. _N_ω-arginine
dimethylation modulates the interaction between a Gly/Arg-rich peptide from human nucleolin and nucleic acids. _Nucleic Acids Res._ 29, 3377–3384 (2001). Article CAS PubMed PubMed Central
Google Scholar * Arakawa, T., Hirano, A., Shiraki, K., Kita, Y. & Koyama, A. H. Stabilizing and destabilizing effects of arginine on deoxyribonucleic acid. _Int. J. Biol. Macromol._
46, 217–222 (2010). Article CAS PubMed Google Scholar * Maeda, Y., Iwata, R. & Wada, T. Synthesis and properties of cationic oligopeptides with different side chain lengths that bind
to RNA duplexes. _Biorg. Med. Chem._ 21, 1717–1723 (2013). Article CAS Google Scholar * Mountain, G. A. & Keating, C. D. in _Methods Enzymol_. Vol. 646 (ed. Keating, C. D.) 115–142
(Academic Press, 2021). * Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. _Nat. Methods_ 9, 676–682 (2012). Article CAS PubMed Google Scholar * Ota, N.
et al. Determination of interactions between structured nucleic acids by fluorescence resonance energy transfer (FRET): selection of target sites for functional nucleic acids. _Nucleic
Acids Res._ 26, 735–743 (1998). Article CAS PubMed PubMed Central Google Scholar * Tsuji, A. et al. Direct observation of specific messenger RNA in a single living cell under a
fluorescence microscope. _Biophys. J._ 78, 3260–3274 (2000). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the NASA
Exobiology programme grant no. 80NSSC17K0034 (S.C., M.O.M., P.C.B. and C.D.K.). S.C. was also supported by Future Investigators in NASA Earth and Space Science and Technology (FINESST) under
grant no. 80NSSC19K1531 and no. 80NSSC22K0553. We thank F. Pir Cakmak and H. Fares for helpful discussions, and T. Mal for help with NMR analysis. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Chemistry, The Pennsylvania State University, University Park, PA, USA Saehyun Choi, Philip C. Bevilacqua & Christine D. Keating * Center for RNA Molecular
Biology, The Pennsylvania State University, University Park, PA, USA McCauley O. Meyer & Philip C. Bevilacqua * Department of Biochemistry and Molecular Biology, The Pennsylvania State
University, University Park, PA, USA McCauley O. Meyer & Philip C. Bevilacqua Authors * Saehyun Choi View author publications You can also search for this author inPubMed Google Scholar
* McCauley O. Meyer View author publications You can also search for this author inPubMed Google Scholar * Philip C. Bevilacqua View author publications You can also search for this author
inPubMed Google Scholar * Christine D. Keating View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.O.M. performed the radiolabelled RNA
partitioning experiments. S.C. performed all other experiments. All authors conceived and designed the experiments and analysed the data. S.C. and C.D.K. wrote the manuscript, with input
from P.C.B. and M.O.M. CORRESPONDING AUTHORS Correspondence to Philip C. Bevilacqua or Christine D. Keating. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemistry_ thanks Pilong Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION Supplementary Figs. 1–18, Discussions 1–7 and Tables 1–17. SUPPLEMENTARY DATA 1 Calculation of radiolabelled RNA partitioning in coacervate phases. SOURCE DATA SOURCE DATA FIG. 1
Estimated labelled peptide concentration for all trials. SOURCE DATA FIG. 2 Estimated RNA concentration for all trials. SOURCE DATA FIG. 3 FRET values and its intensities for all trials.
SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Choi, S., Meyer, M.O., Bevilacqua, P.C. _et al._ Phase-specific RNA
accumulation and duplex thermodynamics in multiphase coacervate models for membraneless organelles. _Nat. Chem._ 14, 1110–1117 (2022). https://doi.org/10.1038/s41557-022-00980-7 Download
citation * Received: 15 May 2021 * Accepted: 20 May 2022 * Published: 30 June 2022 * Issue Date: October 2022 * DOI: https://doi.org/10.1038/s41557-022-00980-7 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative