- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
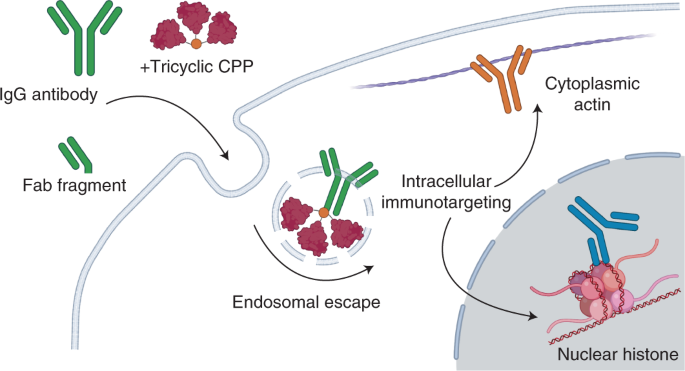
ABSTRACT The intracellular environment hosts a large number of cancer- and other disease-relevant human proteins. Targeting these with internalized antibodies would allow therapeutic
modulation of hitherto undruggable pathways, such as those mediated by protein–protein interactions. However, one of the major obstacles in intracellular targeting is the entrapment of
biomacromolecules in the endosome. Here we report an approach to delivering antibodies and antibody fragments into the cytosol and nucleus of cells using trimeric cell-penetrating peptides
(CPPs). Four trimers, based on linear and cyclic sequences of the archetypal CPP Tat, are significantly more potent than monomers and can be tuned to function by direct interaction with the
plasma membrane or escape from vesicle-like bodies. These studies identify a tricyclic Tat construct that enables intracellular delivery of functional immunoglobulin-G antibodies and Fab
fragments that bind intracellular targets in the cytosol and nuclei of live cells at effective concentrations as low as 1 μM. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more
Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS:
* Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TUMOR-SPECIFIC INTRACELLULAR DELIVERY: PEPTIDE-GUIDED
TRANSPORT OF A CATALYTIC TOXIN Article Open access 17 January 2023 PEPTIDES AS MULTIFUNCTIONAL PLAYERS IN CANCER THERAPY Article Open access 01 June 2023 COMPARISON OF ANTIBODY-SCTRAIL FC
FUSION PROTEINS WITH VARYING VALENCY FOR EGFR AND TRAIL RECEPTORS Article Open access 06 May 2025 DATA AVAILABILITY All the data supporting the findings of this study are available within
the Article, the Supplementary Information or the source data. The data are also available from the corresponding authors upon reasonable request. Source data are provided with this paper.
REFERENCES * Carter, P. J. & Lazar, G. A. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. _Nat. Rev. Drug Discov._ 17, 197–223 (2018). Article CAS PubMed Google
Scholar * Verdine, G. L. & Walensky, L. D. The challenge of drugging undruggable targets in cancer: lessons learned from targeting BCL-2 family members. _Clin. Cancer Res._ 13,
7264–7270 (2007). Article CAS PubMed Google Scholar * Stewart, M. P. et al. In vitro and ex vivo strategies for intracellular delivery. _Nature_ 538, 183–192 (2016). Article CAS PubMed
Google Scholar * Samal, S. K. et al. Cationic polymers and their therapeutic potential. _Chem. Soc. Rev._ 41, 7147–7194 (2012). Article CAS PubMed Google Scholar * Brooks, H., Lebleu,
B. & Vivès, E. Tat peptide-mediated cellular delivery: back to basics. _Adv. Drug Del. Rev._ 57, 559–577 (2005). Article CAS Google Scholar * Peraro, L. & Kritzer, J. A. Emerging
methods and design principles for cell-penetrant peptides. _Angew. Chem. Int. Ed._ 57, 11868–11881 (2018). Article CAS Google Scholar * Pei, D. & Buyanova, M. Overcoming endosomal
entrapment in drug delivery. _Bioconjug. Chem._ 30, 273–283 (2019). Article CAS PubMed Google Scholar * Fu, A., Tang, R., Hardie, J., Farkas, M. E. & Rotello, V. M. Promises and
pitfalls of intracellular delivery of proteins. _Bioconjug. Chem._ 25, 1602–1608 (2014). Article CAS PubMed PubMed Central Google Scholar * Fawell, S. et al. Tat-mediated delivery of
heterologous proteins into cells. _Proc. Natl Acad. Sci. USA_ 91, 664–668 (1994). Article CAS PubMed PubMed Central Google Scholar * Cornelissen, B. et al. Imaging DNA damage in vivo
using γH2AX-targeted immunoconjugates. _Cancer Res._ 71, 4539–4549 (2011). Article CAS PubMed PubMed Central Google Scholar * Singh, K., Ejaz, W., Dutta, K. & Thayumanavan, S.
Antibody delivery for intracellular targets: emergent therapeutic potential. _Bioconjug. Chem_ 30, 1028–1041 (2019). Article CAS PubMed PubMed Central Google Scholar * Brock, R. The
uptake of arginine-rich cell-penetrating peptides: putting the puzzle together. _Bioconj. Chem._ 25, 863–868 (2014). Article CAS Google Scholar * Madani, F., Lindberg, S., Langel, U.,
Futaki, S. & Gräslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. _J. Biophys._ 2011, 414729 (2011). Article PubMed PubMed Central Google Scholar * Dougherty, P.
G., Sahni, A. & Pei, D. Understanding cell penetration of cyclic peptides. _Chem. Rev._ 119, 10241–10287 (2019). Article CAS PubMed PubMed Central Google Scholar * Milletti, F.
Cell-penetrating peptides: classes, origin and current landscape. _Drug Discov. Today_ 17, 850–860 (2012). Article CAS PubMed Google Scholar * Nischan, N. et al. Covalent attachment of
cyclic TAT peptides to GFP results in protein delivery into live cells with immediate bioavailability. _Angew. Chem. Int. Ed._ 54, 1950–1953 (2015). Article CAS Google Scholar * Herce, H.
D. et al. Cell-permeable nanobodies for targeted immunolabelling and antigen manipulation in living cells. _Nat. Chem._ 9, 762–771 (2017). Article CAS PubMed Google Scholar * Schneider,
A. F. L., Kithil, M., Cardoso, M. C., Lehmann, M. & Hackenberger, C. P. R. Cellular uptake of large biomolecules enabled by cell-surface-reactive cell-penetrating peptide additives.
_Nat. Chem._ 13, 530–539 (2021). Article CAS PubMed Google Scholar * Akishiba, M. et al. Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. _Nat. Chem._ 9, 751–761
(2017). Article CAS PubMed Google Scholar * Ovacik, M. & Lin, K. Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. _Clin. Transl. Sci._
11, 540–552 (2018). Article PubMed PubMed Central Google Scholar * Kauffman, W. B., Fuselier, T., He, J. & Wimley, W. C. Mechanism matters: a taxonomy of cell penetrating peptides.
_Trends Biochem. Sci._ 40, 749–764 (2015). Article CAS PubMed PubMed Central Google Scholar * Herce, H. D. & Garcia, A. E. Molecular dynamics simulations suggest a mechanism for
translocation of the HIV-1 TAT peptide across lipid membranes. _Proc. Natl Acad. Sci. USA_ 104, 20805–20810 (2007). Article CAS PubMed PubMed Central Google Scholar * Lawrence, M. S.,
Phillips, K. J. & Liu, D. R. Supercharging proteins can impart unusual resilience. _J. Am. Chem. Soc._ 129, 10110–10112 (2007). Article CAS PubMed PubMed Central Google Scholar *
Cronican, J. J. et al. Potent delivery of functional proteins into mammalian cells in vitro and in vivo using a supercharged protein. _ACS Chem. Biol._ 5, 747–752 (2010). Article CAS
PubMed PubMed Central Google Scholar * Freire, J. M., Almeida Dias, S., Flores, L., Veiga, A. S. & Castanho, M. A. R. B. Mining viral proteins for antimicrobial and cell-penetrating
drug delivery peptides. _Bioinformatics_ 31, 2252–2256 (2015). Article CAS PubMed Google Scholar * Tung, C.-H., Mueller, S. & Weissleder, R. Novel branching membrane translocational
peptide as gene delivery vector. _Biorg. Med. Chem._ 10, 3609–3614 (2002). Article CAS Google Scholar * Angeles-Boza, A. M., Erazo-Oliveras, A., Lee, Y.-J. & Pellois, J.-P. Generation
of endosomolytic reagents by branching of cell-penetrating peptides: tools for the delivery of bioactive compounds to live cells in _cis_ or _trans_. _Bioconjug. Chem._ 21, 2164–2167
(2010). Article CAS PubMed PubMed Central Google Scholar * Fu, J., Yu, C., Li, L. & Yao, S. Q. Intracellular delivery of functional proteins and native drugs by cell-penetrating
poly(disulfide)s. _J. Am. Chem. Soc._ 137, 12153–12160 (2015). Article CAS PubMed Google Scholar * Erazo-Oliveras, A. et al. Protein delivery into live cells by incubation with an
endosomolytic agent. _Nat. Methods_ 11, 861–867 (2014). Article CAS PubMed PubMed Central Google Scholar * Erazo-Oliveras, A. et al. The late endosome and its lipid BMP act as gateways
for efficient cytosolic access of the delivery agent dfTAT and its macromolecular cargos. _Cell Chem. Biol._ 23, 598–607 (2016). Article CAS PubMed PubMed Central Google Scholar *
Najjar, K. et al. Unlocking endosomal entrapment with supercharged arginine-rich peptides. _Bioconj. Chem._ 28, 2932–2941 (2017). Article CAS Google Scholar * Kez, C., Lin, H., Peter, D.
W. & Arwyn, T. J. Endocytosis, intracellular traffic and fate of cell penetrating peptide based conjugates and nanoparticles. _Curr. Pharm. Des._ 19, 2878–2894 (2013). Article Google
Scholar * Jonkman, J., Brown, C. M., Wright, G. D., Anderson, K. I. & North, A. J. Tutorial: guidance for quantitative confocal microscopy. _Nat. Protoc._ 15, 1585–1611 (2020). Article
CAS PubMed Google Scholar * Herbert, A. GDSC colocalisation plugins http://www.sussex.ac.uk/gdsc/intranet/microscopy/UserSupport/AnalysisProtocol/imagej/colocalisation (2013). * Costes,
S. V. et al. Automatic and quantitative measurement of protein-protein colocalization in live cells. _Biophys. J._ 86, 3993–4003 (2004). Article CAS PubMed PubMed Central Google Scholar
* Ramirez, O., García, A., Rojas, R., Couve, A. & Härtel, S. Confined displacement algorithm determines true and random colocalization in fluorescence microscopy. _J. Microsc._ 239,
173–183 (2010). Article CAS PubMed Google Scholar * Söderberg, O. et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. _Nat. Methods_ 3,
995–1000 (2006). Article PubMed Google Scholar * Rouet, R. et al. Receptor-mediated delivery of CRISPR-Cas9 endonuclease for cell-type-specific gene editing. _J. Am. Chem. Soc._ 140,
6596–6603 (2018). Article CAS PubMed PubMed Central Google Scholar * Qian, Z. et al. Discovery and mechanism of highly efficient cyclic cell-penetrating peptides. _Biochemistry_ 55,
2601–2612 (2016). Article CAS PubMed Google Scholar * Liu, H., Gaza-Bulseco, G., Faldu, D., Chumsae, C. & Sun, J. Heterogeneity of monoclonal antibodies. _J. Pharm. Sci._ 97,
2426–2447 (2008). Article CAS PubMed Google Scholar * Delavoie, F., Soldan, V., Rinaldi, D., Dauxois, J. Y. & Gleizes, P. E. The path of pre-ribosomes through the nuclear pore
complex revealed by electron tomography. _Nat. Commun._ 10, 497 (2019). Article CAS PubMed PubMed Central Google Scholar * Paci, G., Zheng, T., Caria, J., Zilman, A. & Lemke, E. A.
Molecular determinants of large cargo transport into the nucleus. _eLife_ 9, e55963 (2020). Article CAS PubMed PubMed Central Google Scholar * Avrameas, A., Ternynck, T., Nato, F.,
Buttin, G. & Avrameas, S. Polyreactive anti-DNA monoclonal antibodies and a derived peptide as vectors for the intracytoplasmic and intranuclear translocation of macromolecules. _Proc.
Natl Acad. Sci. USA_ 95, 5601–5606 (1998). Article CAS PubMed PubMed Central Google Scholar * Gordon, R. E., Nemeth, J. F., Singh, S., Lingham, R. B. & Grewal, I. S. Harnessing SLE
autoantibodies for intracellular delivery of biologic therapeutics. _Trends Biotechnol._ 39, 298–310 (2021). Article CAS PubMed Google Scholar * Bernardes, N. E. & Chook, Y. M.
Nuclear import of histones. _Biochem. Soc. Trans._ 48, 2753–2767 (2020). Article CAS PubMed PubMed Central Google Scholar * Placek, B. J., Harrison, L. N., Villers, B. M. & Gloss,
L. M. The H2A.Z/H2B dimer is unstable compared to the dimer containing the major H2A isoform. _Protein Sci._ 14, 514–522 (2005). Article CAS PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS We thank R. S. Wilson and C. Lang at the Department of Physiology, Anatomy and Genetics, Oxford University, for assistance with microscopy and L. Ittner
and M. Gill for helpful discussions. We acknowledge funding support from Cancer Research UK (CRUK, C5255/A15935), a CRUK grant (C5255/A18085) through the CRUK Oxford Centre, the Medical
Research Council (MC_PC_12004) and the Engineering and Physical Sciences Research Council (EPSRC) Oxford Centre for Drug Delivery Devices (EP/L024012/1). This work has also received support
from the Wellcome Trust (grant no. 106169). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, UK
Ole Tietz, Fernando Cortezon-Tamarit, Sarah Able & Katherine A. Vallis * Centre for Medicines Discovery, University of Oxford, Oxford, UK Rod Chalk Authors * Ole Tietz View author
publications You can also search for this author inPubMed Google Scholar * Fernando Cortezon-Tamarit View author publications You can also search for this author inPubMed Google Scholar *
Rod Chalk View author publications You can also search for this author inPubMed Google Scholar * Sarah Able View author publications You can also search for this author inPubMed Google
Scholar * Katherine A. Vallis View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS O.T. designed, conceived and synthesized the Tat trimers,
designed, conceived and acquired microscopy studies, performed data analysis and wrote the manuscript. F.C.-T. acquired and analysed microscopy data and performed the PLA assay. S.A.
synthesized the IgG and Fab conjugates. R.C. carried out mass spectrometry of the Tat trimers. K.A.V. contributed to conception and design, data analysis and acquired funding and supervised
the study. All authors reviewed and revised the final manuscript. CORRESPONDING AUTHOR Correspondence to Katherine A. Vallis. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemistry_ thanks Wouter Verdurmen and the other, anonymous, reviewer(s) for their contribution to the peer review of this
work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED
DATA FIG. 1 MEMBRANE POROSITY FOLLOWING TREATMENT WITH TAT-TRIMER. (A, B) Addition of 40 μM propidium iodide (PI) 20 min after addition of 1 μM trimer; image at 30 min after start of
experiment. Cells treated with tri-Tat A (A) co-stain with PI; cells treated with tri-cTat B (B) are PI negative. (C) Average fluorescence intensity of PI per cell, 45 min after the start of
the experiment (n = 25). Cells treated with tri-Tat A show significantly higher PI uptake, indicative of pore formation. (D) Cells treated with tri-Tat A (solid line) or tri-cTat B (dotted
line) for 60 min and metabolic activity as an indicator of cell viability assessed using MTT assay after 1 h, 2 h, 4 h, 3 days (n = 3 biologically independent experiments). Data presented as
mean ± standard deviation. Scale bar: 20 μm. Source data SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supporting Information. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1
Statistical source data for the main figures and Extended Data figures. SOURCE DATA FIG. 2 Statistical source data for the main figures and Extended Data figures. SOURCE DATA FIG. 3
Statistical source data for the main figures and Extended Data figures. SOURCE DATA FIG. 4 Statistical source data for the main figures and Extended Data figures. SOURCE DATA FIG. 6
Statistical Source Data for main Figures and Extended Data Figures SOURCE DATA EXTENDED DATA FIG. 1 Statistical source data for the main figures and Extended Data figures. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tietz, O., Cortezon-Tamarit, F., Chalk, R. _et al._ Tricyclic cell-penetrating peptides for efficient delivery of
functional antibodies into cancer cells. _Nat. Chem._ 14, 284–293 (2022). https://doi.org/10.1038/s41557-021-00866-0 Download citation * Received: 16 September 2019 * Accepted: 19 November
2021 * Published: 10 February 2022 * Issue Date: March 2022 * DOI: https://doi.org/10.1038/s41557-021-00866-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative