- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
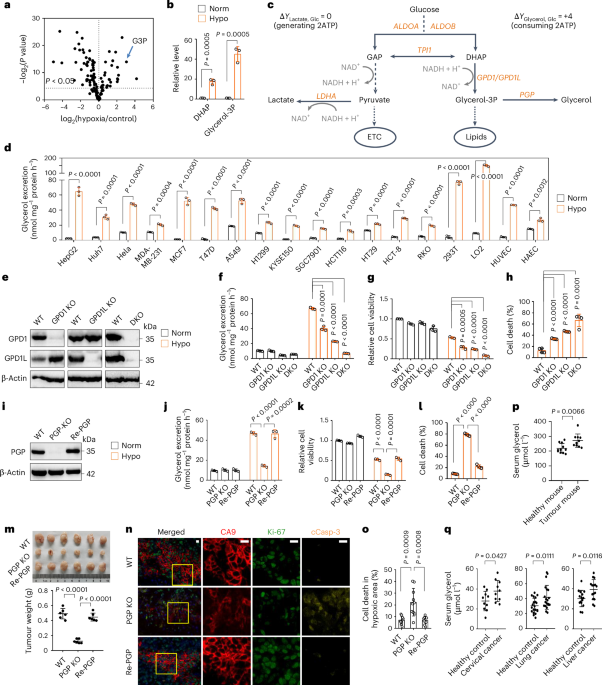
ABSTRACT Glucose metabolism has been studied extensively, but the role of glucose-derived excretory glycerol remains unclear. Here we show that hypoxia induces NADH accumulation to promote
glycerol excretion and this pathway consumes NADH continuously, thus attenuating its accumulation and reductive stress. Aldolase B accounts for glycerol biosynthesis by forming a complex
with glycerol 3-phosphate dehydrogenases GPD1 and GPD1L. Blocking GPD1, GPD1L or glycerol 3-phosphate phosphatase exacerbates reductive stress and suppresses cell proliferation under hypoxia
and tumour growth in vivo. Overexpression of these enzymes increases glycerol excretion but still reduces cell viability under hypoxia and tumour proliferation due to energy stress. AMPK
inactivates aldolase B to mitigate glycerol synthesis that dissipates ATP, alleviating NADH accumulation-induced energy crisis. Therefore, glycerol biosynthesis/excretion regulates the
trade-off between reductive stress and energy stress. Moreover, this mode of regulation seems to be prevalent in reductive stress-driven transformations, enhancing our understanding of the
metabolic complexity and guiding tumour treatment. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more
Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS THE ALTERNATIVE ACTIVITY OF NUCLEAR PHGDH CONTRIBUTES TO TUMOUR GROWTH UNDER NUTRIENT STRESS Article 18 October 2021 TARGETING
ALDOLASE A IN HEPATOCELLULAR CARCINOMA LEADS TO IMBALANCED GLYCOLYSIS AND ENERGY STRESS DUE TO UNCONTROLLED FBP ACCUMULATION Article Open access 20 January 2025 GFAT1-LINKED TAB1
GLUTAMYLATION SUSTAINS P38 MAPK ACTIVATION AND PROMOTES LUNG CANCER CELL SURVIVAL UNDER GLUCOSE STARVATION Article Open access 09 August 2022 DATA AVAILABILITY MS data have been deposited in
ProteomeXchange with the primary accession code PXD056351 (http://proteomecentral.proteomexchange.org)50,51. The human cancer data were derived from the TCGA Research Network at
http://cancergenome.nih.gov/. The dataset derived from this resource that supports the findings of this study is available in Supplementary Table 1. All other data supporting the findings of
this study are available from the corresponding author on reasonable request. Source data are provided with this paper. REFERENCES * Hanahan, D. Hallmarks of cancer: new dimensions. _Cancer
Discov._ 12, 31–46 (2022). CAS PubMed Google Scholar * Terry, A. R. & Hay, N. Fuelling cancer cells. _Nat. Rev. Endocrinol._ 15, 71–72 (2019). CAS PubMed PubMed Central Google
Scholar * Yu, B. et al. Measuring tumor cycling hypoxia and angiogenesis using a side-firing fiber optic probe. _J. Biophotonics_ 7, 552–564 (2014). CAS PubMed Google Scholar *
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. _Nature_ 407, 249–257 (2000). CAS PubMed Google Scholar * Chappell, J. C., Payne, L. B. & Rathmell, W. K.
Hypoxia, angiogenesis, and metabolism in the hereditary kidney cancers. _J. Clin. Invest._ 129, 442–451 (2019). PubMed PubMed Central Google Scholar * Vander Heiden, M. G. &
DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. _Cell_ 168, 657–669 (2017). PubMed Central Google Scholar * Liu, M. et al. Inhibiting both
proline biosynthesis and lipogenesis synergistically suppresses tumor growth. _J. Exp. Med._ 217, e20191226 (2020). PubMed PubMed Central Google Scholar * Yang, R. et al. Identification
of purine biosynthesis as an NADH-sensing pathway to mediate energy stress. _Nat. Commun._ 13, 7031 (2022). CAS PubMed PubMed Central Google Scholar * Titov, D. V. et al. Complementation
of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. _Science_ 352, 231–235 (2016). CAS PubMed PubMed Central Google Scholar * Missiaen, R., Lesner, N. P.
& Simon, M. C. HIF: a master regulator of nutrient availability and metabolic cross-talk in the tumor microenvironment. _EMBO J._ 42, e112067 (2023). CAS PubMed PubMed Central Google
Scholar * Yang, R., Ying, G. & Li, B. Potential of electron transfer and its application in dictating routes of biochemical processes associated with metabolic reprogramming. _Front.
Med._ 15, 679–692 (2021). PubMed Google Scholar * Sun, L., Suo, C., Li, S. T., Zhang, H. & Gao, P. Metabolic reprogramming for cancer cells and their microenvironment: beyond the
Warburg Effect. _Biochim. Biophys. Acta Rev. Cancer_ 1870, 51–66 (2018). CAS PubMed Google Scholar * Mugabo, Y. et al. Identification of a mammalian glycerol-3-phosphate phosphatase: role
in metabolism and signaling in pancreatic beta-cells and hepatocytes. _Proc. Natl Acad. Sci. USA_ 113, E430–E439 (2016). CAS PubMed PubMed Central Google Scholar * Nielsen, S. et al.
Aquaporins in the kidney: from molecules to medicine. _Physiol. Rev._ 82, 205–244 (2002). CAS PubMed Google Scholar * Zhang, L. & Tew, K. D. Reductive stress in cancer. _Adv. Cancer
Res_ 152, 383–413 (2021). CAS PubMed Google Scholar * Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. _Cell_ 162,
552–563 (2015). CAS PubMed PubMed Central Google Scholar * Billiard, J. et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells.
_Cancer Metab._ 1, 19 (2013). PubMed PubMed Central Google Scholar * Kim, W. et al. Polyunsaturated fatty acid desaturation is a mechanism for glycolytic NAD+ recycling. _Cell Metab._ 29,
856–870.e7 (2019). CAS PubMed PubMed Central Google Scholar * Obukowicz, M. G. et al. Novel, selective Δ6 or Δ5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. _J.
Pharmacol. Exp. Ther._ 287, 157–166 (1998). CAS PubMed Google Scholar * Graziano, F. et al. Glycolysis gene expression analysis and selective metabolic advantage in the clinical
progression of colorectal cancer. _Pharmacogenomics J._ 17, 258–264 (2017). CAS PubMed Google Scholar * Finley, L. W. et al. SIRT3 opposes reprogramming of cancer cell metabolism through
HIF1α destabilization. _Cancer Cell_ 19, 416–428 (2011). CAS PubMed PubMed Central Google Scholar * O’Donnell, J. L. et al. Oncological implications of hypoxia inducible factor-1α
(HIF-1α) expression. _Cancer Treat. Rev._ 32, 407–416 (2006). PubMed Google Scholar * Tennant, D. A., Duran, R. V. & Gottlieb, E. Targeting metabolic transformation for cancer therapy.
_Nat. Rev. Cancer_ 10, 267–277 (2010). CAS PubMed Google Scholar * Li, B. et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. _Nature_ 513, 251–255 (2014). CAS
PubMed PubMed Central Google Scholar * Grandjean, G. et al. Definition of a novel feed-forward mechanism for glycolysis-HIF1α signaling in hypoxic tumors highlights aldolase A as a
therapeutic target. _Cancer Res._ 76, 4259–4269 (2016). CAS PubMed PubMed Central Google Scholar * Cascone, T. et al. Increased Tumor Glycolysis Characterizes Immune Resistance to
Adoptive T Cell Therapy. _Cell Metab._ 27, 977–987.e4 (2018). CAS PubMed PubMed Central Google Scholar * Ou, X. et al. Crystal structures of human glycerol 3-phosphate dehydrogenase 1
(GPD1). _J. Mol. Biol._ 357, 858–869 (2006). CAS PubMed Google Scholar * Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components
of type 2 diabetes and the metabolic syndrome. _Cell Metab._ 3, 403–416 (2006). CAS PubMed Google Scholar * Schaffer, B. E. et al. Identification of AMPK phosphorylation sites reveals a
network of proteins involved in cell invasion and facilitates large-scale substrate prediction. _Cell Metab._ 22, 907–921 (2015). CAS PubMed PubMed Central Google Scholar * Hardie, D.
G., Schaffer, B. E. & Brunet, A. AMPK: an energy-sensing pathway with multiple inputs and outputs. _Trends Cell Biol._ 26, 190–201 (2016). CAS PubMed Google Scholar * Steinberg, G. R.
& Hardie, D. G. New insights into activation and function of the AMPK. _Nat. Rev. Mol. Cell Biol._ 24, 255–272 (2023). CAS PubMed Google Scholar * Park, S. H. et al.
Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. _J. Appl Physiol. (1985)_ 92, 2475–2482 (2002). CAS PubMed Google Scholar * Kim, J. & Guan, K. L.
mTOR as a central hub of nutrient signalling and cell growth. _Nat. Cell Biol._ 21, 63–71 (2019). CAS PubMed Google Scholar * Liu, Q. et al. Discovery of
1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1_H_)-one as a highly potent, selective mammalian target of rapamycin (mTOR)
inhibitor for the treatment of cancer. _J. Med. Chem._ 53, 7146–7155 (2010). CAS PubMed PubMed Central Google Scholar * Zhou, G. et al. Role of AMP-activated protein kinase in mechanism
of metformin action. _J. Clin. Invest._ 108, 1167–1174 (2001). CAS PubMed PubMed Central Google Scholar * Li, M. et al. Aldolase B suppresses hepatocellular carcinogenesis by inhibiting
G6PD and pentose phosphate pathways. _Nat. Cancer_ 1, 735–747 (2020). CAS PubMed Google Scholar * Liu, G. et al. Fructose-1,6-bisphosphate aldolase b depletion promotes hepatocellular
carcinogenesis through activating insulin receptor signaling and lipogenesis. _Hepatology_ 74, 3037–3055 (2021). CAS PubMed Google Scholar * He, X. et al. Loss of hepatic aldolase B
activates Akt and promotes hepatocellular carcinogenesis by destabilizing the Aldob/Akt/PP2A protein complex. _PLoS Biol._ 18, e3000803 (2020). CAS PubMed PubMed Central Google Scholar *
He, J. et al. Downregulation of ALDOB is associated with poor prognosis of patients with gastric cancer. _Onco Targets Ther._ 9, 6099–6109 (2016). PubMed PubMed Central Google Scholar *
Sanders, E. & Diehl, S. Analysis and interpretation of transcriptomic data obtained from extended Warburg effect genes in patients with clear cell renal cell carcinoma. _Oncoscience_ 2,
151–186 (2015). PubMed PubMed Central Google Scholar * Bu, P. et al. Aldolase B-Mediated Fructose Metabolism Drives Metabolic Reprogramming of Colon Cancer Liver Metastasis. _Cell Metab._
27, 1249–1262.e4 (2018). CAS PubMed PubMed Central Google Scholar * Chen, W. L. et al. Enhanced fructose utilization mediated by SLC2A5 is a unique metabolic feature of acute myeloid
leukemia with therapeutic potential. _Cancer Cell_ 30, 779–791 (2016). PubMed PubMed Central Google Scholar * Zhou, P. et al. High dietary fructose promotes hepatocellular carcinoma
progression by enhancing O-GlcNAcylation via microbiota-derived acetate. _Cell Metab._ 35, 1961–1975.e6 (2023). CAS PubMed Google Scholar * Cui, Y. et al. Fructose-induced mTORC1
activation promotes pancreatic cancer progression through inhibition of autophagy. _Cancer Res._ 83, 4063–4079 (2023). PubMed PubMed Central Google Scholar * Wang, Y. et al. Coordinative
metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. _Nat. Commun._ 10, 201 (2019). PubMed PubMed Central Google Scholar * Qian, X. et al. Conversion
of PRPS hexamer to monomer by AMPK-mediated phosphorylation inhibits nucleotide synthesis in response to energy stress. _Cancer Discov._ 8, 94–107 (2018). CAS PubMed Google Scholar *
Theret, M. et al. AMPKα1–LDH pathway regulates muscle stem cell self-renewal by controlling metabolic homeostasis. _EMBO J._ 36, 1946–1962 (2017). CAS PubMed PubMed Central Google Scholar
* Burg, J. S. & Espenshade, P. J. Regulation of HMG-CoA reductase in mammals and yeast. _Prog. Lipid Res._ 50, 403–410 (2011). CAS PubMed PubMed Central Google Scholar * Li, L. et
al. ALKBH5–PYCR2 positive feedback loop promotes proneural–mesenchymal transition via proline synthesis In GBM. _J. Cancer_ 14, 1579–1591 (2023). CAS PubMed PubMed Central Google Scholar
* Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. _Nucleic Acids Res._ 50, D1522–D1527 (2022). CAS PubMed Google Scholar * Ma, J. et al. iProX: an
integrated proteome resource. _Nucleic Acids Res._ 47, D1211–D1217 (2019). PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank X. Liu (Metabolomics Facility at Tsinghua
University Branch of China National Center for Protein Sciences, China) for technical help. This work was supported by the National Natural Science Foundation of China (82325038 and 82030093
to B.L.) and China Postdoctoral Science Foundation (GZB20230454 to R.Y.) AUTHOR INFORMATION Author notes * These authors contributed equally: Xuewei Zhai, Ronghui Yang. AUTHORS AND
AFFILIATIONS * Beijing Institute of Hepatology, Beijing Youan Hospital, Capital Medical University, Beijing, China Xuewei Zhai, Ronghui Yang, Zihao Guo, Pengjiao Hou, Xuexue Li, Ziwen Lu,
Luxin Qiao & Binghui Li * Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Capital Medical University, Beijing, China Qiaoyun Chu, Yanxia Fu, Jing Niu
& Binghui Li * Department of Cancer Cell Biology and National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China Changsen Bai
& Binghui Li Authors * Xuewei Zhai View author publications You can also search for this author inPubMed Google Scholar * Ronghui Yang View author publications You can also search for
this author inPubMed Google Scholar * Qiaoyun Chu View author publications You can also search for this author inPubMed Google Scholar * Zihao Guo View author publications You can also
search for this author inPubMed Google Scholar * Pengjiao Hou View author publications You can also search for this author inPubMed Google Scholar * Xuexue Li View author publications You
can also search for this author inPubMed Google Scholar * Changsen Bai View author publications You can also search for this author inPubMed Google Scholar * Ziwen Lu View author
publications You can also search for this author inPubMed Google Scholar * Luxin Qiao View author publications You can also search for this author inPubMed Google Scholar * Yanxia Fu View
author publications You can also search for this author inPubMed Google Scholar * Jing Niu View author publications You can also search for this author inPubMed Google Scholar * Binghui Li
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.L. conceived the study and designed experiments; X.Z. and R.Y. performed experiments;
Q.C. prepared some constructs and cell lines; Z.G., P.H., X.L., C.B. and Z.L. collected and analysed data; L.Q., Y.F. and J.N. provided conceptual advice and gave technical support; B.L.
wrote the manuscript; X.Z. and R.Y. edited the manuscript. CORRESPONDING AUTHOR Correspondence to Binghui Li. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Cell Biology_ thanks Lluis Fajas, Constantinos Koumenis and the other, anonymous, reviewer(s) for their contribution to the peer review
of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 HYPOXIA PROMOTES GLYCEROL 3-PHOSPHATE BIOSYNTHESIS. A, The relative abundance of metabolites of glycolysis in HeLa cells under
normoxia, hypoxia, or antimycin A (AntA, 2 μM) treatment for 8 h. B, The relative abundance of glycerol 3-phosphate and its precursor DHAP in HeLa cells under normoxia, hypoxia, or AntA (2
μM) treatment for 8 h. C, Isotopomer tracing analysis of glycerol 3-phosphate biosynthesis in HeLa cells cultured with 13C6-glucose (25 mM) or 13C5-glutamine (2 mM) for 8 h. D,E, Using
N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) to modify the hydroxyl groups of glycerol and glycerol 3-phosphate and detect them by gas chromatography-mass spectrometry (GC-MS). F,
Isotopomer tracing analysis of excreted glycerol in HeLa cells cultured with 13C6-glucose (25 mM) under normoxia or hypoxia for 8 h. G,H, The levels of excreted glycerol determined by GC-MS
or a liquid sample glycerol assay kit in HeLa cells cultured under normoxia and hypoxia for 24 h. Values are shown as mean ± SD, n = 3 biologically independent samples, two-tailed Student’s
t-tests. Source data EXTENDED DATA FIG. 2 HYPOXIA PROMOTES GLUCOSE UPTAKE AND LACTATE EXCRETION. A, Glucose uptake of several cell types including tumor cells and endothelial cells cultured
under normoxia and hypoxia for 24 h. B, Lactate excretion of several cell types including tumor cells and endothelial cells cultured under normoxia and hypoxia for 24 h. C-H, The glycerol
excretion, glucose uptake, and lactate excretion in HeLa and A549 cells cultured under hypoxia as indicated. I, Western blot analysis of PGP, GPD1, GPD1L, GPD2, ALDOA, ALDOB, TPI1, and
β-Actin expression in different cells as indicated. J, Immunoblot verification of GPD2 knockout in HeLa and A549 cells. K, Effects of GPD2 knockout on glycerol excretion of HeLa cells
cultured under normoxia and hypoxia for 24 h. Values are shown as mean ± SD, n = 3 biologically independent samples (a-h, k), two-tailed Student’s t-tests. In i, data are verified in two
replicates with similar results. Source data EXTENDED DATA FIG. 3 EFFECTS OF GLYCEROL EXCRETION ON CELL VIABILITY UNDER HYPOXIA. A, Effects of GPD1 and/or GPD1L knockout on colony formation
in HeLa cells cultured for 10 days. B, Effects of PGP knockout and PGP re-expression on colony formation in HeLa cells cultured for 10 days. C, Effects of PGP knockout and PGP re-expression
on glycerol excretion of A549 cells cultured under normoxia and hypoxia for 24 h. D, Effects of PGP knockout and PGP re-expression on cell viability of A549 cells cultured under normoxia and
hypoxia for 48 h. E, Effects of PGP knockout and PGP re-expression on colony formation of A549 cells cultured for 10 days. F, Effects of PGP knockout and PGP re-expression on glycerol
excretion of HepG2 cells cultured under normoxia and hypoxia for 24 h. G, Effects of PGP knockout and PGP re-expression on cell viability of HepG2 cells cultured under normoxia and hypoxia
for 48 h. H, Effects of PGP knockout and PGP re-expression on colony formation of HepG2 cells cultured for 10 days. I, Analysis of the mRNA levels of aquaporins (AQPs) based on the data from
Cancer Cell Line Encyclopedia (CCLE). J, Effects of AQP3 knockout on glycerol excretion and cell viability of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h (glycerol
excretion) or 72 h (cell proliferation). Values are shown as mean ± SD, n = 3 biologically independent samples, two-tailed Student’s t-tests. Source data EXTENDED DATA FIG. 4 EFFECTS OF NADH
ACCUMULATION ON GLUCOSE UPTAKE, LACTATE EXCRETION, AND GLYCEROL EXCRETION. A, Immunoblot verification of CHOP and ATF4 activation in GPD1/GPD1L DKO, PGP KO and PGP re-expression HeLa cells
cultured under normoxia or hypoxia for 24 h. B, Effects of different concentrations of αKB on glucose uptake and lactate excretion of HeLa, A549, and HepG2 cells under hypoxia for 24 h. C,
Effects of different concentrations of LDHi on glucose uptake and lactate excretion of HeLa, A549, and HepG2 cells under normoxia for 24 h. D, Glucose uptake and lactate excretion of HeLa,
A549 and HepG2 cells cultured as indicated for 24 h. E, Effects of different concentrations of SC-26196 on the ratios of NADH/NAD+, glycerol excretion, glucose uptake, and lactate excretion
of HeLa, A549, and HepG2 cells cultured under normoxia for 8 h (NADH/NAD+ ratios) or 24 h (glycerol excretion, glucose uptake, and lactate excretion). F, Effects of αKB (2 mM) on cell
viability of HeLa PGP KO or GPD1/GPD1L DKO cells cultured under hypoxia for 48 h. Values are shown as mean ± SD, n = 3 biologically independent samples (b-f), two-tailed Student’s t-tests.
In a, data are verified in three replicates with similar results. Source data EXTENDED DATA FIG. 5 EFFECTS OF GLUCOSE UPTAKE ON GLYCEROL EXCRETION. A, Western blots for the expression of
PKM2, LDHA, GAPDH, and β-Actin in HeLa and A549 cells treated with normoxia, hypoxia, or AntA (2 μM) for 24 h. B, Effects of HIF2α knockdown on glycerol excretion of HeLa and A549 cells
cultured under hypoxia for 24 h. C, Immunoblot verification of GLUT1 knockdown in HeLa and A549 cells. D-F, Effects of GLUT1 knockdown on glucose uptake, lactate excretion, and glycerol
excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h. G, Immunoblot verification of GLUT1 over-expression in HeLa and A549 cells. H-J, Effects of GLUT1
over-expression on glucose uptake, lactate excretion, and glycerol excretion of HeLa and A549 cells cultured under normoxia, hypoxia, or AntA (2 μM) treatment for 24 h. Values are shown as
mean ± SD, n = 3 biologically independent samples (b, d-f, h-j), two-tailed Student’s t-tests. In a, data are verified in two replicates with similar results. Source data EXTENDED DATA FIG.
6 EFFECTS OF PGP, GPD1, OR GPD1L OVER-EXPRESSION ON GLUCOSE UPTAKE, LACTATE EXCRETION, GLYCEROL EXCRETION, AND CELL GROWTH. A, Effects of PGP, GPD1, or GPD1L over-expression on glucose
uptake and lactate excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h. B, Immunoblot verification of LKB1 expression in HeLa, A549, SK-Hep-1, and HCC-LM3 cells. C,
Immunoblot verification of over-expression of PGP, GPD1L, or GPD1 in SK-Hep-1 and HCC-LM3 cells. D, Effects of PGP, GPD1, or GPD1L over-expression on glycerol excretion of SK-Hep-1 and
HCC-LM3 cells cultured under normoxia and hypoxia for 24 h. E, Effects of PGP, GPD1, or GPD1L over-expression on cell viability of SK-Hep-1 and HCC-LM3 cells cultured with low nutrient
medium (10% fetal bovine serum medium containing 2 mM of glucose, without glutamine and pyruvate) under normoxia and hypoxia for 8 h. F, Effects of PGP, GPD1, or GPD1L over-expression on
cell viability of SK-Hep-1 and HCC-LM3 cells cultured in normal or nutrient-deprived media. Nutrient-deprived media contained 10% fetal bovine serum but without glucose, glutamine, and
pyruvate. G, Effects of PGP, GPD1L, or GPD1 over-expression on AMPK activation in SK-Hep-1 and HCC-LM3 cells cultured in nutrient-deprived medium. H, Tumor formation ability in nude mice of
SK-Hep-1 cells with PGP, GPD1, or GPD1L over-expression. Values are shown as mean ± SD, n = 3 biologically independent samples (a,d,e,f) or n = 5 biologically independent mice (h),
two-tailed Student’s t-tests. In g, data are verified in two replicates with similar results. Source data EXTENDED DATA FIG. 7 EFFECTS OF ALDOLASES AND AMPK ON GLUCOSE UPTAKE, LACTATE
EXCRETION, AND GLYCEROL EXCRETION. A, Effects of ALDOA over-expression on glycerol excretion, glucose uptake, and lactate excretion of HeLa and A549 cells cultured under normoxia and hypoxia
for 24 h. B, Effects of ALDOC over-expression on glycerol excretion, glucose uptake, and lactate excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h. C, Effects of
A769662 treatment on glycerol excretion, glucose uptake, and lactate excretion of A549, SK-Hep-1, and HCC-LM3 cells cultured under normoxia and hypoxia for 12 h. D, Effects of Compound C on
AMPK pathway in HeLa cells. E, Effects of Compound C on glycerol excretion, glucose uptake, and lactate excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 12 h. F,
Effects of AMPKα knockdown and AMPKα1 re-expression on glycerol excretion, glucose uptake, and lactate excretion of A549 cells cultured under normoxia and hypoxia for 24 h. G.
Immunoprecipitation (IP) analysis of the interaction between endogenous ALDOB, GPD1 and GPD1L in HepG2 cells with anti-ADLOB antibody. Rabbit IgG was used as a negative control. WCL,
whole-cell lysate. pALDOB T245, AMPK, pAMPK T172, ACC1 and pACC1 S79 were also blotted. Values are shown as mean ± SD, n = 3 biologically independent samples (a-c, e-f), two-tailed Student’s
t-tests. In d,g, data are verified in two replicates with similar results. Source data EXTENDED DATA FIG. 8 INACTIVATION OF ADLOB BY AMPK-MEDIATED PHOSPHORYLATION. A, HEK293T cells were
transfected with vector, Flag-GPD1, or Flag-GPD1L plasmids, and then cultured under normoxia or hypoxia for 8 h. IP assays were performed using anti-FLAG affinity M2 beads followed by
immunoblotting for the phosphorylation of GPD1 or GPD1L. B, Mass spectrometry was used to detect the phosphorylation site of ALDOB in HeLa cells after treatment with A769662 for 8 h. C,
Conservation of the phosphorylation site in ALDOB among different species. Amino acid residues around Thr245 are shown. D, The activity of GST-ALDOB-WT, T245A, and T245D mutants purified
from an _E. coli_ expression system incubated with or without active AMPK as indicated. Values are shown as mean ± SD, data are verified in two replicates with similar results (a) and n = 3
biologically independent samples (d). Source data EXTENDED DATA FIG. 9 THE TRADE-OFF REGULATION BETWEEN REDUCTIVE STRESS AND ENERGY STRESS. A, Effects of αKB and _Lb_NOX on AMPK activation
of A549 cells cultured under respiratory chain inhibition or hypoxia for 24 h. αKB, 2 mM; Dox, 0.1 μg/mL. B,C, Effects of αKB and _Lb_NOX on cellular NADH/NAD+ ratio (b) and ATP/AMP ratio
(c) of A549 cells cultured under respiratory chain inhibition or hypoxia for 24 h. αKB, 2 mM; Dox, 0.1 μg/mL. D, Effects of Torin-1 (0.1 μM) and Rapamycin (1 μM) on AMPK activation of A549
cells cultured under respiratory chain inhibition or hypoxia for 24 h. E,F, Effects of Torin-1 (0.1 μM) and Rapamycin (1 μM) on cellular NADH/NAD+ ratio (e) and ATP/AMP ratio (f) of A549
cells cultured under respiratory chain inhibition or hypoxia for 24 h. G, Effects of Compound C (2 μM) on cell death of A549 cells cultured in nutrient-deprived medium under respiratory
chain inhibition or hypoxia for 12 h. H, Immunoblot verification of AMPKα knockout and AMPKα re-expression in HeLa cells. I, The effect of metformin (100 mg/kg/day, i.g.) or Compound C (20
mg/kg/day, i.p.) treatment alone or in combination on tumor formation ability in nude mice of HeLa cells. Metformin (Metf, 4 mM) and Ant A (2 μM) were used. Values are shown as mean ± SD, n
= 3 biologically independent samples (b,c,e,f,g) or n = 6 biologically independent mice (i), two way ANOVA (i), and two-tailed Student’s t-tests for others. In a,d, data are verified in two
replicates with similar results. Source data EXTENDED DATA FIG. 10 THE WORKING MODEL FOR TRADE-OFF BETWEEN REDUCTIVE STRESS AND ENERGY STRESS. A, Schematic diagram illustrating hypoxic
regulation of glycerol biosynthesis. Hypoxia promotes glycerol biosynthesis and excretion by inducing NADH accumulation and glucose uptake. Glycerol biosynthesis consumes NADH to reduce
reductive stress, but this process is accompanied by ATP consumption and thus may potentially provoke an energy crisis. Cells have evolved a negative feedback loop to suppress glycerol
synthesis through AMPK-mediated phosphorylation of ALDOB. B, The trade-off regulation between reductive stress and energy stress. Reductive stress induced by hypoxia or ETC inhibition
primarily contributes to energy stress and AMPK activation. To alleviate reductive stress, some metabolic pathways are promoted to consume NADH, along with ATP. In turn, the activated AMPK
negatively regulates these metabolic reactions to prevent catastrophic energy depletion. SUPPLEMENTARY INFORMATION REPORTING SUMMARY PEER REVIEW FILE SUPPLEMENTARY TABLE 1 Survival _P_-value
analysis based on TCGA datasets. SOURCE DATA SOURCE DATA FIG. 1 Unprocessed western blots. SOURCE DATA FIG. 1 Statistical source data. SOURCE DATA FIG. 2 Unprocessed western blots. SOURCE
DATA FIG. 2 Statistical source data. SOURCE DATA FIG. 3 Unprocessed western blots. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Unprocessed western blots. SOURCE DATA FIG.
4 Statistical source data. SOURCE DATA FIG. 5 Unprocessed western blots. SOURCE DATA FIG. 5 Statistical source data. SOURCE DATA FIG. 6 Unprocessed western blots. SOURCE DATA FIG. 6
Statistical source data. SOURCE DATA FIG. 7 Unprocessed western blots. SOURCE DATA FIG. 7 Statistical source data. SOURCE DATA FIG. 8 Unprocessed western blots. SOURCE DATA FIG. 8
Statistical source data. SOURCE DATA EXTENDED DATA FIG. 1 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 2 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 2 Unprocessed
western blots. SOURCE DATA EXTENDED DATA FIG. 3 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 3 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 4 Statistical source data.
SOURCE DATA EXTENDED DATA FIG. 4 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 5 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 5 Unprocessed western blots. SOURCE DATA
EXTENDED DATA FIG. 6 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 6 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 7 Statistical source data. SOURCE DATA EXTENDED DATA
FIG. 7 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 8 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 8 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 9
Statistical source data. SOURCE DATA EXTENDED DATA FIG. 9 Unprocessed western blots. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive
rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed
by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhai, X., Yang, R., Chu, Q. _et al._ AMPK-regulated glycerol
excretion maintains metabolic crosstalk between reductive and energetic stress. _Nat Cell Biol_ 27, 141–153 (2025). https://doi.org/10.1038/s41556-024-01549-x Download citation * Received:
20 November 2023 * Accepted: 29 September 2024 * Published: 02 January 2025 * Issue Date: January 2025 * DOI: https://doi.org/10.1038/s41556-024-01549-x SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative