- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
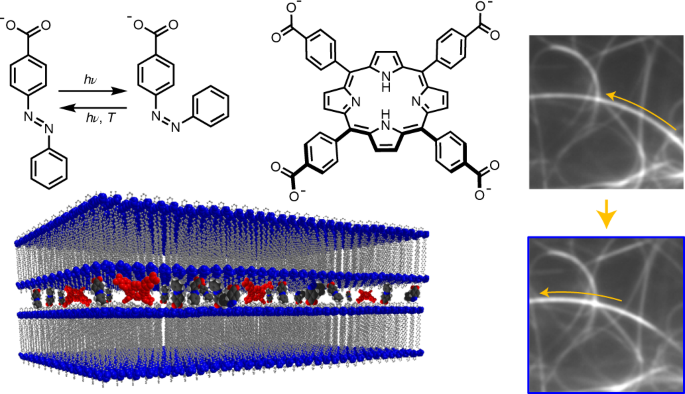
ABSTRACT The micron-scale movement of biomolecules along supramolecular pathways, mastered by nature, is a remarkable system requiring strong yet reversible interactions between components
under the action of a suitable stimulus. Responsive microscopic systems using a variety of stimuli have demonstrated impressive relative molecular motion. However, locating the position of a
movable object that travels along self-assembled fibres under an irresistible force has yet to be achieved. Here, we describe a purely supramolecular system where a molecular ‘traveller’
moves along a ‘path’ over several microns when irradiated with visible light. Real-time imaging of the motion in the solvated state using total internal reflection fluorescence microscopy
shows that anionic porphyrin molecules move along the fibres of a bis-imidazolium gel upon irradiation. Slight solvent changes mean movement and restructuring of the fibres giving
microtoroids, indicating control of motion by fibre mechanics with solvent composition. The insight provided here may lead to the development of artificial travellers that can perform
catalytic and other functions. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal
Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS SELF-REGULATED NON-RECIPROCAL MOTIONS IN SINGLE-MATERIAL MICROSTRUCTURES Article 04 May 2022 EXERTING PULLING FORCES IN FLUIDS BY DIRECTIONAL
DISASSEMBLY OF MICROCRYSTALLINE FIBRES Article Open access 29 July 2024 PROPAGATING WAVE IN A FLUID BY COHERENT MOTION OF 2D COLLOIDS Article Open access 19 November 2021 DATA AVAILABILITY
All data supporting the findings are included in the manuscript and Supplementary Information. REFERENCES * Sasaki, K., Kaya, M. & Higuchi, H. A unified walking model for dimeric motor
proteins. _Biophys. J._ 115, 1981–1992 (2018). Article CAS Google Scholar * Kassem, S. et al. Artificial molecular motors. _Chem. Soc. Rev._ 46, 2592–2621 (2017). Article CAS Google
Scholar * Baroncini, M., Silvi, S. & Credi, A. Photo- and redox-driven artificial molecular motors. _Chem. Rev._ 120, 200–268 (2020). Article CAS Google Scholar * Pezzato, C., Cheng,
C., Stoddart, J. F. & Astumian, R. D. Mastering the non-equilibrium assembly and operation of molecular machines. _Chem. Soc. Rev._ 46, 5491–5507 (2017). Article CAS Google Scholar *
García-López, V. et al. Molecular machines open cell membranes. _Nature_ 548, 567–572 (2017). Article Google Scholar * Dattler, D. et al. Design of collective motions from synthetic
molecular switches, rotors, and motors. _Chem. Rev._ 120, 310–433 (2020). Article CAS Google Scholar * Haq, S. et al. A small molecule walks along a surface between porphyrin fences that
are assembled in situ. _Angew. Chem. Int. Ed._ 54, 7101–7105 (2015). Article CAS Google Scholar * Abbasi-Pérez, D. et al. Controlling the preferential motion of chiral molecular walkers
on a surface. _Chem. Sci._ 10, 5864–5874 (2019). Article Google Scholar * Kudernac, T. et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface. _Nature_
479, 208–211 (2011). Article CAS Google Scholar * Xing, Y., Liu, B., Chao, J. & Wang, L. DNA-based nanoscale walking devices and their applications. _RSC Adv._ 7, 47425–47434 (2017).
Article CAS Google Scholar * Samperi, M., Pérez-García, L. & Amabilino, D. B. Quantification of energy of activation to supramolecular nanofibre formation reveals enthalpic and
entropic effects and morphological consequence. _Chem. Sci._ 10, 10256–10266 (2019). Article CAS Google Scholar * Samperi, M., Limón, D., Amabilino, D. B. & Pérez-García, L. Enhancing
singlet oxygen generation by self-assembly of a porphyrin entrapped in supramolecular fibres. _Cell Rep. Phys. Sci._ 1, 100030 (2020). Article Google Scholar * Bandara, H. M. D. &
Burdette, S. C. Photoisomerization in different classes of azobenzene. _Chem. Soc. Rev._ 41, 1809–1825 (2012). Article CAS Google Scholar * Kathan, M. & Hecht, S. Photoswitchable
molecules as key ingredients to drive systems away from the global thermodynamic minimum. _Chem. Soc. Rev._ 46, 5536–5550 (2017). Article CAS Google Scholar * Tecilla, P. & Bonifazi,
D. Configurational selection in azobenzene‐based supramolecular systems through dual‐stimuli processes. _ChemistryOpen_ 9, 538–553 (2020). Article CAS Google Scholar * Mattheyses, A. L.,
Simon, S. M. & Rappoport, J. Z. Imaging with total internal reflection fluorescence microscopy for the cell biologist. _J. Cell Sci._ 123, 3621–3628 (2010). Article CAS Google Scholar
* Canevet, D., del Pino, A. P., Amabilino, D. B. & Salle, M. Varied nanostructures from a single multifunctional molecular material. _J. Mater. Chem._ 21, 1428–1437 (2011). Article
CAS Google Scholar * Kim, Y., Li, W., Shin, S. & Lee, M. Development of toroidal nanostructures by self-assembly: rational designs and applications. _Acc. Chem. Res._ 46, 2888–2897
(2013). Article CAS Google Scholar * Datta, S. et al. Self-assembled poly-catenanes from supramolecular toroidal building blocks. _Nature_ 583, 400–405 (2020). Article CAS Google
Scholar * Carl, N., Müller, W., Schweins, R. & Huber, K. Controlling self-assembly with light and temperature. _Langmuir_ 36, 223–231 (2020). Article CAS Google Scholar * Limón, D.
et al. Microscale coiling in bis-imidazolium supramolecular hydrogel fibres induced by the release of a cationic serine protease inhibitor. _Chem. Commun._ 3, 4509–4512 (2017). Article
Google Scholar * Cao, H., Jiang, J., Zhu, X., Duan, P. & Liu, M. Hierarchical co-assembly of chiral lipid nanotubes with an azobenzene derivative: optical and chiroptical switching.
_Soft Matter_ 7, 4654–4660 (2011). Article CAS Google Scholar * Bortolus, P. & Monti, S. Cis-trans photoisomerization of azobenzene. Solvent and triplet donors effects. _J. Phys.
Chem._ 83, 648–652 (1979). Article CAS Google Scholar * Nath, N. K., Panda, M. K., Sahoo, S. C. & Naumov, P. Thermally induced and photoinduced mechanical effects in molecular single
crystals—a revival. _CrystEngComm_ 16, 1850–1858 (2014). Article CAS Google Scholar * Pilz da Cunha, M., van Thoor, E. A. J., Debije, M. G., Broer, D. J. & Schenning, A. P. H. J.
Unravelling the photothermal and photomechanical contributions to actuation of azobenzene-doped liquid crystal polymers in air and water. _J. Mater. Chem. C_ 7, 13502–13509 (2019). Article
CAS Google Scholar * Fredy, J. W. Molecular photoswitches mediating the strain-driven disassembly of supramolecular tubules. _Proc. Natl Acad. Sci. USA_ 114, 11850–11855 (2017). Article
CAS Google Scholar * Sathyanarayanan, G. et al. Drug-loaded supramolecular gels prepared in a microfluidic platform: distinctive rheology and delivery through controlled
far-from-equilibrium mixing. _ACS Omega_ 2, 8849–8858 (2017). Article CAS Google Scholar * Kubota, R., Nakamura, K., Torigoe, S. & Hamachi, I. The power of confocal laser scanning
microscopy in supramolecular chemistry: in situ real-time imaging of stimuli-responsive multicomponent supramolecular hydrogels. _ChemistryOpen_ 9, 67–79 (2020). Article CAS Google Scholar
* Kageyama, Y. Light-powered self-sustainable macroscopic motion for the active locomotion of materials. _ChemPhotoChem_ 3, 327–336 (2019). Article CAS Google Scholar * Aprahamian, I.
The future of molecular machines. _ACS Cent. Sci._ 6, 347–358 (2020). Article CAS Google Scholar * Zheng, L. et al. Catalytic transport of molecular cargo using diffusive binding along a
polymer track. _Nat. Chem._ 11, 359–366 (2019). Article CAS Google Scholar * Muller-Deku, A. et al. Photdoswitchable paclitaxel-based microtubule stabilisers allow optical control over
the microtubule cytoskeleton. _Nat. Commun._ 11, 4640 (2020). Article Google Scholar * Casal-Dujat, L. et al. Gemini imidazolium amphiphiles for the synthesis, stabilization, and drug
delivery from gold nanoparticles. _Langmuir_ 28, 2368–2381 (2012). Article CAS Google Scholar * Datta-Gupta, N. & Bardos, T. J. Synthetic porphyrins. I. Synthesis and spectra of some
_para_-substituted _meso_-tetraphenylporphines. _J. Heterocycl. Chem._ 3, 495–502 (1966). Article CAS Google Scholar * Schindelin, J. et al. Fiji: an open-source platform for
biological-image analysis. _Nat. Methods_ 9, 676–682 (2012). Article CAS Google Scholar * Laine, R. F. et al. NanoJ: a high-performance open-source super-resolution microscopy toolbox.
_J. Phys. D-Appl. Phys._ 52, 163001 (2019). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS All the authors thank the School of Life Sciences Imaging (SLIM) in Nottingham
for access to the optical microscope and the Nanoscale and Microscale Research Centre (nmRC) for facilitating access to electron microscopes. D.B.A. thanks the Telluride Conference on
Molecular Rotors, Motors, and Switches for inspiring this research. We warmly thank M. Amabilino i Pérez for assistance with graphics (D.B.A.). A.R.M. acknowledges funding from the European
Union’s Horizon 2020 research and innovation programme under Marie–Skłodowska–Curie grant agreement no. 793424. The microscope facility was established using the BB/L013827/1 fund. This work
was supported by the Engineering and Physical Sciences Research Council (EPSRC) under grants EP/M005178/1 and EP/N024818/1 (D.B.A.), EU ERDF (FEDER) funds, Spanish Government grants
TEC2017-85059-C3-2-R and PID2020-115663GB-C3-2 (L.P.-G.) and the University of Nottingham (B.B., M.S.) including work under the Anne McLaren fellowship scheme (L.P.-G.) and the Propulsion
Futures Beacon of Excellence (D.B.A.). AUTHOR INFORMATION Author notes * Mario Samperi Present address: Istituto di Tecnologie Avanzate per l’Energia “Nicola Giordano” - CNR-ITAE, Messina,
Italy AUTHORS AND AFFILIATIONS * School of Pharmacy, University of Nottingham, Nottingham, United Kingdom Mario Samperi & Lluïsa Pérez-García * School of Chemistry, GSK Carbon Neutral
Laboratories for Sustainable Chemistry, University of Nottingham, Nottingham, United Kingdom Mario Samperi, Bilel Bdiri, Charlotte D. Sleet, Ajith R. Mallia & David B. Amabilino * SLIM
Imaging Unit, School of Life Sciences, University of Nottingham, Nottingham, United Kingdom Robert Markus * Departament de Farmacologia, Toxicologia i Química Terapèutica, Universitat de
Barcelona, Barcelona, Spain Lluïsa Pérez-García * Institut de Nanociència i Nanotecnologia IN2UB, Universitat de Barcelona, Barcelona, Spain Lluïsa Pérez-García Authors * Mario Samperi View
author publications You can also search for this author inPubMed Google Scholar * Bilel Bdiri View author publications You can also search for this author inPubMed Google Scholar * Charlotte
D. Sleet View author publications You can also search for this author inPubMed Google Scholar * Robert Markus View author publications You can also search for this author inPubMed Google
Scholar * Ajith R. Mallia View author publications You can also search for this author inPubMed Google Scholar * Lluïsa Pérez-García View author publications You can also search for this
author inPubMed Google Scholar * David B. Amabilino View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Author contributions are defined based
on the CRediT (Contributor Roles Taxonomy) and listed alphabetically. Conceptualization: D.B.A. and L.P.-G. Data curation: M.S. Formal analysis: M.S., D.B.A. and L.P.-G. Funding acquisition:
D.B.A. and L.P.-G. Investigation: M.S., B.B., A.R.M., R.M. and C.D.S. Methodology: D.B.A., M.S., R.M. and L.P.-G. Project administration: D.B.A. and L.P.-G. Supervision: D.B.A. and L.P.-G.
Validation: D.B.A. and L.P.-G. Writing original draft: M.S., D.B.A. and L.P.-G. Writing review and editing: M.S., L.P.-G. and D.B.A. CORRESPONDING AUTHOR Correspondence to David B.
Amabilino. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Chemistry_ thanks the anonymous
reviewers for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 GEL FORMATION. (A) A picture of gel samples of 1·2Br obtained with water:ethanol ratio 5:5 without guest molecules (Gel), with incorporated
azobenzene (Gel@Azo), porphyrin (Gel@TCPP) and both (Gel@TCPP@Azo). The scale bar represents 1 cm. Gel samples were always prepared by addition of MilliQ water to an ethanolic solution of
1·2Br, giving a final amphiphile concentration of 8 mM. For TCPP-containing gels, aqueous solutions of the TCPP sodium salt were prepared by dispersing the desired amount of solid TCPP in
water followed by the addition of 4 equivalents of sodium hydroxide (from a 0.1 M stock solution). For AZO-containing gels, an ethanolic solution of 4-(phenylazo)benzoic acid (AZO)was
premixed with 1·2Br, and the equimolar equivalent of sodium hydroxide was added in water in order to incorporate the AZO as its sodium salt. The final concentration of TCPP and AZO within
the gels was always 60 μM and 2 mM, respectively. (B) Gelation time of the samples at room temperature (approximately 22 °C) obtained with solvent ratio 5:5 (blue bars) and 7:3 (red bars).
EXTENDED DATA FIG. 2 RHEOLOGY OF THE MULTICOMPONENT GELS. Shear stress profiles displaying storage (G’, black curve) and loss (G”, red curve) moduli obtained for gels in water-ethanol ratio
5:5 (top box) and 7:3 (bottom box). EXTENDED DATA FIG. 3 X-RAY POWDER DIFFRACTOGRAMS OF XEROGELS. X-ray powder diffractograms of xerogels made from gel with no guests (black lines), Gel@TCPP
(red lines), Gel@Azo (green lines) and Gel@TCPP@Azo (blue lines) in water-ethanol ratio 5:5 (A) and 7:3 (B). EXTENDED DATA FIG. 4 ISOMERISATION OF AZO IN SOLUTION AND IN THE MULTICOMPONENT
GEL. UV-Visible absorption spectra of: Top. AZO 50 μM in homogeneous ethanol solution as prepared (black line) and sequential irradiation under light at 405 and 365 nm, and; Below.
Gel@TCPP@Azo in 5:5 water:ethanol under light irradiation at 405 and 365 nm. EXTENDED DATA FIG. 5 WAVELENGTH DEPENDENCE OF MOLECULAR MOTION. TIRF images of Gel@TCPP@Azo in water-ethanol 5:5
before (A-B-C-D) and after (E-F-G-H) irradiation (for a total of 8.3 minutes) with light at 405, 488, 561, 642 nm in the central region. Scale bar represents 10 μm. The difference maps below
show the areas where changes of intensity can be appreciated (note that the white and black intensities on the four maps have different intensities and do not indicate amount of change).
Some fibres in the irradiated area are darker in the difference map (so there is more porphyrin after irradiation). Those fibres are apparently above the focal plane, deeper into the sample
compared with the layer imaged. In principle, this can occur because laser intensity is higher at the slide-sample interface, then decreases as we look deeper into the sample. It is evidence
for movement of TCPP into the sample as well as to the sides of the irradiated area. EXTENDED DATA FIG. 6 RING FORMATION FROM FIBRES. Zoomed SRRF images of Gel@TCPP@Azo in water-ethanol 7:3
during irradiation at 405 nm. The frames are taken from Supplementary Video 9. Scale bar represents 2 μm, all images are to the same scale. EXTENDED DATA FIG. 7 TCPP FLUORESCENCE IN THE
GELS. The steady state fluorescence spectra (excitation wavelength 405 nm) in both 5:5 and 7:3 water:ethanol gels show (top row) that there is a quenching of the porphyrin fluorescence in
Gel@TCPP@Azo compared with Gel@TCPP for both solvent mixtures, as a result of energy transfer from the TCPP to the AZO chromophore. The middle row shows the time evolution of the same
Gel@TCPP samples under continuous irradiation at 405 nm, where a modest bleaching is observed over time for both solvent systems. The bottom row shows the evolution of the fluorescence of
Gel@TCPP@Azo under continuous irradiation, where at 5:5 water:ethanol a slight bleaching is observed (similar to Gel@TCPP), while for the 7:3 mixture an increase in fluorescence is observed,
so that after 60 minutes the intensity is actually higher than that for Gel@TCPP after the same time. EXTENDED DATA FIG. 8 ISOMERISATION OF A MAJORITY OF THE CIS-AZO ENHANCES MOTION. TIRF
micrographs from two regions at time zero with irradiation at 405 nm (top) and after the end of Supplementary Movie 13 (left) and Supplementary Video 15 (right) of sample Gel@TCPP@Azo where
AZO was enriched in the _cis_ isomer by photoisomerization prior to gel preparation. Scale bar represents 5 μm on the left hand series and 2 μm in the right hand series, all images in a
series are to the same scale. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION List of videos, Supplementary Figs. S1–S19, Tables S1–S6 and Synthetic Procedures and Characterization.
SUPPLEMENTARY VIDEO 1 TIRF video of an irradiation experiment performed on sample Gel@TCPP@Azo obtained in water:ethanol ratio 5:5. Real time 8.3 min, 20 frames per second (fps).
SUPPLEMENTARY VIDEO 2 TIRF video of an irradiation experiment performed on sample Gel@TCPP@Azo obtained in water:ethanol ratio 7:3. Real time 8.3 min, 20 fps. SUPPLEMENTARY VIDEO 3 TIRF
video of the central irradiation experiment performed on sample Gel@TCPP@Azo obtained in water:ethanol ratio 5:5. Real time 8.3 min, 20 fps. SUPPLEMENTARY VIDEO 4 TIRF video of the central
irradiation experiment performed on sample Gel@TCPP@Azo obtained in water:ethanol ratio 6:4. Real time 8.3 min, 20 fps. SUPPLEMENTARY VIDEO 5 Video of an irradiation experiment performed on
sample Gel@TCPP@Azo 5:5 using light at 405 nm. Real time 8.3 min, 20 fps. SUPPLEMENTARY VIDEO 6 Video of the irradiation experiment performed on sample Gel@TCPP@Azo 5:5 using light at 488
nm. Real time 5 min, 10 fps. SUPPLEMENTARY VIDEO 7 Video of the irradiation experiment performed on sample Gel@TCPP@Azo 5:5 using light at 561 nm. Real time 8.3 min, 20 fps. SUPPLEMENTARY
VIDEO 8 Video of the irradiation experiment performed on sample Gel@TCPP@Azo 5:5 using light at 642 nm. Real time 8.2 min, 20 fps. SUPPLEMENTARY VIDEO 9 SRRF video of the irradiation
experiment performed on sample Gel@TCPP@Azo obtained in water:ethanol ratio 7:3. Real time 1.7 min, 10 fps. SUPPLEMENTARY VIDEO 10 TIRF video of the irradiation experiment performed on
sample Gel@TCPP obtained in water:ethanol ratio 5:5. Real time 8.3 min, 10 fps. SUPPLEMENTARY VIDEO 11 TIRF video of the irradiation experiment performed on sample Gel@TCPP obtained in
water:ethanol ratio 7:3. Real time 1.7 min, 10 fps. SUPPLEMENTARY VIDEO 12 TIRF video of the irradiation experiment performed on sample Gel@TCPP@AzoH (prepared from the sodium salt of TCPP
but with no additional base to deprotonate the AZO compound) obtained in water:ethanol ratio 5:5. Real time 8.3 min, 10 fps. SUPPLEMENTARY VIDEO 13 TIRF video of an irradiation experiment
performed on sample Gel@TCPP@Azo in 5:5 water:ethanol with a starting _trans_:_cis_ ratio of approximately 35:65 (prepared by irradiating the ethanol stock solution containing the AZO used
for the preparation of the gel at 365 nm). Real time 50 s, 5 fps. SUPPLEMENTARY VIDEO 14 TIRF video of the irradiation experiment performed on sample Gel@TCPP@Biph obtained in water:ethanol
ratio 7:3. Real time 8.3 min, 20 fps. SUPPLEMENTARY VIDEO 15 TIRF video of an irradiation experiment performed on sample Gel@TCPP@Azo in 5:5 water:ethanol with a starting _trans_:_cis_ ratio
of approximately 35:65 (prepared by irradiating the ethanol stock solution containing the AZO used for the preparation of the gel at 365 nm). Real time 120 s, 10 fps. SUPPLEMENTARY VIDEO 16
TIRF video of an irradiation experiment performed on sample Gel@TCPP@Azo obtained in water:ethanol ratio 5:5. Real time 8.3 min, 20 fps. SUPPLEMENTARY VIDEO 17 TIRF video of an irradiation
experiment performed on sample Gel@TCPP@Azo obtained in water:ethanol ratio 7:3. Real time 8.3 min, 10 fps. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Samperi, M., Bdiri, B., Sleet, C.D. _et al._ Light-controlled micron-scale molecular motion. _Nat. Chem._ 13, 1200–1206 (2021). https://doi.org/10.1038/s41557-021-00791-2 Download
citation * Received: 04 August 2020 * Accepted: 19 August 2021 * Published: 11 October 2021 * Issue Date: December 2021 * DOI: https://doi.org/10.1038/s41557-021-00791-2 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative





