- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The translation of messenger RNA (mRNA) is a fundamental process in gene expression, and control of translation is important to regulate protein synthesis in cells. The primary
hallmark of eukaryotic mRNAs is their 5′ cap, whose molecular contacts to the eukaryotic translation initiation factor eIF4E govern the initiation of translation. Here we report 5′ cap
analogues with photo-cleavable groups (FlashCaps) that prohibit binding to eIF4E and resist cleavage by decapping enzymes. These compounds are compatible with the general and efficient
production of mRNAs by in vitro transcription. In FlashCap-mRNAs, the single photocaging group abrogates translation in vitro and in mammalian cells without increasing immunogenicity.
Irradiation restores the native cap, triggering efficient translation. FlashCaps overcome the problem of remaining sequence or structure changes in mRNA after irradiation that limited
previous designs. Together, these results demonstrate that FlashCaps offer a route to regulate the expression of any given mRNA and to dose mRNA therapeutics with spatio-temporal control.
SIMILAR CONTENT BEING VIEWED BY OTHERS RATIONAL DESIGN OF MICRORNA-RESPONSIVE SWITCH FOR PROGRAMMABLE TRANSLATIONAL CONTROL IN MAMMALIAN CELLS Article Open access 08 November 2023
OPTOCHEMICAL CONTROL OVER MRNA TRANSLATION BY PHOTOCAGED PHOSPHORODIAMIDATE MORPHOLINO OLIGONUCLEOTIDES IN VIVO Article Open access 16 April 2025 ENGINEERED POLY(A)-SURROGATES FOR
TRANSLATIONAL REGULATION AND THERAPEUTIC BIOCOMPUTATION IN MAMMALIAN CELLS Article Open access 04 January 2024 MAIN Messenger RNAs (mRNAs) have recently entered the public stage as most
versatile medical modalities. Prominent examples are the mRNA-based vaccines by Moderna and BioNTech/Pfizer that code for spike proteins to protect against infection by SARS-CoV-2 (ref. 1).
The mRNA technology is not limited to vaccination, and can also greatly improve, for example, therapy for autoimmune diseases or personalized cancer treatment2. Translation of mRNA into
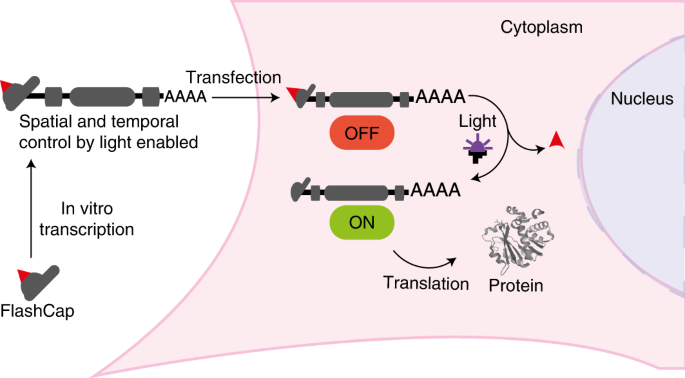
proteins is one of the fundamental and highly conserved processes in the cell and works for endogenous and exogenous transcripts (Fig. 1a). Its regulation is essential in cell
differentiation, cell proliferation and localized translation3,4, but is also relevant for pathologies5. In mRNA therapy, however, one cannot currently control when and where mRNA has an
effect, that is, when and where it is translated into proteins, which then have a pharmacological effect. A hallmark of eukaryotic mRNAs is their 5′ cap, which, in its simplest form (cap 0),
links an _N_7-methylated guanosine to the first transcribed nucleotide via a 5′-5′ triphosphate bridge (Fig. 1b). Higher-order cap structures contain additional methyl groups6. The 5′ cap
plays a key role in translation initiation, as the _N_7-methylated guanosine is essential for recognition by the translation initiation factor eIF4E (Fig. 1c). Importantly, the molecular
contacts with the cap are sequence-independent, that is, they are identical for all mRNAs7. The 5′ cap is also crucial for many mRNA processing and quality control steps and protects
eukaryotic mRNAs from degradation by exonucleases3,5,8. Dedicated decapping enzymes (Dcp1-2, DcpS) are required for mRNA turnover and homeostasis9,10. Together with the poly(A) tail at the
3′ end, the 5′ cap forms an mRNA ‘closed-loop’, facilitated by interactions between the cap-binding eIFs and the poly(A)-binding protein (PABP). The closed loop promotes recruitment of the
small ribosomal subunit (40S) and the complex enters the next initiation stages, leading to formation of the 80S ribosome and translation (Fig. 1a)5. RNA without the 5′ cap is barely
translated and is highly immunogenic11,12,13. Therefore, production of mRNAs for biological studies and therapeutic applications routinely involves in vitro transcription in the presence of
synthetic cap analogues to obtain 5′-capped mRNAs14,15,16 (Fig. 1d). In nature, the initiation phase of translation is the target of multiple types of regulatory intervention, enabling
confinement of gene expression to a certain time span and cell region, for example, in neurons or multicellular organisms5,17. The ability to control translation by external
triggers—especially by light—would greatly enrich our ability to dissect cellular processes at the molecular level with high spatio-temporal precision. The directed release of mRNAs for
translation at a certain time and destination would also provide an avenue to control the pharmacokinetics of mRNA therapeutics. In this context, it would be important to avoid a drastic
increase of immunogenicity. However, methods to control gene expression externally at the mRNA level are scarce. Natural mechanisms triggering mRNA translation by light are still unknown,
and only one example of integrating photo-sensitive units in translation has been reported so far18. Chemical approaches to directly photocage RNAs provide control of several RNA-regulated
processes involving short regulatory RNAs, such as small interfering RNAs, microRNAs, morpholinos and aptamers19,20,21,22,23. The chemical or chemo-enzymatic synthesis of long mRNAs,
however, suffers from low yields24. Moreover, the installation of multiple modifications in the mRNA does not necessarily impede the ribosome25. Previous approaches towards controlling mRNA
translation by light required tags26, multiple photocaging groups27 or photoswitches28,29,30,31—including photoswitches at the 5′ cap—that left the RNA altered. Remaining chemical
modifications in the mRNA might affect the properties of the mRNA, as shown for natural modifications32,33. Additional sequence elements may alter mRNA interactions, potentially disrupting
the regulatory processes of mRNA turnover34. In this Article we report optochemical control of mRNA translation in eukaryotic cells. Our approach is based on a synthetic cap analogue
(FlashCap) that efficiently interferes with the initiation stage of translation. Irradiation of FlashCap-mRNAs liberates an unaltered cap 0-mRNA molecule that is accessible for translation
into hundreds of protein copies (Fig. 1a,d). This concept capitalizes on a single photocaging group at a defined position to leverage strong effects on the translation of ~1,000-nt-long
mRNAs. It is generally applicable, as synthetic 5′ cap analogues are routinely used in the production of mRNAs by in vitro transcription for research and therapeutic purposes. FlashCaps are
therefore an efficient and readily applicable solution to make mRNA studies controllable by light, without requiring new production steps and without introducing artefacts into measurements.
RESULTS To achieve a strong effect on translation, we analysed the molecular interactions between the 5′ cap and the translation initiation factor eIF4E (Fig. 1c), as well as previous work
on the effect of cap modifications on binding16,28,35,36,37. We anticipated that the installation of a sterically demanding residue (such as a photo-cleavable group) at the N2 position of
the guanosine should interfere with the direct hydrogen bonding to E103 that is required for proper positioning of the 5′ cap (Fig. 1c). At the same time, photo-deprotection should rapidly
reconstitute the natural cap 0 and initiate translation. We therefore developed a synthesis route to 5′ caps with photo-cleavable groups at the N2 position of the cap guanosine (Fig. 2). To
promote cap 0 release, we connected the photo-cleavable group via a self-immolative carbamate linkage. Photo-cleavage releases CO2, driving the deprotection reaction. Starting from guanosine
(3), we first protected the three hydroxyl groups using trimethylsilyl (TMS) chloride. In a one-pot reaction, we then converted the free amino group of the guanosine to isocyanate, which
was directly reacted with the _ortho_-nitrobenzyl (ONB) alcohol 4C as the photo-cleavable group or the redshifted derivatives 3,4-dimethoxy-2-nitrobenzyl (DMNB), 6-nitropiperonyl (NP) or
6-nitropiperonyl-methyl (NPM) alcohol (4A–D; Supplementary Fig. 22). During workup in THF with aqueous ammonia, the TMS groups were removed to obtain the photocaged guanosines, 5A–D. The
photocaged guanosines were then monophosphorylated at the 5′-OH to give 6A,B, methylated to 7A,B and coupled to guanosine-5′-diphosphate imidazolide, prepared from guanosine diphosphate
(GDP) as previously described38. We measured the absorption spectra of the synthesized guanosines with photo-cleavable groups at the N2 position (5A–D). ONB guanosine (5C) showed only low
absorbance above 300 nm, DMNB guanosine (5A) showed an absorption maximum at 350 nm, and NP (5D) and NPM (5B) guanosine were slightly redshifted with a maximum at 360 nm (Fig. 3a), in line
with literature on the respective photo-cleavable groups20,39,40. To choose the most suitable photo-cleavable group for biological applications, we irradiated 5A–C in aqueous solution at
neutral pH and analysed their decrease as well as formation of the native guanosine using HPLC and liquid chromatography mass spectrometry (LC-MS; Fig. 3b, Extended Data Fig. 1 and
Supplementary Fig. 3). Time-dependent analyses revealed that at 365 nm (light-emitting diode (LED), 140 mW cm−2), short irradiation (5–15 s) was sufficient to remove the photo-cleavable
group in 10 µl of a 500 µM solution of 5A,B,D and release the free guanosine (Fig. 3b and Extended Data Fig. 1). At 405 nm, the NP, NPM and DMNB groups were efficiently removed after 60 s,
more efficiently than the ONB group (Fig. 3b and Extended Data Fig. 1). At 420 nm, the NPM group was completely removed after 120 s (Fig. 3b and Extended Data Fig. 1). We therefore chose
DMNB and NPM groups for further studies and synthesized the respective cap 0 analogues. The resulting FlashCaps contain the DMNB (1) or the NPM (2) group at the N2 position connected via a
carbamate functionality (Fig. 2). Their absorption spectra above 300 nm and their uncaging kinetics were similar to the respective photocaged guanosines (5A,B) (Fig. 3a and Extended Data
Fig. 1) and formation of cap 0 was confirmed (Supplementary Figs. 7–10). We also assessed the biological stability of the carbamate linkage by incubating cap 0 (0) or FlashCaps (1, 2) in
cell lysate followed by HPLC analysis (Fig. 3c and Supplementary Fig. 4). FlashCaps exhibited high stabilities over 30 h, similar to the cap 0, suggesting that the carbamate linkage is not
the primary point of degradation in lysate. Next, we evaluated how the photo-cleavable groups affect interaction of the 5′ cap with eIF4E. Binding measurements of FlashCaps and Cy5-labelled
eIF4E using microscale thermophoresis (MST) did not result in a binding curve in the case of the photocaged caps (1, 2) (Fig. 3d,e). Under identical conditions, a _K_d value of 0.3 µM was
determined for cap 0 (Supplementary Fig. 5), in line with the literature35,41. Importantly, after light-induced removal of the photocaging groups from 1 or 2, the characteristic binding
curve and a _K_d value in a similar range to cap 0 was obtained (Fig. 3d,e), indicating efficient formation of cap 0 (Supplementary Table 2). We also investigated how the photo-cleavable
groups affected interactions with cap-modifying enzymes. DcpS is a pyrophosphatase hydrolysing the cap structure to m7GMP and GDP in eukaryotic cells (Fig. 3f)8. Similar to the results with
eIF4E, DcpS (H277N)—a binding but non-cleaving variant of the decapping enzyme—interacted with cap 0 but not with FlashCaps (Supplementary Fig. 6). However, if FlashCaps were briefly
irradiated before the assay was performed, the _K_d value of the DcpS variant was in the same range as for cap 0 (Supplementary Fig. 6), indicating light-induced liberation of functional cap
0. We also tested whether the photo-cleavable groups would affect the enzymatic degradation of cap structures (Fig. 3f,g). Catalytically active DcpS-WT rapidly cleaved cap 0 into m7GMP and
GDP, resulting in >50% degradation within 15 min (Fig. 3g)8. In contrast, FlashCaps 1 and 2 remained almost completely intact during that time (~98% undigested cap), demonstrating that
the photo-cleavable groups abrogate enzymatic cleavage of FlashCaps (Fig. 3f). As expected, the DcpS-mediated cleavage of FlashCaps was triggered in situ by irradiation with light (365 nm,
30 s), confirming that light-mediated release of the photo-cleavable group renders the reconstituted cap 0 readily available to enzymatic conversion (Fig. 3f). Taken together, these data
demonstrate that FlashCaps efficiently impede the interaction with cap-binding proteins and cap-degrading enzymes and that irradiation by light releases fully functional cap 0 that is
readily recognized by cap-binding partners in vitro. Next, we were interested in whether FlashCaps are suitable for the preparation of long mRNAs containing a photocaged 5′ cap
(FlashCap-mRNAs) using standard molecular biology methods. In vitro transcription (IVT) using phage T7 RNA polymerase and synthetic cap analogues is routinely used to produce capped mRNAs
for biological studies42 and therapeutic applications43. The cap analogue is incorporated as the first G by transcriptional priming, yielding capped and uncapped RNA. The latter can be
removed by enzymatic treatment with polyphosphatase and XRN1 (ref. 16). Comparative evaluation of IVT with FlashCaps or cap 0 revealed that all tested 5′ caps yielded intact mRNAs (Fig. 4a).
The yield and capping efficiency in the presence of 1 or 2 were slightly lower but in the same range as for cap 0, according to our analysis of four different mRNAs (Fig. 4b). These data
show that transcriptional priming with FlashCaps is efficient and that 1 and 2 can be routinely used for IVT with T7 polymerase to produce long FlashCap-mRNAs with yields comparable to cap
0. We then probed the interaction of long FlashCap-mRNAs with cap-binding proteins or cap-modifying enzymes. In the major mRNA turnover pathway, the decapping enzyme Dcp1/2 cleaves mRNA to
release the 5′ monophosphorylated mRNA, which is degraded by the exoribonuclease XRN1 (refs. 9,10). We tested this cap-dependent decay in vitro by treating cap 0-mRNA and FlashCap-mRNA with
Dcp1/2 followed by XRN1 digestion (Fig. 4c). This treatment completely degraded cap 0-mRNA, whereas FlashCap-mRNAs with 1 or 2 remained intact (Fig. 4c). When FlashCap-mRNAs were irradiated
before the enzymatic treatment, they became susceptible to enzymatic degradation, indicating light-dependent release of the free cap 0, which is recognized by Dcp1/2. In control reactions,
which were irradiated but not treated with the enzymes, mRNAs with cap 0 or FlashCaps remained intact, confirming that irradiation alone does not degrade long mRNAs (Fig. 4c). As FlashCaps
abrogate eIF4E binding, which is the rate-limiting step for translation initiation, we were curious as to how FlashCap-mRNA would impact translation. We therefore tested in vitro translation
(IVTL) of luciferase-mRNAs with cap 0 and FlashCaps using rabbit reticulocyte lysate. To our delight, the translation of FlashCap-mRNAs was drastically reduced (Fig. 4d), in line with
results from our eIF4E-binding studies (Fig. 3d,e). FlashCap-RLuc-mRNAs with 1 or 2 exhibited only 2–4% of luciferase activity relative to cap 0-mRNA (Fig. 4d). However, if the
FlashCap-mRNAs were irradiated, translation was increased by 15–20-fold, reaching 41 ± 2% (1) (s.d., _n_ = 3) or 59 ± 11% (2) (s.d., _n_ = 3) relative to the native cap 0. Under the same
irradiation conditions, the IVTL of cap 0-mRNAs was only slightly reduced (to 91 ± 5%; s.d., _n_ = 3) and the mRNAs remained intact (Fig. 4a,d). Taken together, these data demonstrate that
FlashCap-mRNAs are translationally muted and efficiently activated by brief irradiation with light. The released mRNAs are intact, functional and contain a 5′ cap 0, but no sequence changes
or remaining chemical modifications. Next we investigated the translation of FlashCap-mRNAs in cultured mammalian cells using _Gaussia_ luciferase (GLuc) or enhanced green fluorescent
protein (eGFP) as the secreted or intracellular reporter. Luciferase activity for HeLa cells transfected with FlashCap-mRNAs or controls was normalized to cap-dependent translation of cap
0-mRNA. The cap-dependent translation of FlashCap-mRNAs with 1 or 2 was reduced to 6 ± 1% (s.e.m., _n_ = 3) and 2 ± 2% (s.e.m., _n_ = 3), respectively (Fig. 5a). Half of the cell samples
were briefly irradiated 6 h after transfection. Irradiation strikingly increased the luciferase signal of cells transfected with FlashCap-mRNA, resulting in 72 ± 8% (s.e.m., _n_ = 3) in the
case of 1 and 54 ± 4% (s.e.m., _n_ = 3) in the case of 2. This corresponds to a remarkable 12–27-fold irradiation-dependent increase in translation. A 32-fold increase was observed when
FlashCap-mRNA with 2 was irradiated before transfection. The irradiation itself only slightly decreased the translation (77 ± 7%; s.e.m., _n_ = 3) in HeLa cells, as shown by controls with
cap 0-mRNA. Of note, the absolute amount of cap-dependent translation triggered by light almost reaches the level of irradiated cells transfected with control mRNA (72 ± 8%, s.e.m., _n_ =
3), supporting the notion that intracellular uncaging is efficient and fully functional mRNA is generated (Fig. 5a). Taken together, these data demonstrate that irradiation efficiently
releases cap 0-mRNA and triggers translation in living cells transfected with FlashCap-mRNAs, without compromising cell viability and mRNA integrity (Fig. 3a and Supplementary Fig. 13). In
current mRNA-based therapeutics, modified nucleosides are widely used to increase translation32 and reduce immunogenicity33,44. To test whether FlashCaps are compatible with such
modifications, we produced FlashCap-mRNAs containing 5-methylcytosine (m5C) and N1-methyl-pseudouridine (m1Ψ). As expected, these internal RNA modifications increased the amount of protein
produced in all cases (Supplementary Fig. 14)32. Normalized to control-mRNAs containing the same modifications, the light-dependent turn-on effect of FlashCap-mRNAs remained in the same
range both in vitro (Fig. 4e) and in cells (Supplementary Fig. 15). Light-induced translation of FlashCap-mRNAs was also achieved in HEK293T cells, demonstrating their functionality in
different human cell lines (Extended Data Fig. 2 and Supplementary Fig. 16). To assess the effect of FlashCaps and light on translation for a different mRNA and using a different assay, we
co-transfected HeLa cells with differently capped eGFP-mRNAs and cap 0-mScarlet-I-mRNAs as internal reference. Imaging by confocal microscopy revealed that a green fluorescent signal was
barely detectable when using FlashCap-eGFP-mRNA with 2 (Fig. 5b). Control cells transfected with cap0-eGFP-mRNA showed bright fluorescence under the same conditions. However, if the cells
transfected with FlashCap-mRNA were irradiated, strong green fluorescence was visible, comparable to cells transfected with cap 0-mRNA (Fig. 5b and Extended Data Fig. 3). Similarly,
irradiation of FlashCap-mRNA before transfection strongly increased the fluorescence. Quantification of the microscopy images confirmed a notable increase, supporting the data obtained by
the luminescence assay (Supplementary Fig. 19). Furthermore, we tested a transcript coding for Rheb, a guanosine-5′-triphosphate-binding protein that is ubiquitously expressed in humans. A
western blot confirmed that FlashCap-Rheb-mRNA was muted, but efficiently translated upon irradiation (Extended Data Fig. 4), indicating that FlashCaps are compatible with biologically
relevant mRNAs. To analyse the effect of irradiation on translation also on the single-cell level, we performed flow cytometry of HeLa cells transfected with differently capped eGFP-mRNAs
(Fig. 5c). Direct comparison revealed a marked increase in eGFP-positive cells when FlashCap-mRNA-containing cells had been irradiated. FlashCap-mRNAs with 1 or 2 then led to 36.0% or 41.4%
eGFP-positive cells. These values are close to the 48.6% observed for the positive control (cap 0-mRNA; Fig. 5c). Without irradiation, FlashCap-mRNAs led to a substantially lower fraction of
eGFP-positive cells (18–19%), albeit higher than the negative control (4%). This can be attributed to partial uncaging during this long experiment, as the same FlashCap-eGFP-mRNA shows no
relevant background in confocal laser scanning microscopy (CLSM) images (Fig. 5b), nor in western blots (Extended Data Fig. 4). The histograms unambiguously show that the eGFP intensity of
the irradiated samples is much higher compared to non-irradiated samples. Taken together, the flow cytometry data show, on a single-cell level, that the eGFP fluorescence intensity is
increased for FlashCap-mRNAs in response to irradiation (Fig. 5c). The data independently confirm the findings from luminescence, western blot and microscopy analyses, showing that
irradiation of FlashCap-mRNAs highly increases the translation of a variety of reporter mRNAs. A key feature of light-triggered processes is the exquisite and facile spatio-temporal control.
Using a CLSM set-up, we tested whether brief irradiation of a predefined circle with a diameter of 120 µm using the 405-nm laser would activate translation in a subset of cells. Indeed, we
observed that cells transfected with FlashCap-eGFP-mRNA containing 2 developed green fluorescence exclusively in the circled area (Fig. 6a). These data show that FlashCap-mRNAs enable
control of translation in a subset of cells. Spatial control of translation on a micrometre scale can be readily achieved using a commercial CLSM set-up. mRNA therapeutics have recently
gained enormous interest. For the use of modified mRNAs in vivo in humans it is important to estimate the effects on the stability of the mRNA as well as on the elicited immune response.
Previous studies reported that untranslated mRNAs are subject to degradation as part of a quality control mechanism45. To assess whether translationally muted FlashCap-mRNAs are prone to
degradation, we determined the stability of mRNAs in cells in comparison to cap 0-mRNAs (Fig. 6b). Using quantitative real-time PCR with reverse transcription (RT–qPCR), we compared the
amount of differently capped mRNAs at 4 h and 10 h after transfection. We observed similar levels of remaining mRNA 10 h after transfection, suggesting that the half-life of mRNAs is not
affected by the photo-cleavable group at the 5′ cap (Fig. 6b and Supplementary Fig. 21). To assess the effect of FlashCaps on the immune response, we used reporter HEK-NF-ĸB cell lines
overexpressing a nuclear factor (NF)-ĸB-driven Firefly luciferase and different Toll-like receptors (TLRs)46. The control cell line (Null) has no TLR overexpressed and provides a measure for
the activation of endogenously expressed pathogen recognition receptors. The FlashCap-mRNAs did not exhibit a substantial increase in response to TLR3, TLR7 or TLR8, nor to the control cell
line in comparison to cap 0-mRNA (Fig. 6c). This was observed both for the unirradiated and the irradiated forms. These data suggest that the application and activation of FlashCap-mRNAs
can be expected to elicit an immune response similar to cap 0-mRNAs and may thus prove suitable for application in therapeutic mRNAs. CONCLUSIONS With the approval of mRNAs as a therapeutic
modality, the number of studies on mRNA aiming to improve the technology and addressing other diseases can be expected to rise, both in the field of basic research as well as in preclinical
and clinical studies. However, so far, no strategy exists to efficiently time the expression of the administered mRNA, nor to control the delivery and uptake into certain tissues without
alterations remaining in the mRNA. Even in cell culture, the administration and liberation of exogenous mRNA currently cannot be efficiently controlled in space and time. We developed a
technique to control the translation of any given mRNA by light. FlashCaps are 5′ cap analogues containing a single photocaging group connected via a self-immolative carbamate linkage,
leading to fast and efficient liberation of the natural cap 0 structure, as demonstrated by multiple assays in vitro. FlashCaps are compatible with common molecular biology techniques. They
are simply added instead of the synthetic 5′ cap analogue to the in vitro transcription to make any mRNA of interest with efficiencies similar to the cap 0-mRNA. The resulting FlashCap-mRNAs
are (1) translationally muted in vitro and in cells, (2) contain only a single photo-cleavable group, (3) release native cap 0-mRNA, (4) do not require changes in sequence or permanent
chemical alterations and (5) are not immunogenic. We demonstrate the functionality of FlashCap-mRNAs in two different cell lines and for light-activated translation into both intracellular
(eGFP, RLuc, Rheb) and secreted (GLuc) proteins. The irradiation conditions required to release cap 0-mRNA are compatible with cell viability and translation, and the photo-cleavable groups
have even proven compatible with animal models in previous studies47,48. An up to 32-fold light-induced increase of translation was observed in HeLa cells. We also confirmed that
translationally muted FlashCap-mRNAs are not preferentially degraded and do not elicit an increased immune response compared to cap 0-mRNAs. FlashCaps are therefore a highly efficient and
readily applicable solution to make mRNA studies controllable by light, without requiring new production steps and without introducing permanent artefacts. METHODS ABSORBANCE SPECTRA
ANALYSIS The analysis of the absorbance properties of the photocaged guanosines was performed using a quartz cuvette (Hellma) together with an FP-8500 fluorescence spectrometer (Jasco). The
respective guanosines were dissolved in water at a final concentration of 100 µM. For the absorbance measurements, 20 µl of the solution was further diluted in water to give a final volume
of 100 µl (20 µM), which was transferred into the cuvette followed by the absorbance measurement. Values are normalized to the highest measured value of each measurement. IRRADIATION OF
SAMPLES LEDs (LED Engin) were used to irradiate mRNA samples, guanosines, cells and cap analogues. The UV-A LED (_λ_max = 365 nm) and the blue-light LEDs (_λ_max = 405 nm, _λ_max = 420 nm)
were operated at 5 V and 600 mA input power (the respective output power is shown in Supplementary Fig. 1). Irradiation was performed in a custom-made LED set-up at 23 °C. The samples were
irradiated in a PCR tube or a cell culture dish (Supplementary Fig. 2) unless stated otherwise. Samples were irradiated at 365 nm (142 mW cm−2) for 30 s, 405 nm (142 mW cm−2) for 60 s or 420
nm (52 mW cm−2) for 120 s, unless otherwise noted. GUANOSINE AND DINUCLEOTIDE IRRADIATION STUDIES The respective guanosines or cap analogues were dissolved in ddH2O if possible (if needed,
organic solvents were added to increase solubility) to give a solution with a final concentration of 500 µM. The solution (10 µl) was transferred into a PCR tube and irradiated as described
above. Subsequently, the solution was analysed by HPLC. HPLC ANALYSIS HPLC analysis and purification of cap analogues were performed on an Agilent1260 Infinity HPLC system equipped with a
diode array detector (DAD) (190–640 nm) using a Nucleodur C18 Pyramid reversed-phase column (5 μm, 125 × 4 mm) from Macherey–Nagel. Elution was carried out at a flow rate of 1 ml min−1 by
applying a linear gradient from buffer A (50 mM ammonium acetate, pH 6.0) to buffer B (1:1 buffer A:acetonitrile). If other conditions were used, this is described in the respective section.
MST MEASUREMENTS MST measurements were performed on a Monolith NT.115 series instrument (NanoTemper). Before the thermophoresis measurements, proteins were labelled by incubation with
Cy5-NHS (Lumiprobe) for 30 min at room temperature (r.t.). Unreacted dye was separated from the protein using PD SpinTrap G-25 gel filtration columns (GE Healthcare) according to the
manufacturer’s protocol. Serial dilutions of the cap analogues (starting from 200 µM of cap analogue) in MST reaction buffer (20 mM HEPES, 50 mM KCl, 0.2 mM EDTA, 0.01% Triton-X, 700 µM
mercaptoethanol, 0.01% Tween-20, pH 8) were prepared and mixed with an equal volume of the labelled protein (~50 nM). The mixture was filled into premium coated capillaries (4 μl) and
directly measured. The MST power was set to 30–40%, the LED power to 20% red (excitation, 625 nm; emission, 680 nm). Thermophoresis measurements were performed with the following settings:
fluorescence before (5 s), MST on (30 s), fluorescence after (3 s). The capillaries were measured three times in direct succession as technical replicates. MST data were normalized to
baseline differences and _K_d values were calculated using nonlinear regression assuming a Hill coefficient of 1.0 (GraphPad Prism). MST is known to produce occasional outliers. This was
handled as follows: 16 data points were measured per binding curve and at least 12 data points were used for each fit. YDCPS HYDROLYSIS ASSAY The hydrolytic activity of yDcpS (New England
Biolabs) was assayed using the following experimental conditions: 50 mM Tris-HCl containing 1 mM Mg(Ac)2, 30 mM (NH4)2SO4 and 1 mM dithiothreitol (final pH 8.0) at 37 °C. Together with the
respective cap analogue, an internal standard (either adenine monophosphate or 4,5,7-trihydroxy-3-phenylcoumarin, with a final concentration of 200 µM) was added. Finally, 20 U of yDcpS
were added. The hydrolysis process was started by incubation at 37 °C. At 0, 5, 10, 15, 30, 45 and 60 min of the hydrolysis, 10-µl aliquots of the reaction mixture were withdrawn and the
reaction was stopped by heat inactivation of the enzyme (10 min at 90 °C). The samples were then subjected to analytical HPLC and analysed at 260 nm. Hydrolysis products were identified by
comparison of their retention times with those of reference standards. EXPRESSION AND PURIFICATION OF MTAN, LUXS, HTGS, HDCPS AND EIF4E The enzymes 5'-methylthioadenosine nucleosidase
(MTAN), LuxS, hDcpS H277N, eIF4E and hTgs were produced and purified as previously described16,35,49,50. IN VITRO TRANSCRIPTION The DNA template required for the in vitro transcription was
synthesized by PCR, in which the DNA sequence coding for eGFP, Firefly luciferase (FLuc), Gaussia luciferase (GLuc) and Renilla luciferase (RLuc) were amplified from pMRNA vectors containing
the respective sequence. After purification (NucleoSpin Gel and PCR Clean-up, Macherey–Nagel), the resulting linear dsDNA was used as template (200 ng). The runoff template is an
alternative to the PCR-DNA template that was used for the GLuc, mScarlet-I and eGFP-mRNAs used in cell studies and fluorescence microscopy. Plasmid DNA (3 µg) was incubated with 1×
FastDigest buffer (Thermo Fisher) and 3 µl of PacI FastDigest enzyme for 10 min at 37 °C, followed by inactivation at 65 °C for 10 min. Subsequently, the ends were dephosphorylated by adding
3 µl of FastAP and incubation at 37 °C for 15 min and inactivation at 65 °C for 5 min. The product was purified using the NucleoSpin Gel and PCR Clean-up kit (Macherey–Nagel). The
concentration was measured at 260 nm with a Tecan Infinite M1000 PRO instrument. The resulting linear dsDNA was used as template (400 ng). The in vitro transcription was performed with T7
polymerase (Thermo Scientific) in transcription buffer (40 mM Tris/HCl, 25 mM NaCl, 8 mM MgCl2, 2 mM spermidine(HCl)3) by adding either an A/C/UTP (0.5 mM) mix or A/m5C/m1ΨTP mix (0.5 mM),
guanosine-5'-triphosphate (0.25 mM), the respective cap analogue (1 mM), T7 RNA polymerase (50 U; Thermo Scientific) and pyrophosphatase (0.1 U; Thermo Scientific) for 4 h at 37 °C.
After the reaction, the DNA template was digested in the presence of 2 U of DNase I for 1 h at 37 °C and then mRNAs were purified using the RNA Clean & Concentrator-5 kit (Zymo
Research). To digest non-capped RNAs, 10 U of the RNA 5′-polyphosphatase (Epicentre) as well as the supplied reaction buffer were added to purified mRNAs. After an incubation period of 30
min at 37 °C, 0.5 U of the 5′–3′ exoribonuclease XRN1 (NEB) and MgCl2 (5 mM) were added. The reaction mixture was incubated for 60 min at 37 °C. Subsequently, capped mRNAs were purified
using the RNA Clean & Concentrator-5 kit (Zymo Research). IN VITRO LUMINESCENCE ASSAY For in vitro translation, the Retic Lysate IVT kit (Invitrogen), a eukaryotic cell-free protein
expression system, was used. In a total volume of 15 µl, 40 ng of the FLuc–mRNA (capped as indicated), 50 µM l-methionine and 150 mM potassium acetate were mixed with 8.5 µl of the
reticulocyte lysate and incubated for 90 min at 30 °C. Samples were mixed with 8.5 µl of the reticulocyte lysate and incubated for 90 min at 30 °C. Afterwards, 2 µl of the respective
translation mix was further used in a luminescence assay. The translation efficiencies of the differently capped FLuc–mRNAs were measured using a luciferase assay based on the Beetle-juice
Luciferase Assay Firefly (pjk). Luciferase activity was determined after adding 50 µl of freshly prepared substrate solution to the translation mixture. Luminescence was assessed using a
Tecan Infinite M1000 PRO microplate reader with an integration time of 3 s. Differently capped mRNAs were used. ApppG-capped mRNA represents cap-independent translation and was subtracted as
background from the other samples. All values were normalized to m7GpppG-capped mRNA. MAMMALIAN CELL CULTURE HeLa cells (Merck) were cultured in MEM Earle’s medium (PAN) supplemented with
l-glutamine (2 mM, PAN), non-essential amino acids (1%, PAN), penicillin and streptomycin (1%, PAN) and fetal calf serum (FCS; 10%, PAN) under standard conditions (5% CO2, 37 °C). HEK293T
cells (DSMZ) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; PAN) supplemented with l-glutamine (2 mM, PAN), penicillin and streptomycin (1%, PAN) and FCS (10%, PAN) under
standard conditions (5% CO2, 37 °C). HEK-NF-κB cells (TRON) were cultured under standard conditions (5% CO2, 37 °C) in DMEM supplemented with FCS (10%), HEPES buffer (1%), l-glutamine (1%),
non-essential amino acids (1%) and sodium pyruvate (1%). For selection, the following antibiotics were added to the culture of the HEK-NF-κB-Null, HEK-NF-κB-TLR7 and HEK-NF-κB-TLR8 cell
lines: blasticidin (10 µg ml−1), Zeocin (100 µg ml−1) and Geneticin (G418; 250 µg ml−1). The HEK-NF-ĸB-TLR3 cell line was cultured in the absence of Geneticin. All of the cell lines
overexpress an NF-κB driven Firefly luciferase, which allows the detection of NF-κB production in a luminescence assay and can be used as an indicator for the induction of an immune
response46. Additionally, the cell lines HEK-NF-κB-TLR3, HEK-NF-κB-TLR7 and HEK-NF-κB-TLR8 overexpress the respective TLRs. STABILITY ASSAY OF 5′ CAPS IN CELL LYSATE For preparation of HeLa
cell lysate, HeLa cells were cultured as mentioned above. At 24 h before cell lysis, 3 × 106 cells were seeded on a Petri dish (90 mm). The cells were collected and pelleted by
centrifugation. The cell pellets were stored at −80 °C. For cell lysis, the medium was removed and the cells were washed with 1× phosphate buffered saline (PBS), then lysed with CelLytic M
reagent (1.5 ml, Sigma Aldrich) according to the manufacturer’s instructions and stored at −80 °C. The lysis mixture was centrifuged (11,000 r.p.m., 3 min, 4 °C) and the supernatant was used
for the cell lysate stability assay. To the cell lysate were added the respective cap analogue (500 µM) and 4,5,7-trihydroxy-3-phenylcoumarin (100 µM) as internal standard, followed by
incubation for different periods of time (0, 0.5, 1, 5, 18 and 30 h) at 37 °C. The samples were analysed by HPLC. MTT ASSAY HeLa cells (Merck) were cultured as mentioned above. One day
before transfection, the cells were seeded in a 96-well plate (30,000 cells per well) and cultured in minimal essential medium (MEM) with antibiotics. The cells were transfected with mRNA
(100 ng) in Opti-MEM (10 µl) using Lipofectamine MessengerMAX transfection reagent (0.3 µl) in Opti-MEM (9.7 µl). The cells were incubated with the mRNA/Lipofectamine MessengerMAX mixture
for 6 h at 37 °C in a total volume of 100 µl. The samples were irradiated under the indicated conditions. Subsequently, the cell medium with the transfection agent was replaced by fresh
medium and the cells were incubated overnight at 37 °C in medium. At 24 h post transfection, MTT solution (16.5 mg MTT in 3.3 ml of PBS) was added to the 96-well plate (12.5 µl per well).
After 4 h of incubation at 37 °C, the supernatant was removed and 0.04 M HCl in isopropanol was added to the wells. After incubation for 1.5 h at r.t., 100 µl of the supernatant was placed
in a new 96-well plate and absorption at 550 nm was measured using the Tecan Infinite M1000 PRO plate reader. IN-CELL LUMINESCENCE ASSAY HeLa or HEK293T cells were cultured as mentioned
above. One day before transfection, the cells were seeded in a 96-well plate (30,000 cells per well) and cultured in MEM with antibiotics. The cell were transfected with mRNA (100 ng) in
Opti-MEM (10 µl) using Lipofectamine MessengerMAX transfection reagent (0.3 µl) in Opti-MEM (9.6 µl). The cells were incubated with the mRNA/Lipofectamine MessengerMAX mixture for 6 h at 37
°C in a total volume of 100 µl. The samples were irradiated at 365 nm for 30 s if not stated otherwise. Subsequently, the cell medium with the transfection agent was replaced with fresh
medium and the cells were incubated overnight at 37 °C in medium. At 24 h post transfection, the supernatant was collected. To perform the luminescence measurement, a Gaussia-Juice
Luciferase Assay kit (PJK) was used. The supernatant of the previously prepared samples was transferred to a 96-well plate (5 µl of supernatant per well). Afterwards, 50 µl of a reaction
mixture (PJK Reconstruction buffer and Coelenterazine) was added to the wells and the luminescence activity was measured using a Tecan Infinite M1000 PRO plate reader. The activity in
relative light units (RLU) was determined with an integration time of 3 s. Differently capped mRNAs were used. ApppG-capped mRNA represents cap-independent translation and was subtracted as
background from the other samples. All values were normalized to m7GpppG-capped mRNA. CLSM For microscopic imaging, HeLa cells were cultured as mentioned above. One day before transfection,
2 × 105 cells were seeded on glass coverslips in a 12-well plate in 1 ml of medium (indicated in cell culture section). Cells were transfected using 1.5 µl of Lipofectamine MessengerMAX
(Invitrogen) in Opti-MEM (48.5 μl) and eGFP-mRNA (containing m5C and m1Ψ; 1 μg) in Opti-MEM (50 μl). In the case of mScarlet-I/eGFP co-transfection, a total amount of 1 µg mRNA (eGFP 800 ng,
mScarlet-I 200 ng) was used. For confocal microscopy the runoff plasmid of pRNA2-(A)128 (Addgene) with eGFP or mScarlet-I was used as template for in vitro transcription. At 24 h post
transfection, cells were fixed with 300 μl per well of 4% paraformaldehyde in PBS for 10 min at r.t. After washing, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 1:10 in
PBS). After washing with PBS and water, the coverslips were mounted on microscopy slides using Aqua-Poly/mount (Polysciences). A Leica TCS SP8 CLSM was used to image fixed cells with a ×63
water immersion objective lens (HC PL APO ×63/1.20 W CORR UVIS CS2). Images were captured at a green channel for eGFP fluorescence (_λ_ex = 488 nm, _λ_em = 492–558 nm), a red channel for
mScarlet-I fluorescence (_λ_ex = 568 nm, _λ_em = 583–693 nm), a blue channel for DAPI (_λ_ex = 358 nm, _λ_em = 443–510 nm) and at the differential interference correlation channel. The
objectives used in this study were HC PL APO ×63/1.20 W CORR UVIS CS2 and HC PL FLUOSTAR ×10/0.30 Ph1 objectives, the laser was a diode laser (405 nm; 8.3 mW, laser power in the focus plane
with a ×10 objective), and the detectors were photomultiplier (Hamamatsu R 9624) HyD detectors. For all microscopy images, hyperstacking and background subtraction were performed with ImageJ
(30 pixels). RNA ISOLATION AND RT–QPCR For RT–qPCR, HeLa cells (Merck), were cultured as described above. One day before transfection, 2 × 105 cells were seeded in medium (1 ml) in a
12-well plate. Cells were transfected using 1.5 µl of Lipofectamine MessengerMAX (Invitrogen) in Opti-MEM (48.5 μl). 1 µg of RLuc-mRNA in Opti-MEM (50 μl) was prepared. The cells were
incubated with the mRNA/Lipofectamine MessengerMAX mixture for 4 h at 37 °C in a total volume of 1 ml. Subsequently, the cell medium with the transfection agent was replaced with fresh
medium. The cells were collected at 4 h or 10 h post transfection by adding 500 µl of lysis buffer (10 mM Tris-HCl (pH 8), 150 mM NaCl, 0.5 mM EDTA, 0.1% NP40). The RNA was isolated from the
cell lysate via phenol/chloroform extraction. The isolated total RNA was incubated with DNase I (2 U) in DNase reaction buffer (1×) in a total volume of 20 µl for 30 min at 37 °C to digest
the remaining DNA. Addition of EDTA (final concentration 5 mM) and incubation for 2 min at 65 °C was used to inactivate the enzymes. For reverse transcription, 1× RT buffer, dNTPs (final
concentration 0.5 mM) with random hexamer primer (5 μM) and Maxima H Minus reverse transcriptase (25 U) were mixed for 10 min at 25 °C followed by 30 min at 50 °C and finally 5 min at 85 °C.
The resulting complementary DNA (cDNA) was diluted 1:3 in ddH2O and 3 µl of the diluted cDNA was added into a 96-well qPCR plate. 17 µl of Mastermix, containing forward primer (0.5 μM),
reverse primer (0.5 μM) and 1× iTaq Universal SYBR Green Supermix (Bio-Rad), was added to the provided cDNA in the 96-well plate (Supplementary Table 1). The following PCR program was
applied: (1) initial denaturation (95 °C for 3 min), (2) denaturation (95 °C for 5 s), (3) elongation (55 °C for 30 s), (4) plate read, (5) 39 × cycle (2)–(4), (6) melt curve (60 °C–95 °C,
0.5 °C per 4 s) and (7) plate read. Quantitative real-time PCR measurements were performed on a Bio-Rad CFX96TM Real-Time System with a C1000TM Touch Thermal Cycler. Data analysis was
performed with the CFX Manager 3.1 (Bio-Rad). FLOW CYTOMETRY For flow cytometry, HeLa cells were cultured as mentioned above. One day before transfection, 2 × 105 cells were seeded in a
12-well plate in 1 ml of medium. Cells were transfected using 1.5 µl of Lipofectamine MessengerMAX (Invitrogen) in Opti-MEM (48.5 μl) and 1 µg eGFP-mRNA in Opti-MEM (50 µl). The cells were
incubated with the mRNA/Lipofectamine MessengerMAX mixture for 4 h at 37 °C in a total volume of 1 ml. The samples were irradiated at 365 nm for 30 s. Subsequently, the cell medium with the
transfection agent was replaced with fresh medium and the cells were incubated overnight at 37 °C. At 24 h post transfection, the cells were collected with trypsin/EDTA and washed with PBS.
The cell suspension was filtered through a 40-µm filter to avoid cell clumps. The eGFP signal was measured with a flow cytometer (Beckman Coulter Cytomics FC 500). During flow cytometry,
10,000 cells (total cell count) were measured per sample. Analysis was performed with the CxP Analysis Software. DETECTION OF IMMUNOGENICITY IN HEK-NF-ĸB CELLS HEK-NF-ĸB cells were cultured
as mentioned above. One day before transfection, 1.5 × 105 cells were seeded in medium (500 µl) in a 24-well plate. Cells were transfected using Metafectene Pro (2 μl; Biontex) in PBS (28
μl) and (non-irradiated or irradiated) RLuc-mRNA (500 ng) in PBS (30 μl). At 20 h post transfection, the cells were collected and washed with PBS. The pellets were resuspended in 50 µl of
PBS and used for the luminescence assay. The luminescence measurement was performed using the Beetle-Juice Luciferase assay Firefly kit (pjk). The reagents were prepared as suggested by the
manufacturer. The 50 µl of cell suspension were mixed with 50 µl of 2× Lysis Juice. After incubation for 15 min at 37 °C (450 U min−1), 20 µl of the cell lysate was transferred to a 96-well
plate (in duplicates), then 50 µl of the freshly prepared Firefly reaction mixture was injected into the well with an acquisition time of 3,000 ms. The samples were normalized to the
m7GpppG-capped mRNA. WESTERN BLOTS HEK293T and HeLa cells were cultured as mentioned above. One day before transfection, 2 × 105 cells were seeded in a 12-well plate in 1 ml of medium. Cells
were transfected using 1.5 µl of Lipofectamine MessengerMAX (Invitrogen) in Opti-MEM (48.5 μl) and 1 µg of eGFP-mRNA in Opti-MEM (50 µl). At 4 h post transfection, the cells were irradiated
(142 mW cm−2, 365 nm, 30 s) and the transfection medium was replaced with fresh medium. At 24 h post transfection, the cells were collected and washed with PBS. The cells were lysed with
CelLytic M (Sigma Aldrich). To determine the protein concentration of the cell lysate, a Bradford assay was performed using BSA calibration standards and a dilution of cell lysate (1:25),
then 50 µl of the sample was incubated (10 min, r.t., exclusion of light) with 1× Roti-Quant (Roth) staining solution (200 μl) and the extinction at 595 nm was determined. The proteins (40
µg) were separated via tris-glycine–PAGE (12% polyacrylamide (PAA) gel, 120 V, 1.5 h, r.t.). The proteins were transferred onto a nitrocellulose membrane Roti-NC (Roth) in a semi-dry
transfer buffer with 90 mA for 75 min at r.t. To validate protein transfer, a Ponceau S (0.5% Ponceau S + 1% glacial acetic acid) stain was performed. The membrane was cut into two
appropriate pieces for subsequent antibody treatment and washed with 1× PBS + 0.01% Tween (PBST). Blocking of the membrane was performed in blocking buffer (3% BSA in PBS) for 1 h at r.t.,
followed by incubation with the respective primary antibodies—anti-eGFP mouse monoclonal antibody (Santa Cruz Biotechnology) and anti-nucleolin mouse monoclonal antibody (Thermo Fisher
Scientific)—overnight at 4 °C and three times washing with PBST for 5 min at r.t. The membrane pieces were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody
(polyclonal rabbit anti-mouse immunoglobulins/HRP; Dako Diagnostica) for 1 h at r.t. and then washed three times with PBST. For chemiluminescence detection, the EZ-ECL chemiluminescence
detection kit (Biological Industries) was used and the results were analysed with a Chemo Star Advanced Fluorescence & ECL imager (Intas). DECAPPING ASSAY The RNA was prepared as
mentioned above. A 1-µg sample of capped eGFP-mRNA was mixed with mRNA decapping enzyme reaction buffer (NEB; final concentration 1×) in a total volume of 19.7 µl. The mixture was irradiated
(except for the control without irradiation). Then either 0.3 µl of mRNA decapping enzyme (NEB) or 0.3 µl of H2O as negative control was added. After incubation for 30 min at 37 °C, 1 µl of
XRN1 and 2.5 µl of MgCl2 were added to each Eppendorf tube (to the controls as well) and incubated for 1 h at 37 °C, then 2 µl of each sample were loaded on a 7.5% PAA gel. RiboRuler Low
Range (Thermo Fisher) was used as a marker. REMETHYLATION ASSAY A solution of LuxS (5 µM), MTAN (5 µM), the corresponding cap analogue (400 µM), SAM (6 mM) and hTgs (20 µM) in buffer (5 mM
Tris-HCl, 10 mM MgCl2, 5 mM KCl, pH 8.0) was incubated at 37 °C. At 0, 5, 15, 30 and 60 min of the methylation reaction, 10-µl aliquots of the reaction mixture were withdrawn and the
reaction was stopped by heat inactivation of the enzyme (10 min at 90 °C). The samples were then analysed by HPLC, monitoring the absorbance at 260 nm. Methylation products were assigned by
comparison of their retention times with those of reference standards. STATISTICAL ANALYSIS For statistical analysis of the luminescence data, an unpaired, parametric, two-tailed Student’s
_t_-test was used. When compared with the m7GpppG mRNA, an additional Welch correction was used (*_P_ < 0.05, **_P_ < 0.01, ***_P_ < 0.001). REPORTING SUMMARY Further information on
research design is available in the Nature Research Reporting Summary linked to this Article. DATA AVAILABILITY The data generated or analysed during this study are included in this Article
and its Supplementary Information. Protein structures and models used for the figures are available under PDB accession code 1EJ1. Source data are provided with this paper. CODE
AVAILABILITY No custom software was used in this study. The following software was used for data collection: Leica Application Suite X (3.5.6.21594), CxP Analysis Software, CFX Manager
Software V 3.1 (Bio-Rad), Qualitative Navigator B.08.00, Agilent ChemStation for LC 3D systems Rev. B.04.03 and Agilent OpenLab CDS ChemStation Edition Rev. C.01.10. The following software
was used for data analysis: Origin (2016b), Origin (2021b), Origin (2019b), GraphPad Prism 7, ImageJ (Version 20160205), MestReNova 14, LasX (Version), Excel 2016 and PerkinElmer ChemDraw
20.1. REFERENCES * Zhang, C., Maruggi, G., Shan, H. & Li, J. Advances in mRNA vaccines for infectious diseases. _Front. Immunol._ 10, 594 (2019). Article CAS PubMed PubMed Central
Google Scholar * Sahin, U., Kariko, K. & Tureci, O. mRNA-based therapeutics—developing a new class of drugs. _Nat. Rev. Drug Discov._ 13, 759–780 (2014). Article CAS PubMed Google
Scholar * Besse, F. & Ephrussi, A. Translational control of localized mRNAs: restricting protein synthesis in space and time. _Nat. Struct. Mol. Biol._ 9, 971–980 (2008). Article CAS
Google Scholar * Jansen, R. P., Niessing, D., Baumann, S. & Feldbrugge, M. mRNA transport meets membrane traffic. _Trends Genet._ 30, 408–417 (2014). Article CAS PubMed Google
Scholar * Shirokikh, N. E. & Preiss, T. Translation initiation by cap-dependent ribosome recruitment: recent insights and open questions. _Wiley Interdiscip. Rev. RNA_ 9, e1473 (2018).
PubMed Google Scholar * Mikkola, S., Salomaki, S., Zhang, Z., Maki, E. & Lonnberg, H. Preparation and properties of mRNA 5′-cap structure. _Curr. Org. Chem._ 9, 999–1022 (2005).
Article CAS Google Scholar * von der Haar, T., Gross, J. D., Wagner, G. & McCarthy, J. E. G. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. _Nat. Struct.
Mol. Biol._ 11, 503–511 (2004). Article PubMed CAS Google Scholar * Liu, H. D., Rodgers, N. D., Jiao, X. & Kiledjian, M. The scavenger mRNA decapping enzyme DcpS is a member of the
HIT family of pyrophosphatases. _EMBO J._ 21, 4699–4708 (2002). Article CAS PubMed PubMed Central Google Scholar * Charenton, C. et al. Structure of the active form of Dcp1–Dcp2
decapping enzyme bound to m7GDP and its Edc3 activator. _Nat. Struct. Mol. Biol._ 23, 982–986 (2016). Article CAS PubMed Google Scholar * Deshmukh, M. V. et al. mRNA decapping is
promoted by an RNA-binding channel in Dcp2. _Mol. Cell_ 29, 324–336 (2008). Article CAS PubMed Google Scholar * Goubau, D. et al. Antiviral immunity via RIG-I-mediated recognition of RNA
bearing 5′-diphosphates. _Nature_ 514, 372–375 (2014). Article CAS PubMed PubMed Central Google Scholar * Nallagatla, S. R. et al. 5′-triphosphate-dependent activation of PKR by RNAs
with short stem-loops. _Science_ 318, 1455–1458 (2007). Article CAS PubMed Google Scholar * De Gregorio, E., Preiss, T. & Hentze, M. W. Translational activation of uncapped mRNAs by
the central part of human eIF4G is 5′ end-dependent. _RNA_ 4, 828–836 (1998). Article PubMed PubMed Central Google Scholar * Wojtczak, B. A. et al. 5′-Phosphorothiolate dinucleotide cap
analogues: reagents for messenger RNA modification and potent small-molecular inhibitors of decapping enzymes. _J. Am. Chem. Soc._ 140, 5987–5999 (2018). Article CAS PubMed Google Scholar
* Kauffman, K. J. et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. _Nano Lett._ 15, 7300–7306
(2015). Article CAS PubMed Google Scholar * Holstein, J. M., Anhauser, L. & Rentmeister, A. Modifying the 5′-cap for click reactions of eukaryotic mRNA and to tune translation
efficiency in living cells. _Angew. Chem. Int. Ed._ 55, 10899–10903 (2016). Article CAS Google Scholar * Gebauer, F. & Hentze, M. W. Molecular mechanisms of translational control.
_Nat. Struct. Mol. Biol._ 5, 827–835 (2004). Article CAS Google Scholar * Weber, A. M. et al. A blue light receptor that mediates RNA binding and translational regulation. _Nat. Chem.
Biol._ 15, 1085–1092 (2019). Article CAS PubMed PubMed Central Google Scholar * Govan, J. M. et al. Optochemical control of RNA interference in mammalian cells. _Nucleic Acids Res._ 41,
10518–10528 (2013). Article CAS PubMed PubMed Central Google Scholar * Dhamodharan, V., Nomura, Y., Dwidar, M. & Yokobayashi, Y. Optochemical control of gene expression by
photocaged guanine and riboswitches. _Chem. Commun._ 54, 6181–6183 (2018). Article CAS Google Scholar * Shestopalov, I. A., Sinha, S. & Chen, J. K. Light-controlled gene silencing in
zebrafish embryos. _Nat. Chem. Biol._ 3, 650–651 (2007). Article CAS PubMed Google Scholar * Bardhan, A., Deiters, A. & Ettensohn, C. A. Conditional gene knockdowns in sea urchins
using caged morpholinos. _Dev. Biol._ 475, 21–29 (2021). Article CAS PubMed PubMed Central Google Scholar * Tang, X. J., Maegawa, S., Weinberg, E. S. & Dmochowski, I. J. Regulating
gene expression in zebrafish embryos using light-activated, negatively charged peptide nucleic acids. _J. Am. Chem. Soc._ 129, 11000–11001 (2007). Google Scholar * Keyhani, S., Goldau, T.,
Bluemler, A., Heckel, A. & Schwalbe, H. Chemo-enzymatic synthesis of position-specifically modified RNA for biophysical studies including light control and NMR spectroscopy. _Angew.
Chem. Int. Ed._ 57, 12017–12021 (2018). Article CAS Google Scholar * Hoernes, T. P. et al. Translation of non-standard codon nucleotides reveals minimal requirements for codon-anticodon
interactions. _Nat. Commun._ 9, 4865 (2018). Article PubMed PubMed Central CAS Google Scholar * Zhang, D., Jin, S., Piao, X. & Devaraj, N. K. Multiplexed photoactivation of mRNA
with single-cell resolution. _ACS Chem. Biol._ 15, 1773–1779 (2020). Article CAS PubMed PubMed Central Google Scholar * Ando, H., Furuta, T., Tsien, R. Y. & Okamoto, H.
Photo-mediated gene activation using caged RNA/DNA in zebrafish embryos. _Nat. Genet._ 28, 317–325 (2001). Article CAS PubMed Google Scholar * Ogasawara, S. Duration control of protein
expression in vivo by light-mediated reversible activation of translation. _ACS Chem. Biol._ 12, 351–356 (2017). Article CAS PubMed Google Scholar * Rotstan, K. A. et al. Regulation of
mRNA translation by a photoriboswitch. _eLife_ 9, e51737 (2020). Article CAS PubMed PubMed Central Google Scholar * Zhang, D., Zhou, C. Y., Busby, K. N., Alexander, S. C. & Devaraj,
N. K. Light-activated control of translation by enzymatic covalent mRNA labeling. _Angew. Chem. Int. Ed._ 57, 2822–2826 (2018). Article CAS Google Scholar * Ogasawara, S. Control of
cellular function by reversible photoregulation of translation. _ChemBioChem_ 15, 2652–2655 (2014). Article CAS PubMed Google Scholar * Mauger, D. M. et al. mRNA structure regulates
protein expression through changes in functional half-life. _Proc. Natl Acad. Sci. USA_ 116, 24075–24083 (2019). Article CAS PubMed PubMed Central Google Scholar * Kariko, K.,
Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. _Immunity_ 23,
165–175 (2005). Article CAS PubMed Google Scholar * Garcia, J. F. & Parker, R. MS2 coat proteins bound to yeast mRNAs block 5′ to 3′ degradation and trap mRNA decay products:
implications for the localization of mRNAs by MS2-MCP system. _RNA_ 21, 1393–1395 (2015). Article CAS PubMed PubMed Central Google Scholar * Anhauser, L. et al. A benzophenone-based
photocaging strategy for the N7 position of guanosine. _Angew. Chem. Int. Ed._ 59, 3161–3165 (2020). Article CAS Google Scholar * Rydzik, A. M. et al. Synthetic dinucleotide mRNA cap
analogs with tetraphosphate 5′,5′ bridge containing methylenebis(phosphonate) modification. _Org. Biomol. Chem._ 7, 4763–4776 (2009). Article CAS PubMed Google Scholar *
Grudzien-Nogalska, E. et al. Synthetic mRNAs with superior translation and stability properties. _Methods Mol. Biol._ 969, 55–72 (2013). Article CAS PubMed Google Scholar * Jemielity, J.
et al. Novel ‘anti-reverse’ cap analogs with superior translational properties. _RNA_ 9, 1108–1122 (2003). Article CAS PubMed PubMed Central Google Scholar * Aujard, I. et al.
_o_-Nitrobenzyl photolabile protecting groups with red-shifted absorption: syntheses and uncaging cross-sections for one- and two-photon excitation. _Chemistry_ 12, 6865–6879 (2006). Article
CAS PubMed Google Scholar * Bohacova, S. et al. Protected 5-(hydroxymethyl) uracil nucleotides bearing visible-light photocleavable groups as building blocks for polymerase synthesis of
photocaged DNA. _Org. Biomol. Chem._ 16, 1527–1535 (2018). Article CAS PubMed Google Scholar * Liu, W. et al. Structural basis for nematode eIF4E binding an m(2,2,7)G-Cap and its
implications for translation initiation. _Nucleic Acids Res._ 39, 8820–8832 (2011). Article CAS PubMed PubMed Central Google Scholar * Anhauser, L., Huwel, S., Zobel, T. &
Rentmeister, A. Multiple covalent fluorescence labeling of eukaryotic mRNA at the poly(A) tail enhances translation and can be performed in living cells. _Nucleic Acids Res._ 47, e42 (2019).
Article PubMed PubMed Central CAS Google Scholar * Kormann, M. S. et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. _Nat. Biotechnol._ 29,
154–157 (2011). Article CAS PubMed Google Scholar * Kariko, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity
and biological stability. _Mol. Ther._ 16, 1833–1840 (2008). Article CAS PubMed Google Scholar * Jia, L. et al. Decoding mRNA translatability and stability from the 5′ UTR. _Nat.
Struct. Mol. Biol._ 27, 814–821 (2020). Article CAS PubMed Google Scholar * van Dülmen, M., Muthmann, N. & Rentmeister, A. Chemo-enzymatic modification of the 5′ cap maintains
translation and increases immunogenic properties of mRNA. _Angew. Chem. Int. Ed._ 60, 13280–13286 (2021). Article CAS Google Scholar * Lucas, T. et al. Light-inducible antimiR-92a as a
therapeutic strategy to promote skin repair in healing-impaired diabetic mice. _Nat. Commun._ 8, 15162 (2017). Article PubMed PubMed Central Google Scholar * Chen, C. et al.
Dextran-conjugated caged siRNA nanoparticles for photochemical regulation of RNAi-induced gene silencing in cells and mice. _Bioconjug. Chem._ 30, 1459–1465 (2019). Article CAS PubMed
Google Scholar * Muthmann, N. et al. Combining chemical synthesis and enzymatic methylation to access short RNAs with various 5′ caps. _ChemBioChem_ 20, 1693–1700 (2019). Article CAS
PubMed PubMed Central Google Scholar * Holstein, J. M., Stummer, D. & Rentmeister, A. Enzymatic modification of 5′-capped RNA with a 4-vinylbenzyl group provides a platform for
photoclick and inverse electron-demand Diels-Alder reaction. _Chem. Sci._ 6, 1362–1369 (2015). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This project has
received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 772280; A.R.). We gratefully
acknowledge funding by the DFG (RE2796/7-1; A.R.). We thank TRON (Translational Oncology at the University Medical Center of the Johannes Gutenberg University Mainz) for providing us with
the HEK-NF-ĸB cell lines and D. Kümmel (Westfälische Wilhelms Universität Münster) for providing the human Rheb plasmid DNA. We thank A.-M. Dörner, H. Schepers, N. Kück, A. Bollu and M.
Dittmar for excellent technical and experimental assistance (all Westfälische Wilhelms Universität Münster). The NMR and mass measurements were supported by K. Bergander, the NMR and MS
facility of the Organic Chemistry Institute at the Westfälische Wilhelms Universität Münster. FUNDING Open access funding provided by Westfälische Wilhelms-Universität Münster. AUTHOR
INFORMATION Author notes * These authors contributed equally: Nils Klöcker, Florian P. Weissenboeck, Melissa van Dülmen, Petr Špaček. AUTHORS AND AFFILIATIONS * Institute of Biochemistry,
Westfälische Wilhelms-Universität Münster, Münster, Germany Nils Klöcker, Florian P. Weissenboeck, Melissa van Dülmen, Petr Špaček, Sabine Hüwel & Andrea Rentmeister Authors * Nils
Klöcker View author publications You can also search for this author inPubMed Google Scholar * Florian P. Weissenboeck View author publications You can also search for this author inPubMed
Google Scholar * Melissa van Dülmen View author publications You can also search for this author inPubMed Google Scholar * Petr Špaček View author publications You can also search for this
author inPubMed Google Scholar * Sabine Hüwel View author publications You can also search for this author inPubMed Google Scholar * Andrea Rentmeister View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS N.K. designed and performed the biochemical experiments. M.v.D. designed and performed the biomolecular and cell experiments.
F.P.W. and P.S. designed and performed the chemical syntheses. S.H. performed cell experiments. A.R. conceived and supervised the project. N.K., F.P.W., M.v.D., P.S. and A.R. evaluated the
data and discussed the results. N.K., F.P.W., M.v.D., P.S. and A.R. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Andrea Rentmeister. ETHICS DECLARATIONS COMPETING INTERESTS
P.S., N.K., F.W. and A.R. are the inventors on a European patent application (EP1184349.5, pending) of the University of Münster covering the synthesis and use of photo-cleavable 5′-cap
analogues as well as RNA molecules comprising photo-cleavable 5′-cap analogues. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemistry_ thanks Ivan Dmochowski and the other, anonymous,
reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 IRRADIATION STUDIES OF PHOTOCAGED GUANOSINES AND FLASHCAPS. Photo-cleavage reaction by the example of 5C (A).
Illustration of the decrease of caged guanosine (G) after irradiation with 365 nm (B), 405 nm (C) or 420 nm (D) and the decrease of FlashCaps after irradiation with 365 nm (E), 405 nm (F) or
420 nm (G) for various periods of time. The samples (500 µM) were analysed via HPLC after irradiation via LED (365 nm (142 mW/cm2), 405 nm (142 mW/cm2); 420 nm (60 mW/cm2)). The percentage
of uncaged guanosine or FlashCap was calculated by integration of the resulting peaks. Data points and error bars denote mean values ± standard deviation for _n_ = 3 independent replicates.
Source data EXTENDED DATA FIG. 2 IN-CELL TRANSLATION ASSAY SHOWING LUCIFERASE ACTIVITY OF FLASHCAP-GLUC-MRNAS NORMALIZED TO M7GPPPG-MRNA. The samples were either irradiated before
transfection (++), in cells (+) or left untreated (−). A, Measured and normalized luminescence values, which were obtained from the cell media of HeLa cells that were previously transfected
with differently capped GLuc-mRNA (containing m5C and m1Ψ) either irradiated or not irradiated in a 96 well plate. The _P_ value for 1(+) versus 1(−) is 4.66 × 10−4. The _P_ value for 2(+)
versus 2(-) is 5.70 × 10−5. The _P_ value for 2(++) versus 2(−) is 1.16 × 10−5. B, Measured and normalized luminescence values, which were obtained from the cell media of HEK293T cells that
were previously transfected with differently capped GLuc-mRNA and either irradiated or not irradiated in a 96 well plate. The _P_ value for 1(+) versus 1(−) is 3.57 × 10−3. The _P_ value for
2(+) versus 2(−) is 1.7 × 10−4. The _P_ value for 2(++) versus 2(−) is 4.3 × 10−4. C, Measured and normalized luminescence values, which were obtained from the cell media of HEK293T cells
that were previously transfected with differently capped GLuc-mRNA (containing m5C and m1Ψ) and either irradiated or not irradiated in a 96 well plate. The _P_ value for 1(+) versus 1(−) is
9.05 × 10−4. The _P_ value for 2(+) versus 2(−) is 9.32 × 10−4. The _P_ value for 2(++) versus 2(−) is 2.09 × 10−4. Statistical significance was determined by two-tailed t-test. Data and
error bars represent average and standard error of the mean of three independent (_n_ = 3) cell experiments. Significance-levels were defined as p < 0.05:*, p < 0.01:**, p <
0.001:***. Source data EXTENDED DATA FIG. 3 REPRESENTATIVE 630X MAGNIFICATION CONFOCAL MICROSCOPY IMAGES OF IRRADIATED (405 NM, 420 NM) AND NON-IRRADIATED HELA CELLS TRANSFECTED WITH EGFP-
AND MSCARLET-I-MRNA WITH DAPI STAINING. HeLa cells were transfected with differently capped eGFP-mRNA containing m5C and m1Ψ and m7GpppG-capped mScarlet-I-mRNA containing m5C and m1Ψ.
Untransfected cells served as control. ApppG-capped mRNA represents cap-independent translation. The m7GpppG-capped eGFP-mRNA (0) served as positive control. The NPM-(2) caged eGFP-mRNA was
either not irradiated or irradiated in cells (405 nm, 60 s/420 nm 180 s). The top two rows show the 630x magnification (63x objective) of the red channel (mScarlet-I) or the green channel
(eGFP) while the bottom two show the DAPI staining and DIC channel. Shown is one representative experiment of three independent experiments (_n_ = 3). Source data EXTENDED DATA FIG. 4
WESTERN BLOTS OF EGFP AND RHEB_EGFP. The eGFP, Rheb_eGFP and Nucleolin protein levels of HEK293T and HeLa cell samples transfected with either ApppG-, m7GpppG- or NPM-capped mRNA were
analyzed via Western blotting at 24 h post transfection. Irradiation of transfected cells was performed 4 h post transfection at 365 nm for 30 sec. As marker, the prestained PageRuler
(ThermoFisher) was used and as primary antibodies anti-nucleolin-antibody or anti-eGFP-antibody were used, respectively. Additionally, a HRP secondary antibody was used. Shown is one
representative gel from _n_ = 3 independent experiments. Source data SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–80, synthetic procedures, references for
synthetic procedures and Tables 1–4. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 3 Statistical source data SOURCE DATA FIG. 4 Statistical source data and unprocessed gels SOURCE DATA FIG.
5 Statistical source data and microscopy images SOURCE DATA FIG. 6 Statistical source data and microscopy images SOURCE DATA EXTENDED DATA FIG. 1 Statistical source Ddta SOURCE DATA
EXTENDED DATA FIG. 2 Statistical source data SOURCE DATA EXTENDED DATA FIG. 3 Microscopy images SOURCE DATA EXTENDED DATA FIG. 4 Unprocessed and uncropped western blots RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Klöcker, N.,
Weissenboeck, F.P., van Dülmen, M. _et al._ Photocaged 5′ cap analogues for optical control of mRNA translation in cells. _Nat. Chem._ 14, 905–913 (2022).
https://doi.org/10.1038/s41557-022-00972-7 Download citation * Received: 03 November 2021 * Accepted: 11 May 2022 * Published: 20 June 2022 * Issue Date: August 2022 * DOI:
https://doi.org/10.1038/s41557-022-00972-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative

:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)








