- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT During early embryogenesis, fast mitotic cycles without interphase lead to a decrease in cell size, while scaling mechanisms must keep cellular structures proportional to cell size.
For instance, as cells become smaller, if the position of nuclear envelope reformation (NER) did not adapt, NER would have to occur beyond the cell boundary. Here we found that NER position
in anaphase scales with cell size via changes in chromosome motility, mediated by cytoplasmic flows that themselves scale with cell size. Flows are a consequence of friction between viscous
cytoplasm and bulky cargo transported by dynein on astral microtubules. As an emerging property, confinement in cells of different sizes yields scaling of cytoplasmic flows. Thus, flows
behave like a cell geometry sensor: astral microtubules approach the boundary causing flow velocity changes, which then affect the velocity of chromosome separation, thus scaling NER.
SIMILAR CONTENT BEING VIEWED BY OTHERS CENTRIPETAL NUCLEAR SHAPE FLUCTUATIONS ASSOCIATE WITH CHROMATIN CONDENSATION IN EARLY PROPHASE Article Open access 12 July 2023 INTRACELLULAR SOFTENING
AND INCREASED VISCOELASTIC FLUIDITY DURING DIVISION Article 21 October 2021 CHROMATIN COMPACTION DURING CONFINED CELL MIGRATION INDUCES AND RESHAPES NUCLEAR CONDENSATES Article Open access
18 November 2024 MAIN Chromosome separation during anaphase is tightly coordinated with nuclear envelope reformation (NER) so that NER occurs after complete segregation of the DNA yet before
separating chromosomes reach the edge of the cell (cell pole). NER positioning is regulated by a mechanism in which Aurora B kinase, localized at the spindle midzone, and PP1/PP2A
phosphatases act on chromosomal substrates1,2,3. After substrates are phosphorylated at the midzone, their phosphorylation levels decay exponentially as a function of time. NER occurs only
at a particular time, below a defined phosphorylation threshold and, because chromosomes are moving while being dephosphorylated, at a defined distance from the midzone1. Therefore, (1) the
time of NER is set by the dephosphorylation rate and (2) the position of NER is set by the dephosphorylation rate and the velocity with which chromosomes separate from the midzone. NER is
positioned according to a temporal model (dephosphorylation rate), which becomes a spatial model by chromosome velocity. RESULTS POSITION OF NER SCALES WITH CELL SIZE Can this mechanism
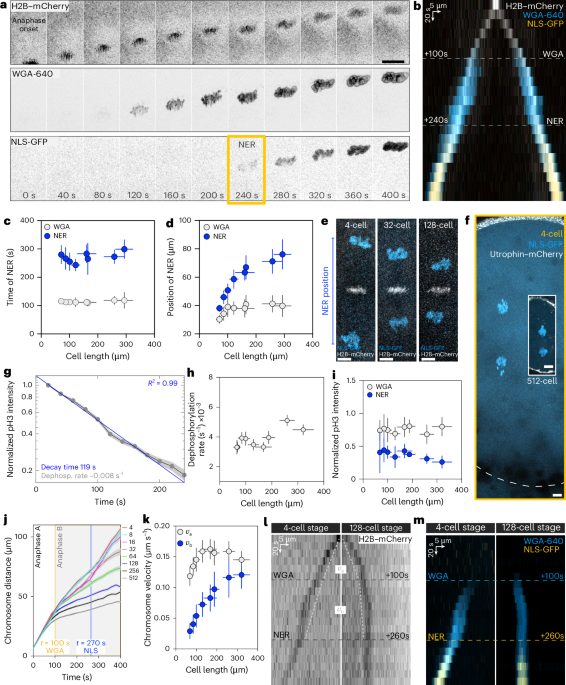
adjust the position of NER to cell size? We studied this during early zebrafish embryogenesis, where cells undergo nine rapid mitotic cycles (~10 min each) without interphase4. In these
cleavage divisions, cell length along the axis of chromosome separation decreases by around tenfold (Extended Data Fig. 1a–c). In the one-cell stage, cells are 580 ± 52 µm long and the
distance between the two sets of separating chromosomes when NER occurs is 91 ± 14 µm, which is longer than the cell length at the 512-cell stage (68 ± 11 µm). Therefore, the NER position
must adapt to cell size. To study how NER adapts to cell size, we systematically measured NER timing and position during anaphase from 4- to 512-cell stage, ranging from 300 μm to 70 μm in
cell length. First, we used fluorescent wheat germ agglutinin (WGA-640) to label glycosylated nucleoporins, the first factors known to be recruited to the chromosomes during anaphase5,6,7
(Fig. 1a,b). To then monitor NER, when the nuclear envelope is sealed and has a functional nuclear transport system, we followed the nuclear accumulation of green fluorescent protein
(GFP)-NLS (where NLS is the nuclear localization signal) (Fig. 1a,b). The timings of WGA recruitment and NER do not change with cell size: WGA is recruited 113 ± 3 s after anaphase onset,
and NER occurs at 271 ± 18 s (Fig. 1c). The timing of NER was previously shown to be dependent on Cdk1 downregulation in _Drosophila_ and human culture cells8. However, in zebrafish, Cdk1
inhibition did not affect the timings of WGA or NER (Extended Data Fig. 1d–f). We noticed that WGA timing coincides with the transition from anaphase A to B (Fig. 1a,b with Extended Data
Fig. 2b). Indeed, like in other systems9, here anaphase can be subdivided: in anaphase A, chromosomes approach the spindle poles, while during anaphase B, both chromosomes and spindle poles
move together, with the same velocity (Extended Data Fig. 2a–c). Whereas NER timing is independent of cell size, NER positioning is not. WGA recruitment occurs at approximately the same
distance regardless of size, but NER occurs at distances proportional to cell size, that is, NER scales (Fig. 1d–f; note that WGA scaling is subtle). We wondered whether either the
chromosome phosphorylation threshold for NER or the dephosphorylation rate can explain NER scaling. To address this, we imaged live embryos injected with a fluorescently conjugated antibody
that recognizes phosphorylated Histone H3 (pH3-s10)10, a bona fide substrate of Aurora B. pH3-s10 levels correspond to the phosphorylation state of chromosomes as they move away from the
midzone3,11 (Extended Data Fig. 2d–f). We first confirmed in this system that the phosphorylation levels decay exponentially with time1 (Fig. 1g). The dephosphorylation rate, which
determines the decay time, is the same regardless of size (Fig. 1g,h). In addition, both WGA recruitment and NER occur at defined thresholds of phosphorylation levels regardless of cell size
(75 ± 5% and 37 ± 6% of the midzone level, respectively; Fig. 1i). Because neither the dephosphorylation rate nor the phosphorylation threshold for NER changes, chromosome velocity must be
cell size dependent to explain scaling. During anaphase A and B, chromosomes move at two different velocities, _v_A and _v_B, respectively. In anaphase A, _v_A remains largely constant and
shows marginal scaling in the smallest cells (128-cell stage onwards; Fig. 1j–m). By contrast, in anaphase B, _v_B strongly scales for the full range of cell sizes (Fig. 1j–m). This explains
why the position of WGA recruitment is constant, whereas NER position scales. The scaling of _v_B underlies the scaling of NER positioning, prompting us to study what mediates chromosome
separation during anaphase B. CYTOPLASMIC FLOWS EMERGE IN ANAPHASE During anaphase A, chromosomes approach the spindle poles and the spindle poles do not move (Extended Data Fig. 2a–c). We
showed above that, during anaphase B, the spindle and the chromosomes move together and the chromosomes do not anymore approach the spindle poles (Extended Data Fig. 2a–c), indicating that
the spindle is not used to separate chromosomes in anaphase B. Furthermore, astral microtubules are not in direct contact with the cell cortex during anaphase B, excluding pulling forces
from the cortex (Extended Data Fig. 2g–i). What then drives chromosome separation in anaphase B? Cytoplasmic flows were shown to transport cytoplasmic contents over large distances12,13 or
to position cellular structures such as the mitotic spindle in mouse oocytes or nuclei in _Drosophila_ embryos14,15. We noticed, by looking at mitochondria in early zebrafish embryos, that
flows appear during anaphase. While it is well established that mitochondria are moved by motors on microtubules, a collective movement resembling cytoplasmic flows was indeed prominent
(Fig. 2a). Thus, we imaged mitochondria in transgenic embryos expressing a mitochondrial targeting peptide tagged with GFP16. In anaphase, mitochondria are dispersed in the cytoplasm
(Extended Data Fig. 3a–d) and can be used to analyse cytoplasmic flows. Particle imaging velocimetry (PIV) analysis revealed flow patterns with flow velocity maximal along the axis of
chromosome separation (velocity; Fig. 2b) and vortexes around the anaphase spindle (vorticity; Fig. 2c). Flows emerge at anaphase onset and become stronger in anaphase B (Fig. 2d and
Supplementary Video 1). To study whether other organelles are also moved by cytoplasmic flows, we monitored endogenous lipid droplets (Extended Data Fig. 3e–k). We also studied injected
lipid droplets, which lack motor proteins and must drift following the flows (Extended Data Fig. 3l,m). In both cases, motility corresponds to the mitochondria flow patterns, validating
mitochondria movement as a proxy for cytoplasmic flows. This also indicates that organelles, such as mitochondria and endogenous lipid droplets, or other objects, such as exogenous lipid
droplets, are displaced in the cytoplasm together with the local flows, raising the possibility that chromosomes could, too. In anaphase B, the speed and orientation of the mitochondria flow
field adjacent to chromosomes (Methods) matched those of chromosomes themselves (_v_flow = 0.08 ± 0.02 µm s−1 versus _v_chromo = 0.11 ± 0.04 µm s−1; Fig. 2e and Extended Data Fig. 3n–s).
Also, chromosomes move parallel to neighbouring lipid droplets (Extended Data Fig. 3t–v). These neighbouring lipid droplets lack dedicated structures, like the centromere on chromosomes,
that could bind to a specific set of microtubules. Since droplets near the chromosomes and chromosomes themselves move with parallel trajectories, flows could move chromosomes. However,
flows could themselves be produced by the active movement of chromosomes in a passive fluid. This creates a conundrum: does chromosome movement generate flows, or do flows move chromosomes?
MICROTUBULES ARE ESSENTIAL FOR CYTOPLASMIC FLOWS We addressed this by studying cells dividing without DNA during cleavage divisions, which we observed occasionally (_n_ = 7). The origin of
this phenomenon is currently not understood but has been reported before17,18,19. To follow mitosis in the absence of DNA in these cells, one-cell-stage embryos were injected with
fluorescently tagged Histone H1 and α-tubulin to monitor chromosomes and microtubules. Cells without DNA did not form a mitotic spindle but generated two asters during anaphase (Fig. 2f and
Supplementary Video 2). The absence of DNA was not due to failure of labelling since neighbouring cells, used as internal controls, have chromosomes and mitotic spindles (Fig. 2f). Cells
without chromosomes progress through mitosis, undergo cytokinesis and produce daughter cells through at least four cell cycles (two cell cycles shown in Extended Data Fig. 4). Remarkably,
they show cytoplasmic flows and vortexes like cells with chromosomes (Fig. 2b,c,g–i and Supplementary Video 3). Therefore, cytoplasmic flows are not a passive consequence of the displacement
of chromosomes. Cytoplasmic flows have been shown to be mediated by active mechanisms such as actin polymerization, contraction of the cortical actomyosin network or the movement of
molecular motors on microtubules12,13,14,15. We first looked at actin. From anaphase onset onwards, bulk actin progressively depolymerizes in the cytoplasm and remains mostly at the cell
cortex (Extended Data Fig. 5a), as previously reported20,21. In this scenario, a decreased concentration of bulk actin might not contribute substantially to the generation of cytoplasmic
flows in the centre of the cell. Complete actin depolymerization causes loss of embryo shape and ultimately leads to cytoplasmic leakage outside the cell. Thus, we performed a short
incubation with latrunculin B, which only partially affected cortical actin while keeping embryo shape but completely inhibited cytoplasmic actin polymerization (Extended Data Fig. 5b).
Under these conditions, the flow pattern is maintained (Fig. 3a–c,e–h), but the timing of the flows is changed: flows are initiated and slowed down earlier compared with control embryos
(Fig. 3d). This is explained by the fact that, in control embryos, actin depolymerization might fluidize the cytoplasm, allowing flows to emerge mostly during anaphase B (Extended Data Fig.
5a). Upon the downregulation of actin, cytoplasmic actin is already reduced in anaphase A, allowing flows to emerge earlier. Importantly, changes in flow dynamics upon actin downregulation
did not affect the position and scaling of NER (Extended Data Fig. 5c–f). These data exclude cytoplasmic actin filaments as the origin of the flows and suggest that flows are rather
downregulated by the existence of an actin network. We next focused on the role of astral microtubules in anaphase B. To visualize the displacement within the microtubule network, we
performed tubulin speckle imaging, in which the microtubule lattice is stochastically labelled4,22,23. Speckles showed marked flows during anaphase, with velocity and vortexes like those
observed with labelled mitochondria (Fig. 3i–m,p). These results are consistent with the idea that the displacement of astral microtubules generates the anaphase cytoplasmic flows. To study
whether microtubules themselves generate flows, we used a photo-activatable microtubule depolymerizing drug (SBTub3-P)24 at the metaphase–anaphase transition. Under these conditions, the
mitotic spindle architecture is maintained and chromosome separation in anaphase A was only mildly affected (WGA position is 37.9 ± 3.5 and 30.9 ± 5.4 µm in control and SBTub3-P,
respectively; four-cell stage) (Fig. 3q and Extended Data Fig. 6a–d). However, during anaphase B, astral microtubule growth was impaired (Extended Data Fig. 6a–d), the mitochondrial flow
showed no vortexes and chromosome velocity was reduced (Fig. 3r–u and Extended Data Fig. 6e). Under these conditions, the actomyosin network should not be affected. This suggests that
microtubules are essential for cytoplasmic flows, while the cortical actin remaining in the latrunculin experiment above (Fig. 3a–h and Extended Data Fig. 5b) does not contribute to the
generation of flows. Instead, the latrunculin experiment suggests that the actin network is a facilitator for flows to emerge. DRAG OF MOTOR-DRIVEN ORGANELLES GENERATES CYTOPLASMIC FLOWS
While tubulin speckles show flows with vortexes, microtubule growth, monitored by EB3-labelled plus-ends, is radial, away from the aster centre (Fig. 3n–p). Microtubule growth occurs on a
different (faster) timescale than the flow of speckles or mitochondria. The difference between the pattern in the flows (vortexes) and growth (radial) is explained because flows are too slow
to have a major impact on the short-lived growth events monitored by EB3. Indeed, microtubule polymerization cannot displace the cytoplasm and induce flows by itself, because polymerization
merely represents the reallocation of the tubulin heterodimer mass into the microtubule filament. Instead, the movement on microtubules of bulky organelles fuelled by motors could generate
flows. Dynein transports cargo, including bulky organelles (mitochondria, endoplasmic reticulum and others), towards the minus ends of microtubules that are concentrated in the aster
centre25,26. The movement of bulky cargo against a viscous cytoplasm causes drag on the cargo, which in turn could displace microtubules in the opposite direction27. Indeed, dynein was shown
to generate large cytoplasmic pulling forces in sea urchin embryos28,29 and _Xenopus laevis_ extracts30. We therefore quantitatively analysed the minus-end motion of dynein with
mitochondria, as an example of a bulky cargo. By high-temporal-resolution imaging, we tracked single fluorescently labelled mitochondria while moving into a cleared bleached area near the
aster centre (Fig. 4a). The analysis of mitochondrial motility, including mean square displacement, shows two types of episodes within tracks in anaphase (Fig. 4b–f and Extended Data Fig.
7): (1) fast directed motion towards the aster centre (_v_ = 0.95 ± 0.3 µm s−1; fast episodes) and (2) a combination of diffusive (_D_ = 0.017 ± 0.02 µm2 s−1) and slow directed motion (_v_ =
0.089 ± 0.05 µm s−1; slow episodes) with the same direction and velocity as cytoplasmic flows. Therefore, mitochondria can be either engaged on microtubules by dynein and moving towards the
aster centre (fast episodes) or disengaged from dynein (slow episodes) and drifting with the cytoplasmic flows. To test the role of dynein itself, we injected p150-CC1, a dominant-negative
fragment of dynactin that inhibits dynein function31,32. In these conditions, the spindle architecture was maintained, as previously reported33. During anaphase A, chromosome velocity was
not affected (Fig. 4k). However, in anaphase B, fast mitochondrial episodes were reduced, flow patterns were abolished and chromosome velocity was strongly affected (Fig. 4g–n). Therefore,
dynein generates flows in the microtubule network by moving bulky cargo. We then studied theoretically whether the transport of a cargo (mitochondria) by motors (dynein) in the presence of
friction drag can generate a considerable movement of a microtubule in the opposite direction (Supplementary Information section 1.1-3). Theory shows that the ratio of the velocities of
mitochondria and microtubule is equal to the inverse of the ratio of their friction coefficients (Supplementary Information section 1.1). Friction depends on the size and shape of the
object. Considering the (longitudinal) friction coefficient34 of a 10-µm-long microtubule4 and that of a mitochondrion with 1 µm diameter, which are both similar (\({\xi
}_{\text{mt},\parallel }\approx {\xi }_{\text{mito}}\approx 400\,{{\mathrm{pN}}}/(\upmu {\mathrm{m}}\,{\mathrm{s}}^{-1})\)), the two velocities are comparable. Therefore, the drag of one
mitochondrion is considerable and can contribute to the collective displacement of the microtubule network. Other organelles could also contribute to the observed flows. Cargo drag as
described above occurs in a scenario with a single aster. In this configuration, microtubules would be displaced away from the aster centre with radial symmetry and the aster centre would
remain in place. Instead, experimentally, we observed a flow pattern where the asters, with the chromosomes therein, move towards the cell poles. This is explained by the fact that astral
microtubules do not extend beyond the midzone, creating an asymmetric aster (Extended Data Fig. 6f,g and Supplementary Fig. 1). In turn, astral asymmetry causes an imbalance in cargo drag
that ultimately leads to polar movement of each aster (Supplementary Information section 1.3). In summary, dynein-dependent motility with friction drag on a cargo, in the context of aster
asymmetry, can generate flows that separate chromosomes in anaphase. What is then causing scaling of these flows? CONFINEMENT SCALES CYTOPLASMIC FLOWS NER scaling is mediated by the scaling
of velocity of chromosome motion, which is indeed mediated by cytoplasmic flows. We therefore studied whether flows scale as cells become smaller. Flow velocity adjacent to chromosomes does
scale (Fig. 5a) and correlates with chromosome velocity in cells of different sizes (Fig. 5b and Extended Data Fig. 8a–g). Importantly, flow velocity in other regions (for example, spindle
midzone) correlated neither with chromosome velocity nor orientation (Extended Data Fig. 8h,i). Which parameter scales to mediate flow scaling? Centrosome size and microtubule dynamics were
shown to scale in metaphase spindles35,36. We measured microtubule density, microtubule growth, aster size and aster growth velocity, in anaphase; none of these experienced a major change in
cells of different sizes (Extended Data Fig. 9a–d) and neither did the density of mitochondria nor the proportion of mitochondria, which gets engaged on dynein-dependent cargo change
(fluorescence recovery after photobleaching (FRAP) analysis; Extended Data Fig. 9e,f). Finally, velocity, run length and processivity of mitochondria engaged with microtubules are the same
regardless of cell size (Extended Data Fig. 9g–i). Thus, changes in these biochemical features underlying cytoplasmic flows are not changed during scaling. Could flows scale by the boundary
effects on the aster, whose size does not scale, in cells of decreasing size (confinement)? We tested this with a numerical study (Supplementary Information section 2) in which we considered
parameters based on values obtained experimentally from our system and other cellular systems (Supplementary Table 1). In confinement conditions (Fig. 5c–h), the polar displacement of
asters led to a flow pattern that moves chromosomes (Fig. 5c), forms vortexes (Fig. 5e,g) and scales with cell size (Fig. 5f,h), so that NER position itself scales (Fig. 5d), as observed
experimentally (Fig. 5a,d). Considering the full range of cell sizes of the zebrafish cleavage-stage embryos (from ~600 µm in the 1-cell stage to 60 µm in the 512-cell stage), the
theoretical analysis yields, as an emerging property, the appearance of an upper limit, where scaling reaches a plateau (Fig. 5d). Such a plateau was described before for metaphase spindle
scaling in zebrafish and _Xenopus_4,37. Indeed, for larger cells (one- and two-cell stage), the observed NER position behaves according to that upper limit. In other words, our theoretical
framework based on confinement captures in quantitative detail the relationship between cell size and NER position, not only when it scales, but also when it does not anymore (Fig. 5d). How
does confinement slow down flows? The movement of an object in a confined system decreases as it gets closer to the boundary (no-slip boundary effect)38. Therefore, flows experience a larger
drag and become slower the closer they are to the boundary. This effect is more prominent in small than in large cells, explaining flow scaling. For the largest cells, however, the distance
to the cell boundary is too large to considerably affect the motion of the asters, thereby explaining the upper limit. Taken together our experimental and theoretical approaches uncover a
scenario where confinement by itself causes scaling of flows and, ultimately, determines NER position. To further test whether the mere size of cells determines the position of NER, we
generated smaller-sized embryos by yolk aspiration in one-cell stage, before streaming of ooplasm from the yolk towards the cell20. We thereby achieved a systematic cell size reduction in
two- and four-cell stages, compared with control embryos (Extended Data Fig. 9j), without significantly affecting any of the parameters that contribute to NER (Fig. 5i and Extended Data Fig.
9k–q). In a scenario where a limiting biochemical factor decays with time during cleavage stages and is essential for scaling, we expect NER positioning to be determined by timing
(developmental stages) rather than by cell size. Conversely, if scaling is defined by confinement, NER position would be determined by the new cell size in the aspiration experiment, not by
the stage. We observed that NER is positioned according to cell size rather than stage (Fig. 5j–l), indicating that confinement drives NER scaling; the aspiration data fall into the control
scaling curve of NER distance as a function of cell size (Fig. 5k). To test which cell size parameter (volume versus cell length) has a stronger impact on NER positioning, we patterned
two-cell-stage embryos in well-defined rectangular agarose templates, achieving an inversion of the cell aspect ratio (Extended Data Fig. 10a,b). Under these conditions, the flow pattern was
maintained: flows were aligned with the axis of chromosome separation, and vortexes were formed in the vicinity of the spindle region (compare Fig. 2a–c with Extended Data Fig. 10d–f). Upon
insertion into the agarose template, cells in two-cell-stage patterned embryos have the same volume as cells in control embryos, while their length halves, matching that of cells in
eight-cell-stage control embryos. Extended Data Fig. 10c shows that NER positioning occurs according to cell length rather than cell volume. DISCUSSION Here, we identified cytoplasmic flows
as a mechanism for chromosome separation in anaphase that provides cell size information and mediates scaling of the position of NER. This mechanism is supported by the following five key
findings: (1) the position of NER scales with cell size (Fig. 1d–f); (2) NER scales because chromosome velocity scales (Fig. 1j,k); (3) chromosomes are moved by cytoplasmic flows that emerge
in anaphase (Fig. 2a–d); (4) flows are generated by the friction drag of bulky cargo moved by dynein when walking on microtubules, against a viscous cytoplasm (Fig. 4b,c); and (5) flows
scale by their physical confinement in cells of decreasing sizes (Fig. 5a,d,k). Cytoplasmic flows in confinement are sufficient to scale chromosome velocity and, consequently, the position
of NER. During anaphase, the position and timing of NER are coupled by chromosome velocity. In our study, we found that the timing of NER, determined by the biochemistry of the
dephosphorylation rate of chromosomal substrates, does not itself scale (Fig. 1g–i). By contrast, NER position does scale because the scaling of velocity, mediated by flows, brings
chromosomes further away in the same time interval. The scaling of velocity cannot be explained by changes in biochemical properties (Extended Data Fig. 9a–i). Instead, flows scale simply by
the physics of confinement. Because flows, in turn, affect chromosome velocity and the position of NER, flows become a ‘sensor’ of the confinement state and, therefore, of the cell size
used for scaling. Our data raise the possibility that cytoplasmic flows could be a general, simple mechanism for the scaling of other cellular structures or processes within the cytoplasm,
such as spindle positioning14,39,40, nuclear spacing or the length of the morphogen gradient in the syncytial blastoderm of flies15,41. METHODS ZEBRAFISH LINES AND MAINTENANCE This study
followed European Union directives (2010/63/EU), the Swiss Animal Protection Act and the Swiss Animal Welfare Ordinance. Zebrafish lines used were maintained in a recirculating system with a
14 h day and 10 h night cycle at 28 °C. To visualize chromosomes, the _Tg(h2afva:h2afva-GFP)_42 and _Tg(Ef1α:H2B-mCherry)_ transgenic lines were used. Mitochondria were visualized with a
_Tg(Ef1α:MLS-GFP)_ transgenic line16. F-actin lines, _Tg(actb1:Utr-GFP)_ and _Tg(actb1:Utr-mCherry)_, were a gift from the laboratory of Carl-Philip Heisenberg20. Microtubules were
visualized either with a transgenic line expressing the microtubule binding domain of Ensconsin tagged with 3 GFPs _Tg(bactin2:HsENSCONSIN17-282-3xEGFP)_, a gift from Martin Wuhr32, or with
a transgenic line expressing human Doublecortin, _Tg(actb2:EGFP-Has.DCX)_43. The AB wild-type strain was used for the injection of labelled markers. LIVE IMAGING For all live imaging
experiments, embryos from 30 mpf (minutes post fertilization) to 3 hpf (hours post fertilization) were manually dechorionated, mounted on 0.7–1.0% low-melting agarose and maintained at 28 °C
in a temperature-controlled chamber. Control embryos that, while imaging, showed errors during mitosis were excluded from subsequent analysis. INJECTED MARKERS AND DRUGS All makers were
injected in the yolk of one-cell-stage embryos. Early NER was visualized with WGA conjugated with a 640 CF®dye (29026; Biotium). Complete NER was observed with NLS-GFP (a gift from the Jan
Brugués laboratory, MPI-CBG, Dresden). Histone H1 protein purified from calf thymus and conjugated with Alexa Fluor 488 (H1-488) was used to visualize chromosomes (H13188; Thermo Fisher
Scientific). The Aurora B phosphorylation gradient was observed using a Fab antibody against phosphorylation at S10 of Histone H3 conjugated with Alexa-488, Alexa-Cy3 or Alexa-Cy5 (injected
a 1:10 dilution from 0.2 μg ml−1 stock). Fab antibodies were a kind gift from the Hiroshi Kimura laboratory, Tokyo Tech, Japan10. Microtubules were visualized with purified/conjugated
α-tubulin-ATTO-565, and microtubule speckles were visualized with a 20:80 ratio of labelled:non-labelled mixture of α-tubulin-ATTO-565. Microtubule plus tips were visualized with purified
EB3–GFP or EB3–mCherry. Both tubulin and EB3 purified proteins were a kind gift from the Charlotte Aumeier laboratory (University of Geneva, Switzerland). Endogenous lipid droplets were
labelled with Nile Red following previous protocols20. SBTub3-P was provided by the laboratory of Oliver Thorn-Seshold (LMU, Munich). P150 purified protein was a kind gift from the Jan
Brugués laboratory (MPI-CBG, Dresden). NER SCALING DESCRIPTION The scaling of the time and position of NER in control, latrunculin B, dinaciclib and aspirated embryos was based on live
imaging data of _Tg(Ef1α: H2B-mCherry)_ embryos, co-injected with NLS-GFP and WGA-640. Live imaging was performed on a 3i Marianas confocal spinning disk set-up based on a Zeiss Z1 stand and
a Yokogawa X1 spinning disk head. Images were acquired with sequential excitation with 488, 561 and 640 nm laser lights, every 20 s, with a Zeiss LD C-APO 40×/1.1 W Korr M27 objective and a
_z_-step of 1.5 μm to a total _z_-stack of ~25 μm, in the centre of the cell, where the mitotic spindle is positioned. For each mitotic cycle, the centre of the _z_-stack was adjusted to
the position of the spindle. LIVE IMAGING OF PH3-S10 Imaging of pH3-s10 was performed in the background of _Tg(Ef1α: H2B-mCherry)_ transgenic embryos. Fab antibody against pH3-s10 conjugated
with Alexa-488 was injected in one-cell-stage embryos combined with WGA-640. For the correlation between timing of NER and pH3 levels, pH3-s10-Alexa-Cy5 and NLS-GFP were used. Imaging was
performed with the same set-up as for the scaling description. LIVE IMAGING OF MITOCHONDRIA FOR PIV ANALYSIS Live imaging was performed on _Tg(Ef1α:MLS-GFP)_ transgenic embryos co-injected
with WGA-640. When mentioned, H1-488 was co-injected, to visualize chromosomes. WGA-640 signal was used to time the beginning of anaphase B and the time window to analyse the flows. The
imaging set-up was the same as for the scaling description, with a time resolution of 10 s and a _z_-stack of 17 μm. The same conditions apply for control, SBTub3-P, p150 and latrunculin B
experiments. INDIVIDUAL MITOCHONDRIA TRACKS The imaging of individual mitochondria tracks was based on _Tg(Ef1α:MLS-GFP)_ transgenic embryos injected with H1-488 to facilitate the staging of
mitosis (metaphase versus anaphase). When mentioned, α-tubulin-ATTO-565 was co-injected to visualize the mitotic spindle. Single _z_-stack movies were acquired in a Zeiss LSM 780 with a
Zeiss C-Apochromat 40×/1.2 W Korr FCS M27, 5× zoom with 0.6 s time interval. FRAP was performed with a square region of interest (ROI) positioned such that a portion or half of the aster
region was bleached. TUBULIN SPECKLE MICROSCOPY For tubulin speckle microscopy, wild-type embryos were co-injected at the one-cell stage with α-tubulin-ATTO-565 in a 20:80 ratio of
labelled:non-labelled and EB3–GFP. Single _z_-stack movies were acquired in a Zeiss LSM 780 in Airyscan mode, with a Zeiss C-Apochromat 40×/1.2 W Korr FCS M27, 4× zoom and 4 s time interval.
MICROTUBULE GROWTH WITH EB3 Wild-type embryos were injected at the one-cell stage with EB3–mCherry purified protein. Single _z_-stack movies were acquired in a Zeiss LSM 780 in Airyscan
mode, with a Zeiss C-Apochromat 40×/1.2 W Korr FCS M27, 5× zoom and 0.5 s time interval. DYNEIN INHIBITION For dynein inhibition, p150 was injected at a final concentration of 7 mg ml−1 in
late-one-cell-stage embryos (after cell expansion). Under these conditions, p150 had a nearly immediate effect: injection in the late one-cell stage ensured that the first mitosis had
already occurred and the effect of p150 would be detected at the two-cell stage. P150 resulted in shorter metaphase spindles but no impact on metaphase chromosome congression. During
anaphase A, chromosomes separated without significant differences from control embryos, but anaphase B was clearly affected. After anaphase, cells failed cytokinesis, resulting in a
four-cell-stage embryo with only two cells, each cell with two spindles. The analysis of p150-injected embryos was restricted to two-cell-stage embryos that showed the phenotype described
above. To analyse flows after dynein inhibition, p150 was co-injected with EB3–mCherry (to analyse the spindle phenotype) and WGA-640 on _Tg(Ef1α:MLS-GFP)_ transgenic embryos. INHIBITION OF
ACTIN POLYMERIZATION Total inhibition of actin polymerization led to the loss of embryo shape and was incompatible with cell viability. We achieved inhibition of cytoplasmic actin without
affecting dramatically embryo shape (and cortical actin) with a 3 min incubation of embryos in Danieau 0.3% solution with latrunculin B (428020; Sigma-Aldrich) at a final concentration of 5
μM. CDK1 INHIBITION For Cdk1 inhibition, embryos were incubated for 5 min in Danieau 0.3% with dinaciclib20 at 200 μm (CAY-14707; Cayman Chemical). With this concentration, entry in mitosis
was delayed, confirming the inhibition of Cdk1 activity. LIGHT-INDUCED MICROTUBULE DEPOLYMERIZATION For light-induced microtubule depolymerization, we used SBTub3-P (ref. 24), a soluble,
non-reversible and 405-nm-activatable version of the initially published photostatin44. SBTub3-P was injected at one-cell-stage embryos. The injection and mounting of embryos was performed
under red-light conditions to avoid premature activation of the drug. Drug activation was performed at the metaphase/anaphase onset with three to four pulses of 405 nm laser light through
the entire _z_-stack of imaging (25 μm). This ensured normal metaphase spindle assembly and proper chromosome congression and did not substantially affect chromosome segregation during
anaphase A. SBTub3-P was combined with live imaging of _Tg(bactin2:HsENSCONSIN17-282-3xEGFP)_ to analyse the effect on microtubules during metaphase and anaphase or live imaging of
_Tg(Ef1α:MLS-GFP)_ transgenic embryos and co-injected with α-tubulin-ATTO-565 and WGA-640 to analyse cytoplasmic flows after induced microtubule depolymerization. ENDOGENOUS LIPID DROPLETS
Endogenous lipid droplets were labelled with Nile Red. Embryos were incubated for 5 min in Danieau 0.3% solution with Nile Red (72485, Sigma-Aldrich) at a final concentration of 10 μM.
EXOGENOUS LIPID DROPLETS Exogenous lipid droplets were assembled according to published protocols45. In brief, in a bovine serum albumin (BSA)-precoated glass flask, 1 ml of
DSPE-PEG(2000)-biotin (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- (biotinyl(polyethylene glycol)-2000) was added at a final concentration of 2 mM. The PEG solution was sonicated for
1 min. With a BSA-precoated tip, 70 μl of FC70 oil was added to the PEG solution. After vigorous shaking and pipetting, the emulsion was formed. The resulting mix is composed of droplets of
various sizes and can be maintained over several weeks at 4 °C. Droplets were fluorescently labelled with Cy3-streptavidin (PA43001, Cytiva Amersham) and injected in the cell of
one-cell-stage embryos. CELLS WITHOUT DNA Cells without DNA were identified initially in _Tg(Ef1α:MLS-GFP)_ transgenic embryos with α-tubulin-ATTO-565 and H1-488 co-injected. One cell showed
no H1-488 labelling and the absence of a metaphase spindle, while the neighbouring cells had both DNA and a metaphase spindle. The absence of DNA or a mitotic spindle was not a consequence
of lack of labelling as all other cells in the same embryo had labelling. In _Xenopus_ egg extracts, the Ran-GTP gradient was shown to be essential for metaphase spindle assembly46. The
gradient is generated on mitotic chromosomes47. Thus, in zebrafish embryos, in the cells without DNA the gradient could be impaired, explaining the lack of mitotic spindle. The fate of these
cells and how they arise in the embryo are not understood. YOLK ASPIRATION Yolk aspiration was performed during the first 30 min after fertilization, before the ooplasm streaming
contribution to the final cell size. A microinjection needle (1 mm glass capillary; TW100F-3, Word Precision Instruments) was assembled on a PicoNozzle kit (version 2, 5430-All, World
Precision Instruments), which is, in turn, connected to a 10 ml syringe. This creates enough vacuum pressure to aspirate yolk material from several embryos. Embryos were displayed in a line,
in a similar fashion as for embryo injection, and aspiration was performed until an approximately 50% reduction of yolk size was observed. For live imaging experiments, embryos from
different genetic backgrounds were aspirated and subsequently injected with different markers. The injection droplet was much reduced (~5 μm) compared with the final size of the embryos and,
therefore, did not change the effect of the aspiration. EMBRYO PATTERNING A 3D-printed template (adapted from Donoughe et al.48) was used to pattern rectangularly shaped boxes (900 × 250 ×
1,500 μm3) on 2% agarose. Two-cell-stage wild-type embryos were always maintained in Danieau 0.3% solution while being inserted into the confined spaces (with the help of forceps) and were
covered with a coverslip to maintain the shape during imaging. IMMUNOFLUORESCENCE Whole embryos with chorions were fixed with 4% paraformaldehyde for at least 5 h at room temperature. After
fixation, embryos were washed 2× with phosphate-buffered saline (PBS)–Tween 0.05% and manually dechorionated. Permeabilization was done with PBS–Triton 0.3% for 10 min, followed by blocking
with 5% BSA in PBS–Tween 0.05% for 1 h, overnight primary antibody incubation, three 5 min washes with PBS–Tween 0.05%, and overnight incubation with secondary antibody. Antibody incubations
were done in 5% BSA in PBS–Tween 0.05%. After secondary antibody, three 10 min washes with PBS–Tween 0.05% were performed. DAPI was added in the last wash. The primary antibody was rabbit
anti-pH3-s10 D2C8 (1:200; 3377; Cell Signalling (lot 7)), and the secondary antibody was anti-rabbit Alexa-488 (1:200). DATA ANALYSIS SYSTEMATIC ANALYSIS OF SCALING OF NER NER position and
timing was systematically analysed in _Tg(Ef1α: H2B-mCherry)_ transgenic embryos from 4- to 512-cell stage co-injected with NLS-GFP and WGA-640. The distance between the two sets of DNA that
separate during anaphase was defined as the chromosome distance. The chromosome distance when WGA or NLS are recruited to chromosomes was defined as WGA or NER, respectively. NER timings
were defined as seconds after anaphase onset (_t_ = 0 s, anaphase onset). One- and two-cell stages were not included in this analysis owing to the low number of replicates. QUANTIFICATION OF
TUBULIN SPECKLES Before tracking, movies were processed with background subtraction and a one-pixel Gaussian blur filter. Speckles were automatically tracked with TrackMate plug-in on
Fiji49 using the DoG (difference of Gaussian) detector and a 0.5 μm blob diameter, and spots were linked with a simple LAP tracker. Only tracks with a minimum of three spots were considered.
Statistical analysis was done with GraphPad Prism. QUANTIFICATION OF EB3 MICROTUBULE GROWTH Single stack movies were acquired every 0.5 s. Before tracking, movies were processed with
background subtraction and a one-pixel Gaussian blur filter. The microtubule growth was tracked with TrackMate plug-in on Fiji50. In brief, a LoG detector with a blob diameter of 1 µm was
used. Tracks were linked with the simple LAP (linear assignment problem) tracker. Obtained tracks were filtered for a minimum of three spots and with a linearity threshold of 0.6.
Statistical analysis was done with GraphPad Prism. QUANTIFICATION OF ASTRAL MICROTUBULE GROWTH Astral microtubule growth velocity was obtained from measurements of aster diameter,
perpendicular to the axis of chromosome separation, for a time interval between 40 s before to 40 s after anaphase onset. DCX–GFP transgenic embryos were used. QUANTIFICATION OF ASTER
ANISOTROPY Aster anisotropy was calculated as the ratio of the distance between the aster centre and the spindle midzone (_d_a) and the radius of the aster (_r_a) (see also the Supplementary
Information). Measurements were performed at 100 s after anaphase onset (corresponding to the start of anaphase B) using DCX–GFP transgenic embryos. ANALYSIS OF PH3-S10 PROFILES Analysis of
the phosphorylation gradient was performed on movies of embryos injected with pH3-s10 fluorescently labelled with 488, 561 or 640, depending on the combination with other markers. Sum
projections were used in the analysis. Fluorescence intensity was measured in a circular ROI of constant size in the chromosome region. This fluorescent signal was background subtracted,
measured with the same ROI outside the chromosome region. Intensity values were further processed in MATLAB with custom-made codes; namely, single intensity profiles were normalized to the
maximum value within the first 80 s after anaphase onset. A single exponential function \(\,\left(\right.C(T)={C}_{0}{{\mathrm{e}}}^{-T/\tau }\)) was fitted to the average curve of all
profiles from all cell stages as a function of time. From the fit we extracted the decay time (_τ_) and the dephosphorylation rate (1/_τ_). pH3-s10 live imaging was performed in _Tg(Ef1α:
H2B-mCherry)_ transgenic embryos to use as the internal control H2B–mCherry. Profile intensities were obtained using the same ROIs and analysis pipeline as for pH3-s10. Indeed, H2B–mCherry
profiles show a minor fluorescence intensity decay that we attribute to chromosome decondensation, compared with the decay of pH3-s10. MSD OF MITOCHONDRIA TRACKS Individual mitochondria were
manually tracked with the Manual Tracking plug-in from Fiji. Only tracks that showed the initial fast episode followed by a slow episode were considered for tracking and analysis. Full
tracks were exported into Excel and manually segmented into fast versus slow episodes. MSD (mean square displacement) analysis was performed using the MSD analyser50, a MATLAB-based code.
Both episodes on each track were fitted with a parabolic function (\({{\rm{MSD}}}\left(t\right)=4{Dt}+{v}^{2}{t}^{2}\)), from which the velocity and diffusion were obtained. PIV ANALYSIS
Flows were visualized with mitochondria present in the cytoplasm, labelled with a MLS–GFP transgenic line16. From the movies obtained, a series of 20 frames, corresponding to the timings
from anaphase onset until 200 s after anaphase onset, were selected for PIV analysis. A subset of three to four _z_-stacks, in the plane of chromosome separation, was used for maximum
projection. Flows were analysed using PIVlab, version 2.55 (ref. 51), a MATLAB-based software. In brief, images were preprocessed with a contrast-limited adaptive histogram equalisation
(CLAHE) algorithm using a 64-pixel window. Because the mitotic spindle and chromosome region excluded mitochondria localization, a mask was drawn manually to exclude these regions from the
PIV analysis. A ROI including the total size of the cell was defined to apply PIV analysis only to the cell of interest. PIV analysis was performed with a fast fourier transform cross
correlation algorithm using three multipass windows of 128 × 128, 64 × 64 and 32 × 32 pixels. A velocity threshold was defined for each obtained flow field to exclude outliers and a minimal
smoothening was applied. The final flow field was averaged from 100–200 s, when the flows are more prominent. To obtain flow velocity near the chromosomes, WGA-640 signal was used as a
marker for chromosome position. A mask was defined manually in the place where WGA labels the chromosomes. Flow velocity ‘near the chromosomes’ corresponds to the velocity values 9 μm above
and below the chromosome mask. STATISTICS AND REPRODUCIBILITY No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous
publications4. The experiments were not randomized and the investigators were not blinded to allocation during experiments and analysis of the data. Normality of the samples was determined
with a D’Agostino and Pearson test. Statistical analysis for two-sample comparison, with normal or non-normal distribution, was performed with a _t_-test or Mann–Whitney test, respectively.
For multiple-group comparison, a parametric one-way analysis of variance or a non-parametric analysis of variance (Kruskal–Wallis) was used, for samples with normal or non-normal
distribution, respectively. All pairwise multiple comparisons were subsequently analysed using either Tukey’s (parametric) or Dunn’s (non-parametric) tests. All statistical analysis was
performed with GraphPad Prism V5 (GraphPad Software). Micrographs are representative of a set of at least two independent experimental rounds. KYMOGRAPHS Kymographs were generated using a
previously published custom-written MATLAB code8. SOFTWARE For image acquisition, Slidebook 6 (3i) and Zeiss Zen were used. Numerical simulations were performed with custom-written code on
Julia version 1.10.5 (version of Julia packages is detailed on GitHub). Image processing and analysis were performed with FiJi (2.9) and MATLAB 2021b (The MathWorks). For statistics and data
analysis, Microsoft Excel for Mac 2023, Prism 10 was used. Figures were assembled using Affinity Photo v1 and Affinity Publisher v1. REPORTING SUMMARY Further information on research design
is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All other data supporting the findings of this study are available from the corresponding
authors upon reasonable request. Source data are provided with this paper. CODE AVAILABILITY Source codes used in the numerical study are available via GitHub at
https://github.com/Lu-Dumoulin/SM_OA_MGG.git. REFERENCES * Afonso, O. et al. Feedback control of chromosome separation by a midzone Aurora B gradient. _Science_ 345, 332–336 (2014). Article
CAS PubMed PubMed Central Google Scholar * Maiato, H., Afonso, O. & Matos, I. A chromosome separation checkpoint: a midzone Aurora B gradient mediates a chromosome separation
checkpoint that regulates the anaphase–telophase transition. _Bioessays_ 37, 257–266 (2015). Article CAS PubMed Google Scholar * Fuller, B. G. et al. Midzone activation of aurora B in
anaphase produces an intracellular phosphorylation gradient. _Nature_ 453, 1132–1136 (2008). Article CAS PubMed PubMed Central Google Scholar * Rieckhoff, E. M. et al. Spindle scaling
is governed by cell boundary regulation of microtubule nucleation. _Curr. Biology_ 30, 4973–4983.e10 (2020). Article CAS Google Scholar * Holt, G. D. et al. Nuclear pore complex
glycoproteins contain cytoplasmically disposed O-linked _N_-acetylglucosamine. _J. Cell Biol._ 104, 1157–1164 (1987). Article CAS PubMed Google Scholar * Onischenko, E. A., Gubanova, N.
V., Kiseleva, E. V. & Hallberg, E. Cdk1 and okadaic acid-sensitive phosphatases control assembly of nuclear pore complexes in _Drosophila_ embryos. _Mol Biol Cell_ 16, 5152–5162 (2005).
Article CAS PubMed PubMed Central Google Scholar * Davis, L. I. & Blobel, G. Identification and characterization of a nuclear pore complex protein. _Cell_ 45, 699–709 (1986).
Article CAS PubMed Google Scholar * Afonso, O. et al. Spatiotemporal control of mitotic exit during anaphase by an aurora B–Cdk1 crosstalk. _eLife_ 8, e47646 (2019). Article CAS PubMed
PubMed Central Google Scholar * Maiato, H. & Lince-Faria, M. The perpetual movements of anaphase. _Cell. Mol. Life Sci._ 67, 2251–2269 (2010). Article CAS PubMed PubMed Central
Google Scholar * Hayashi-Takanaka, Y., Yamagata, K., Nozaki, N. & Kimura, H. Visualizing histone modifications in living cells: spatiotemporal dynamics of H3 phosphorylation during
interphase. _J. Cell Biol._ 187, 781–790 (2009). Article CAS PubMed PubMed Central Google Scholar * Orr, B. et al. An anaphase surveillance mechanism prevents micronuclei formation from
frequent chromosome segregation errors. _Cell Rep._ 37, 109783 (2021). Article CAS PubMed PubMed Central Google Scholar * Tominaga, M. et al. Cytoplasmic streaming velocity as a plant
size determinant. _Dev. Cell_ 27, 345–352 (2013). Article CAS PubMed Google Scholar * Ganguly, S., Williams, L. S., Palacios, I. M. & Goldstein, R. E. Cytoplasmic streaming in
_Drosophila_ oocytes varies with kinesin activity and correlates with the microtubule cytoskeleton architecture. _Proc. Natl Acad. Sci. USA_ 109, 15109–15114 (2012). Article CAS PubMed
PubMed Central Google Scholar * Yi, K. et al. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. _Nat. Cell
Biol._ 13, 1252–1258 (2011). Article CAS PubMed PubMed Central Google Scholar * Deneke, V. E. et al. Self-organized nuclear positioning synchronizes the cell cycle in _Drosophila_
embryos. _Cell_ 177, 925–941 e917 (2019). Article CAS PubMed PubMed Central Google Scholar * Kim, M. J., Kang, K. H., Kim, C. H. & Choi, S. Y. Real-time imaging of mitochondria in
transgenic zebrafish expressing mitochondrially targeted GFP. _Biotechniques_ 45, 331–334 (2008). Article PubMed Google Scholar * Harvey, E. B. Parthenogenetic merogony or cleavage
without nuclei in _Arbacia punctulata_. _Biol. Bull-Us_ 71, 101–121 (1936). Article Google Scholar * Harvey, E. B. Parthenogenetic merogony or development without nuclei of the eggs of sea
urchins from Naples. _Biol. Bull-Us_ 75, 170–188 (1938). Article Google Scholar * Faruki, S., Cole, R. W. & Rieder, C. L. Separating centrosomes interact in the absence of associated
chromosomes during mitosis in cultured vertebrate cells. _Cell Motil. Cytoskel._ 52, 107–121 (2002). Article Google Scholar * Shamipour, S. et al. Bulk actin dynamics drive phase
segregation in zebrafish oocytes. _Cell_ 177, 1463–1479 e1418 (2019). Article CAS PubMed Google Scholar * Field, C. M. et al. Actin behavior in bulk cytoplasm is cell cycle regulated in
early vertebrate embryos. _J. Cell Sci._ 124, 2086–2095 (2011). Article CAS PubMed PubMed Central Google Scholar * Waterman-Storer, C. M. & Salmon, E. D. How microtubules get
fluorescent speckles. _Biophys. J._ 75, 2059–2069 (1998). Article CAS PubMed PubMed Central Google Scholar * Waterman-Storer, C. Fluorescent speckle microscopy (FSM) of microtubules and
actin in living cells. _Curr. Protoc. Cell Biol._ https://doi.org/10.1002/0471143030.cb0410s13 (2002). * Gao, L. et al. A robust, GFP-orthogonal photoswitchable inhibitor scaffold extends
optical control over the microtubule cytoskeleton. _Cell Chem Biol_ 28, 228–241 e226 (2021). Article CAS PubMed Google Scholar * Barbosa, D. J. et al. Dynactin binding to tyrosinated
microtubules promotes centrosome centration in _C. elegans_ by enhancing dynein-mediated organelle transport. _PLoS Genet._ 13, e1006941 (2017). Article PubMed PubMed Central Google
Scholar * Kimura, K. & Kimura, A. Intracellular organelles mediate cytoplasmic pulling force for centrosome centration in the _Caenorhabditis elegans_ early embryo. _Proc. Natl Acad.
Sci. USA_ 108, 137–142 (2011). Article CAS PubMed Google Scholar * Palenzuela, H. et al. In vitro reconstitution of dynein force exertion in a bulk viscous medium. _Curr. Biol._ 30,
4534–4540 e4537 (2020). Article CAS PubMed Google Scholar * Hamaguchi, M. S. & Hiramoto, Y. Analysis of the role of astral rays in pronuclear migration in sand dollar eggs by the
colcemid-Uv method. _Dev. Growth Differ._ 28, 143–156 (1986). Article PubMed Google Scholar * Tanimoto, H., Kimura, A. & Minc, N. Shape–motion relationships of centering microtubule
asters. _J. Cell Biol._ 212, 777–787 (2016). Article CAS PubMed PubMed Central Google Scholar * Pelletier, J. F., Field, C. M., Fuerthauer, S., Sonnett, M. & Mitchison, T. J.
Co-movement of astral microtubules, organelles and F-actin by dynein and actomyosin forces in frog egg cytoplasm. _eLife_ 9, e60047 (2020). Article CAS PubMed PubMed Central Google
Scholar * Quintyne, N. J. et al. Dynactin is required for microtubule anchoring at centrosomes. _J. Cell Biol._ 147, 321–334 (1999). Article CAS PubMed PubMed Central Google Scholar *
Wuhr, M., Tan, E. S., Parker, S. K., Detrich, H. W. 3rd & Mitchison, T. J. A model for cleavage plane determination in early amphibian and fish embryos. _Curr. Biol._ 20, 2040–2045
(2010). Article PubMed PubMed Central Google Scholar * Dalton, B. A., Oriola, D., Decker, F., Julicher, F. & Brugues, J. A gelation transition enables the self-organization of
bipolar metaphase spindles. _Nat. Phys._ 18, 323-+ (2022). Article CAS Google Scholar * Yang, K., Lu, C., Zhao, X. & Kawamura, R. From bead to rod: comparison of theories by measuring
translational drag coefficients of micron-sized magnetic bead-chains in Stokes flow. _PLoS ONE_ 12, e0188015 (2017). Article PubMed PubMed Central Google Scholar * Rathbun, L. I. et al.
PLK1- and PLK4-mediated asymmetric mitotic centrosome size and positioning in the early zebrafish embryo. _Curr. Biol._ 30, 4519–4527.e3 (2020). Article CAS PubMed PubMed Central Google
Scholar * Lacroix, B. et al. Microtubule dynamics scale with cell size to set spindle length and assembly timing. _Dev. Cell_ 45, 496–511 e496 (2018). Article CAS PubMed PubMed Central
Google Scholar * Wuhr, M. et al. Evidence for an upper limit to mitotic spindle length. _Curr. Biol._ 18, 1256–1261 (2008). Article PubMed PubMed Central Google Scholar * Happel, J.
& Brenner, H. _Low Reynolds Number Hydrodynamics: with Special Applications to Particulate Media_ 1st edn (Martinus Nijhoff, 1983). * Xie, J. et al. Contribution of cytoplasm
viscoelastic properties to mitotic spindle positioning. _Proc. Natl Acad. Sci. USA_ 119, e2115593119 (2022). Article CAS PubMed PubMed Central Google Scholar * Najafi, J., Dmitrieff, S.
& Minc, N. Size- and position-dependent cytoplasm viscoelasticity through hydrodynamic interactions with the cell surface. _Proc. Natl Acad. Sci. USA_ 120, e2216839120 (2023). Article
CAS PubMed PubMed Central Google Scholar * Hecht, I., Rappel, W. J. & Levine, H. Determining the scale of the Bicoid morphogen gradient. _Proc. Natl Acad. Sci. USA_ 106, 1710–1715
(2009). Article CAS PubMed PubMed Central Google Scholar * Pauls, S., Geldmacher-Voss, B. & Campos-Ortega, J. A. A zebrafish histone variant H2A.F/Z and a transgenic H2A.F/Z:GFP
fusion protein for in vivo studies of embryonic development. _Dev. Genes Evol._ 211, 603–610 (2001). Article CAS PubMed Google Scholar * Icha, J., Kunath, C., Rocha-Martins, M. &
Norden, C. Independent modes of ganglion cell translocation ensure correct lamination of the zebrafish retina. _J. Cell Biol._ 215, 259–275 (2016). Article CAS PubMed PubMed Central
Google Scholar * Borowiak, M. et al. Photoswitchable inhibitors of microtubule dynamics optically control mitosis and cell death. _Cell_ 162, 403–411 (2015). Article CAS PubMed Google
Scholar * Lucio, A. A., Ingber, D. E. & Campas, O. Generation of biocompatible droplets for in vivo and in vitro measurement of cell-generated mechanical stresses. _Methods Cell. Biol._
125, 373–390 (2015). Article CAS PubMed Google Scholar * Carazo-Salas, R. E. et al. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation.
_Nature_ 400, 178–181 (1999). Article CAS PubMed Google Scholar * Kalab, P., Weis, K. & Heald, R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts.
_Science_ 295, 2452–2456 (2002). Article CAS PubMed Google Scholar * Donoughe, S., Kim, C. & Extavour, C. G. High-throughput live-imaging of embryos in microwell arrays using a
modular specimen mounting system. _Biol. Open_ 7, bio031260 (2018). Article PubMed PubMed Central Google Scholar * Tinevez, J. Y. et al. TrackMate: an open and extensible platform for
single-particle tracking. _Methods_ 115, 80–90 (2017). Article CAS PubMed Google Scholar * Derivery, E. et al. Polarized endosome dynamics by spindle asymmetry during asymmetric cell
division. _Nature_ 528, 280–285 (2015). Article CAS PubMed Google Scholar * Thielicke, W. & Stamhuis, E. J. PIVlab—towards user-friendly, affordable and accurate digital particle
image velocimetry in MATLAB. _J. Open Res. Softw._ 2, e30 (2014). Article Google Scholar * Wuhr, M., Obholzer, N. D., Megason, S. G., Detrich, H. W. 3rd & Mitchison, T. J. Live imaging
of the cytoskeleton in early cleavage-stage zebrafish embryos. _Methods Cell. Biol._ 101, 1–18 (2011). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS We thank D. Basagiannis, E. Derivery, C. Aumeier and H. Maiato for critical reading of the manuscript and all the members of the M.G.-G. laboratory for feedback and
discussions. We thank J. Brugues for constructive discussions throughout the project and for sharing reagents. We thank J. Miesch and C. Aumeier for sharing purified EB3 proteins and tubulin
and the laboratory of H. Kimura for sharing the pH3-s10 Fab fragments. This work was supported by postdoctoral fellowships from EMBO (European Molecular Biology Organization, ALTF-672-2017)
and Marie-Curie (792175/MSCA-IF-2017) to O.A., and M.G.-G. was supported by the DIP (Département de l’Instruction Publique) of the Canton of Geneva, SNSF, the SystemsX EpiPhysX grant, the
ERC (European Research Council, Sara and Morphogen grants) and the Chemical Biology NCCR (National Centres of Competence in Research). FUNDING Open access funding provided by University of
Geneva. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry, Faculty of Sciences, University of Geneva, Geneva, Switzerland Olga Afonso, Ludovic Dumoulin, Karsten Kruse
& Marcos Gonzalez-Gaitan * Department of Theoretical Physics, Faculty of Sciences, University of Geneva, Geneva, Switzerland Ludovic Dumoulin & Karsten Kruse Authors * Olga Afonso
View author publications You can also search for this author inPubMed Google Scholar * Ludovic Dumoulin View author publications You can also search for this author inPubMed Google Scholar *
Karsten Kruse View author publications You can also search for this author inPubMed Google Scholar * Marcos Gonzalez-Gaitan View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS O.A. performed all of the experiments and analyses of the experimental data. L.D. and K.K. developed the theory and performed the numerical simulations.
O.A. conceived of and designed the experiments. O.A. prepared the figures. O.A. and M.G.-G. discussed the data and prepared the manuscript. CORRESPONDING AUTHORS Correspondence to Olga
Afonso or Marcos Gonzalez-Gaitan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Cell Biology_ thanks Nicolas
Minc, Nicoletta Petridou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 SYSTEMATIC ANALYSIS
OF CELL SIZE DURING THE CLEAVAGE STAGE OF ZEBRAFISH EMBRYOS. A, Time-lapse of an embryo during the first 9 cleavage divisions of zebrafish development showing LifeAct-mCherry. Bars, 200 µm.
B and C, Cell length (distance along the axis of chromosome separation) and cell width (distance perpendicular to the axis of chromosome separation) as a function of cell stage,
respectively. Error bars, standard deviation. Center, mean. Cell length, n = 2, 12, 17, 15, 9, 16, 27, 43, 37 and 26 cells from 2, 12, 15, 9, 4, 9, 10, 10, 9 and 7 embryos; cell width, n =
2, 14, 18, 17, 7, 12, 25, 43, 28 and 24 cells from 2, 12, 15, 10, 4, 9, 10, 10, 9 and 7 embryos at 1-, 2-, 4-, 8-, 16-, 32-, 64-, 128-, 256- and 512-cell stages. D, Duration of interphase
(time between consecutive mitoses) for control and dinaciclib treated embryos from 4- to 32-cell stage. Numbers indicate cell stage. Control, n = 4 cells from 4 embryos at 4-, 8-, 16- and
32-cell stages. Dinaciclib, n = 10, 12, 10, 7 cells from 10, 12, 10, 7 embryos at 4-, 8-, 16- and 32-cell stages. E and F, Time of WGA and NER in 4-cell stage control (n = 11) and dinaciclib
treated (n = 6) embryos. ns = not significant. Statistics, two-tailed Mann-Whitney test. Error bars, s.e.m. Source data EXTENDED DATA FIG. 2 ANAPHASE A AND B AND PH3-S10 DYNAMICS IN FIXED
AND LIVE ZEBRAFISH EMBRYOS. A, Time-lapse of a cell (4-cell stage) in anaphase. B, Left, kymograph of the same cell as in (d). Chromosomes, blue. Microtubules, grey. Dashed lines indicated
the approximate velocity of chromosomes and aster center. Right, kymograph of the same cell as in (d). Overlay of chromosomes (blue) and nuclear envelope (WGA, yellow). C, Aster and
chromosome velocity during anaphase A (0–100 s, n=14aster/chromosome sets from 7 embryos) and B (100–200 s, n=11asters and 13 chromosome set from 7 embryos). Only 4-cell stage embryos were
used. p-value = 1 × 10−7. During anaphase A, chromosomes move faster than the aster, while in anaphase B both velocities are comparable. Statistics, multiple analysis two-tailed Mann-Whitney
test. Error bars, s.e.m. D, Staining of pH3-s10 and DAPI on fixed embryos. Bar, 50 µm. Insets, magnification of cell 1 and 2. Cells are in different stages of anaphase showing different
levels of pH3-s10. Bars, 10 µm. E, Kymograph of a cell in anaphase. Right kymograph, H2B-mCherry. Left kymograph, pH3-s10-Cy5. Left images, snapshots at the time points highlighted in the
kymograph. Note both in the kymograph and on the images the decay of pH3-s10 but not of H2B-mCherry. Bars, 20 µm. F, Semi-log plot of normalized fluorescent intensity as a function of time.
Lines are exponential fits of pH3-s10 (yellow, n = 134 cells from 12 embryos, all developmental stages combined) and H2B-mCherry (blue, n=107cells from 12 embryos, all developmental stages
combined) profiles. t = 0 s, anaphase onset. Note the decay of pH3-s10 but not of H2B-mcherry. G and H, Snapshots at t = 0 s (anaphase onset) and t = 100 s (begin of anaphase B) of an 8- and
16-cell stage cell. Astral microtubules are far away from the cell cortex. EMTB-3xGFP, microtubules. Bars, 50 µm. Insets chromosome region. Bars, 20 µm. Blue dashed box, magnification in
(h). I, Magnification of a 4-, 8- and 16-cell stage, corresponding to cells in Extended Data Fig. 6 (b) and Extended Data Fig. 2 (g) and (h), respectively. Bars, 10 µm. Source data EXTENDED
DATA FIG. 3 CHROMOSOMES AND OTHER OBJECTS IN THE CYTOPLASM MOVE WITH THE FLOWS. A, Cell in metaphase. Mitochondria, Mito-GFP. Bar, 20 µm. Yellow box, aster/spindle region. Bar, 10 µm. Blue
box, cytoplasmic region. Mitochondria are homogenous in size and distribution. Bar, 5 µm. B and C, Same cell as in (a) during anaphase. Bars, 20 µm. D, Representative density profiles of
Mito-GFP from ROI (grey shaded line in a, b). E-H, Time projection (e and f; 100–200 s after anaphase onset) and overlayed PIV (g and h) for mitochondria (e and g) and endogenous lipid
droplets (f and h). Yellow arrow (f), droplet analysed in (i). (I) Representative velocity of the droplet highlighted in (f) and local flow around it (mitochondria PIV) as a function of
time. t = 0 s, anaphase onset. J-M, Correlation of velocity (j and l) and angle difference in their trajectories (k and m) between flow velocity (mitochondria PIV) and endogenous (j and k)
or injected lipid droplets (l and m), at each time point in 100–200 s after anaphase onset (n = 15 droplets x 10 time points, from 5 embryos). N and O, Time projection (100–200 s after
anaphase onset) of mitochondria and chromosomes, respectively. Blue arrow, chromosomes analysed in (r). P, Merge of (n) and (o). Note similar movement of chromosomes and flows. Q, PIV
analysis of mitochondria. Background image, same as in (n). Red shaded area, chromosomes. Bars, 20 µm. R, Representative velocity of chromosomes highlighted in (o) and the local flow around
it as a function of time. t = 0 s, anaphase onset. Note that, after 100 s (when flows are stronger), chromosome velocity and local flow are correlated. S, Angle difference between
chromosomes and flow around them. Same dataset as in Fig. 2e. T, Angle difference between chromosomes and droplets around them (within ~5 µm distance). U, Time projection of endogenous lipid
droplets and chromosomes. Dashed white box, one droplet is next to chromosomes. Zoomed in inset, detail of chromosomes and droplet following parallel trajectories. Bar, 50 µm. V, Trajectory
of chromosomes and droplet of the cell in (u). Source data EXTENDED DATA FIG. 4 CELLS WITHOUT DNA GO THROUGH SEVERAL ROUNDS OF MITOSIS. A, Snapshots of an embryo (16-cell stage) with two
cells dividing showing α-tubulin-ATTO-565. Grey shadowed cell, with DNA and a mitotic spindle. Blue shadowed cell (‘cell 1’), without DNA and no mitotic spindle. Bars, 50 µm. Insets,
magnification of the spindle region showing H1-488 and Mito-GFP. Top cell, gey square, with DNA and H1-488 signal. Bottom cell, blue square, without DNA and no H1-488 signal. Bars, 20 µm. B,
Same embryo as in (a) one cell division after (32-cell stage) showing α-tubulin-ATTO-565. The initial cell in (a) (‘cell 1’, boundaries are marked with black line) divided and generated two
daughter cells (‘cell 1.1’ and ‘cell 1.2’). Bars, 50 µm. Insets, magnification of the spindle region of cell 1.1 and cell 1.2, showing Mito-GFP. Bars, 20 µm. Source data EXTENDED DATA FIG.
5 ACTIN IS NOT REQUIRED FOR NER SCALING. A, Time-lapse of a dividing cell (4-cell stage). Utrophin-GFP, actin. Blue dashed box, chromosome localization visualized by WGA-640. Blue arrow
shows the orientation of chromosome separation. Insets, magnifications of the regions in the dashed boxes. Bars, 20 µm. At anaphase onset actin localizes at the cell cortex and in the
cytoplasm. Cytoplasmic localization decreases during anaphase while the cortex localization remains. B, Time-lapse of a dividing cell (4-cell stage) treated with latrunculin B. Already at
anaphase onset there is a very reduced cytoplasmic accumulation of actin. In (a) and (b), dashed grey line, cell boundary. t = 0 s, anaphase onset. Insets, magnifications of the regions in
the dashed boxes. Bars, 20 µm. C and D, Chromosome velocity in control and latrunculin B treated embryos in a big (4-cell stage) and small cell (16-cell stage). Control, 4-cell, \({v}_{A}\)
(0–100 s) n = 26 cells from 26 embryos and \({v}_{B}\) (100–200 s) n = 34 cells from 34 embryos. Latrunculin B, 4-cell, \({v}_{A}\) (0–100 s), n = 5 cells from 5 embryos and \({v}_{B}\)
(100–200 s) n = 8 cells from 8 embryos. Control, 16-cell, \({v}_{A}\) (0–100 s), n = 17 cells from 16 embryos and \({v}_{B}\) (100–200 s), n = 20 cells from 19 embryos. Latrunculin B,
16-cell, \({v}_{A}\) (0–100 s), n = 5 cells from 5 embryos and \({v}_{B}\) (100–200 s), n = 8 cells from 8 embryos. E and F, Position of WGA and NER in control and latrunculin B treated
embryos in a big (4-cell stage) and small cell (16-cell stage). For (e), n = 17, 9, 4 and 10 cells, from 17, 9, 3 and 10 embryos, for control and latrunculin B at 4-cell and control and
latrunculin B at 16-cell stages, respectively. For (f), n = 17, 9, 3 and 9 cells, from 17, 9, 3 and 9 embryos, for control and latrunculin B at 4-cell and control and latrunculin at 16-cell
stages, respectively. Statistics, two-tailed unpaired t-test. Error bars, s.e.m. Source data EXTENDED DATA FIG. 6 ASTRAL MICROTUBULE GROWTH IN ANAPHASE IN CONTROL AND AFTER SBTUB3-P
LIGHT-INDUCED MICROTUBULE DEPOLYMERIZATION. A and B, Time-lapse of a control dividing cell in metaphase and anaphase, respectively. Insets, chromosome region. Blue dashed box in (a),
magnification in (h). C and D, Time-lapse of a dividing cell injected with SBTub3-P before (metaphase) and after (anaphase) 405 laser drug activation, respectively. EMTB-3xGFP, microtubules.
Bars, 50 µm. Insets, chromosome region. Bars, 20 µm. t = 0 s, first time point after 405 laser. A corresponding time point was chosen for the control cell in (a). Note that EMTB-3xGFP shows
a stronger microtubule labelling in the periphery of the aster, as previously shown32,52. E, Flow velocity near the chromosomes (n = 15 and 6 PIV measurements, from 10 and 3 embryos for
control and SBTub-3P, respectively. For each cell, there can be up to two vorticity measurements corresponding to the sets of segregating chromosomes in the dividing cell. F, 4-cell stage
cell showing microtubules (DCX-GFP). Schematics show the variables measured (_d__a_ and _r__a_) to calculate aster anisotropy (_d__a__/r__a_; see Methods for details). G, Aster anisotropy
for a range of cell sizes (n = 6, 6 and 5 asters, from 5, 4 and 3 embryos at 4-, 8- and 16-cell stages, respectively). Statistics, two-tailed one sample t-test. P-value = 2 × 10−5, 6 × 10−5
and 3 × 10−5, for 4-, 8- and 16-cell stage embryos, respectively, indicating that anisotropy is significantly different from 1. Error bars, s.e.m. Source data EXTENDED DATA FIG. 7 FAST AND
SLOW EPISODES OF MITOCHONDRIA TRACKS DURING ANAPHASE. Tracks were manually segmented into fast, (a) and (b), and slow episodes, (c) and (d). A, Fast episodes of mitochondrial tracks for a
collection of 4-cell stage cells (n = 68 tracks, 6 cells from 6 embryos). B, Mean square displacement of individual tracks as a function of delay of the episodes in (a). C, Slow episodes of
mitochondrial tracks for the same cells as in (a). D, Mean square displacement of individual tracks as a function of delay of the episodes in (c). E, Fast episodes of mitochondrial tracks
color coded by velocity. Same data set as in (a). F, Fast episodes of mitochondrial tracks color coded by velocity for all cell stages analyzed (2- to 128-cell stage, n = 365 tracks, 36
cells, from 7 embryos). G, Fast episodes of mitochondrial tracks color coded by the angle difference between each segment of the track and the center of the aster. Angle close to zero means
that fast episodes are radial. Same data set as in (f). H, Fast and slow episodes of mitochondrial tracks color coded by time for the same data set as in (f) and (g). I, Slow episodes of
mitochondrial tracks color coded by velocity. Note the difference in color when compare with (e). Same data set as in (a). J, Slow episodes of mitochondrial tracks color coded by velocity,
for all stages analyzed (same dataset as in (f)). K, Individual tracks of mitochondria from a single cell in anaphase (same cell as in Fig. 4f). Fast episodes (continuous line) are radial
while slow episodes (dashed line) have the direction of the flows (highlighted in grey). From (e) to (k), (0,0), aster center. Source data EXTENDED DATA FIG. 8 FLOW VELOCITY NEAR THE
CHROMOSOMES CORRELATES WITH CHROMOSOME VELOCITY ACROSS ALL CELL SIZES. A-G, Correlation of flow velocity near each set of chromosomes in anaphase and the velocity of chromosomes themselves.
Each dot in the graph corresponds to the correlation in one time point in the 100–200 s after anaphase time window. n = 17, 15, 22, 6, 10, 6 and 10 PIV measurements x 10 time points,
corresponding to the 100–200 s after anaphase time window, from 7, 8, 8, 3, 5, 3, 5 embryos at 4-, 8-, 16-,32-,64-,128-cell stages, respectively. For each cell, there can be up to two
vorticity measurements corresponding to the sets of segregating chromosomes in the dividing cell. H, Flow velocity at the spindle midzone region _versus_ chromosome velocity. Velocities are
not correlated. n = 15 PIV measurments, from 8 embryos at 4-cell stage. I, Angle difference between chromosomes and the flow at the spindle midzone region, for the same dataset as in (h).
Source data EXTENDED DATA FIG. 9 BIOCHEMICAL PROPERTIES OF THE FLOWS DO NOT DECREASE WITH CELL SIZE NEITHER CHANGE IN REDUCED SIZED EMBRYOS. A, Frequency of EB3 comets as a function of cell
stage. n = 3, 2, 3, 3, 3, 2 cells from 3, 2, 3, 3, 3, 2 embryos at 4-, 8-, 16-, 32-, 64- and 128-cell stages, respectively. B, Velocity of EB3 comets as a function of cell stage. n = 675,
1379, 911, 908, 1101 and 828 tracks from 4, 4, 5, 5, 5, 4 cells in 4, 4, 5, 5, 5, 4 embryos at 4-, 8-, 16-, 32-, 64- and 128-cell stages, respectively. P-values are approximate. C, Aster
diameter measured at anaphase onset as a function of cell stage. Images show examples of aster size for some representative stages. n = 7, 7, 6, 7, 6, 6 asters from 4, 4, 3, 4, 4 and 4
cells, in 4, 4, 2, 2, 2 and 2 embryos at 4-, 8-, 16-, 32-, 64- and 128-cell stages, respectively. D, Aster growth velocity during anaphase as a function of cell stage. n = 2, 5, 4, 5, 3, 6
asters from 2, 3, 2, 3, 2 and 3 cells, in 2, 3, 1, 1, 1 and 1 embryo(s) at 4-, 8-, 16-, 32-, 64- and 128-cell stages, respectively. E, Mitochondria density in a 100µm2 reference ROI as a
function of cell stage n = 6, 6, 5, 4, 2, 2 cells from 6, 6, 5, 4, 2 and 2 embryos at 4-, 8-, 16-, 32-, 64- and 128-cell stages, respectively. F-I, Frequency, velocity, run-length and
duration of fast episodes of mitochondria tracks as a function of cell stage. For (f), n = 6, 6, 5, 4, 2, 1 cell from 6, 6, 5, 4, 2 and 2 embryos at 4-, 8-, 16-, 32-, 64- and 128-cell
stages, respectively. For (g), (h) and (i), n = 91, 39, 67, 60, 67, 7 tracks from 7, 7, 6, 5, 4 and 1 cell(s) from 7, 7, 6, 5, 4 and 1 embryo(s). J, Cell length, along the axis of chromosome
separation, in control and aspirated 2- (control, n = 12 cells, 12 embryos; aspiration, n = 13 cells, 12 embryos) and 4-cell stage embryos. (control, n = 17 cells, 15 embryos; aspiration, n
= 11 cells, 10 embryos). K-M, Velocity, run-length and duration of fast episodes of mitochondria tracks in control and aspirated 2- (control, n = 28 tracks, 5 embryos; aspiration, n = 9
tracks, 1 embryo) and control and aspirated 4-cell stage embryos (control, n = 91 tracks, 7 embryos; aspiration, n = 55 tracks, 3 embryos). N Time of WGA in control and aspirated 2-
(control, n = 10 cells, 9 embryos; aspiration, n = 8 cells, 7 embryos) and 4-cell stage embryos (control, n = 11 cells, 11 embryos; aspiration, n = 8 cells, 7 embryos). O, Position of WGA in
control and aspirated 2- (control, n = 14 cells, 14 embryos; aspiration, n = 9 cells, 8 embryos) and 4-cell stage embryos (control, n = 17 cells, 17 embryos; aspiration, n = 8 cells, 7
embryos). P, Time of NER in control and aspirated 2- (control, n = 11 cells, 11 embryos; aspiration, n = 8 cells, 7 embryos) and 4-cell stage embryos (control, n = 11 cells, 11 embryos;
aspiration, n = 8 cells, 7 embryos). Q, Velocity of EB3 comets in 4-cell stage control (n=675tracks, 4 cells from 4 embryos) and aspirated embryos (n=690tracks, 5 cells from 5 embryos).
P-value is approximate. N, sample size. Error bars, s.e.m. Statistics, One-way ANOVA (c, e, f, h, i, j, n, o and p), Kruskal-Wallis tests (a, b, d, g, h, I, j, l and m) for parametric and
non-parametric samples, respectively. For q, two-tailed Mann-Whitney test. ns, not significant. ****p < 0.0001; ***p < 0.001; **p < 0.01. Source data EXTENDED DATA FIG. 10 POSITION
OF NER CORRELATES WITH CELL LENGTH RATHER THAN CELL VOLUME. A, Scheme and brightfield image of a control and a patterned 2-cell stage embryo. Bars, 200 µm B, Aspect ratio of cells in control
(n = 4cells) and patterned 2-cell stage embryos (n = 9cells). C, Position of NER in control 2- (n = 14cells) and 8-cell (n = 14cells) stage embryo and patterned 2-cell stage embryos
(n=10cells). Position of NER correlates with cell length. D, Time projection (100–200 s after anaphase onset) of a patterned cell (2-cell stage) in anaphase showing Mito-GFP and WGA-640.
Bar, 50 µm. E and F, PIV analysis of the cell in (d). Arrows, flow field. Colour code, velocity and vorticity, respectively. Statistics, two-tailed Mann-Whitney test. Error bars, s.e.m.
Source data SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Fig. 1, Discussion, Table 1 and References. REPORTING SUMMARY PEER REVIEW FILE SUPPLEMENTARY VIDEO 1 PIV
analysis of a cell in a four-cell-stage embryo during anaphase. Left: overlay of Mito-GFP and vector field (arrows). Middle: overlay of velocity (colour code) and the vector field (arrows).
Right: overlay of vorticity (colour code) and vector field (arrows). Time is min:s. SUPPLEMENTARY VIDEO 2 Cell division of a control and a cell without DNA. Two neighbour cells in the same
embryo. Left cell: with DNA, shows a mitotic spindle and separates chromosomes in anaphase. Right cell: cell without DNA, does not form a mitotic spindle but asters grow during anaphase.
Time is min:s. SUPPLEMENTARY VIDEO 3 Cell division of a cell without DNA and respective PIV analysis. Left: microtubule labelling showing the absence of a mitotic spindle. Middle: Mito-GFP
labelling used to analyse the flows. Right: overlay of Mito-GFP and the flow field. Time is min:s. SUPPLEMENTARY TABLE 1 Supplementary Table 1. SOURCE DATA SOURCE DATA ALL FIGURES
Statistical source data. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Afonso, O., Dumoulin, L., Kruse, K. _et al._ Cytoplasmic flow is a cell size sensor that scales anaphase. _Nat Cell Biol_ 27, 273–282 (2025).
https://doi.org/10.1038/s41556-024-01605-6 Download citation * Received: 08 January 2024 * Accepted: 22 November 2024 * Published: 31 January 2025 * Issue Date: February 2025 * DOI:
https://doi.org/10.1038/s41556-024-01605-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







