- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
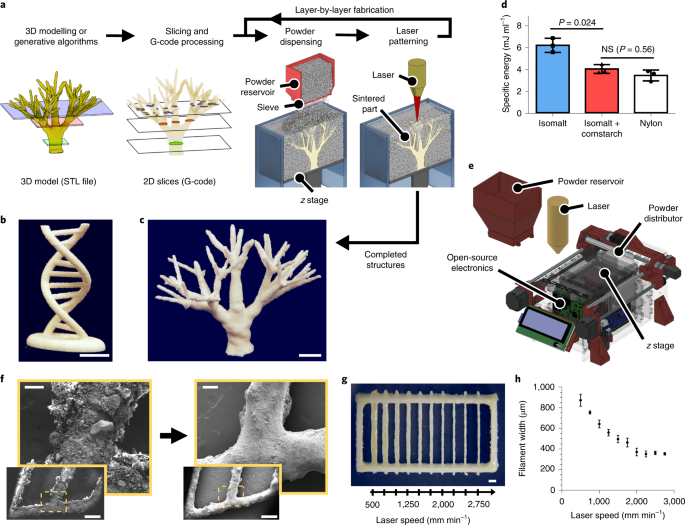
ABSTRACT Sacrificial templates for patterning perfusable vascular networks in engineered tissues have been constrained in architectural complexity, owing to the limitations of
extrusion-based 3D printing techniques. Here, we show that cell-laden hydrogels can be patterned with algorithmically generated dendritic vessel networks and other complex hierarchical
networks by using sacrificial templates made from laser-sintered carbohydrate powders. We quantified and modulated gradients of cell proliferation and cell metabolism emerging in response to
fluid convection through these networks and to diffusion of oxygen and metabolites out of them. We also show scalable strategies for the fabrication, perfusion culture and volumetric
analysis of large tissue-like constructs with complex and heterogeneous internal vascular architectures. Perfusable dendritic networks in cell-laden hydrogels may help sustain thick and
densely cellularized engineered tissues, and assist interrogations of the interplay between mass transport and tissue function. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS SACRIFICIAL CAPILLARY PUMPS TO ENGINEER
MULTISCALAR BIOLOGICAL FORMS Article 11 December 2024 LARGE-SCALE PERFUSED TISSUES VIA SYNTHETIC 3D SOFT MICROFLUIDICS Article Open access 12 January 2023 3D MICROMESH-BASED HYBRID
BIOPRINTING: MULTIDIMENSIONAL LIQUID PATTERNING FOR 3D MICROTISSUE ENGINEERING Article Open access 21 January 2022 DATA AVAILABILITY The main data supporting the results in this study are
available within the paper and its Supplementary Information. Much of the source and analysed data are available in Zenodo (https://doi.org/10.5281/zenodo.3723373). Some source datasets are
too large to be shared in public repositories and are available from the corresponding author on reasonable request. Design files and documentation for our open-source selective laser
sintering hardware and software are available in the Zenodo repository and at https://github.com/MillerLabFTW/OpenSLS. CODE AVAILABILITY A custom Python add-on for Blender to generate
bifurcating vascular structures is available in the Zenodo repository and at https://github.com/MillerLabFTW/IntussusceptionAddon. Image-processing and analysis scripts are also available in
the Zenodo repository. The mutual tree attraction algorithm for generating dendritic networks is closed source, but the generated architectures are included in the Zenodo repository. CHANGE
HISTORY * _ 16 JUNE 2021 A Correction to this paper has been published: https://doi.org/10.1038/s41551-021-00761-6 _ REFERENCES * Zamir, M. Fractal dimensions and multifractility in
vascular branching. _J. Theor. Biol._ 212, 183–190 (2001). CAS PubMed Google Scholar * West, G. B., Brown, J. H. & Enquist, B. J. A general model for the origin of allometric scaling
laws in biology. _Science_ 276, 122–126 (1997). CAS PubMed Google Scholar * West, G. B., Brown, J. H. & Enquist, B. J. A general model for ontogenetic growth. _Nature_ 413, 628–631
(2001). CAS PubMed Google Scholar * Monahan-Earley, R., Dvorak, A. M. & Aird, W. C. Evolutionary origins of the blood vascular system and endothelium. _J. Thromb. Haemost._ 11, 46–66
(2013). PubMed PubMed Central Google Scholar * Novosel, E. C., Kleinhans, C. & Kluger, P. J. Vascularization is the key challenge in tissue engineering. _Adv. Drug Deliv. Rev._ 63,
300–311 (2011). CAS PubMed Google Scholar * Kinstlinger, I. S. & Miller, J. S. 3D-printed fluidic networks as vasculature for engineered tissue. _Lab Chip_ 16, 2025–2043 (2016). CAS
PubMed Google Scholar * Cabodi, M. et al. A microfluidic biomaterial. _J. Am. Chem. Soc._ 127, 13788–13789 (2005). CAS PubMed Google Scholar * Chrobak, K. M., Potter, D. R. & Tien,
J. Formation of perfused, functional microvascular tubes in vitro. _Microvasc. Res._ 71, 185–196 (2006). CAS PubMed Google Scholar * Zhang, B. et al. Biodegradable scaffold with built-in
vasculature for organ-on-a-chip engineering and direct surgical anastomosis. _Nat. Mater._ 15, 669–678 (2016). CAS PubMed PubMed Central Google Scholar * Miller, J. S. The billion cell
construct: will three-dimensional printing get us there? _PLoS Biol._ 12, e1001882 (2014). PubMed PubMed Central Google Scholar * Luo, Y., Lode, A. & Gelinsky, M. Direct plotting of
three-dimensional hollow fiber scaffolds based on concentrated alginate pastes for tissue engineering. _Adv. Healthc. Mater._ 2, 777–783 (2013). CAS PubMed Google Scholar * Christensen,
K. et al. Freeform inkjet printing of cellular structures with bifurcations. _Biotechnol. Bioeng._ 112, 1047–1055 (2015). CAS PubMed Google Scholar * Hinton, T. J. et al.
Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. _Sci. Adv._ 1, e1500758 (2015). PubMed PubMed Central Google Scholar *
Lee, A. et al. 3D bioprinting of collagen to rebuild components of the human heart. _Science_ 365, 482–487 (2019). CAS PubMed Google Scholar * Zhang, R. & Larsen, N. B.
Stereolithographic hydrogel printing of 3D culture chips with biofunctionalized complex 3D perfusion networks. _Lab Chip_ 17, 4273–4282 (2017). CAS PubMed Google Scholar * Meyer, W. et
al. Soft polymers for building up small and smallest blood supplying systems by stereolithography. _J. Funct. Biomater._ 3, 257–268 (2012). PubMed PubMed Central Google Scholar *
Brandenberg, N. & Lutolf, M. P. In situ patterning of microfluidic networks in 3D cell-laden hydrogels. _Adv. Mater._ 28, 7450–7456 (2016). CAS PubMed Google Scholar * Heintz, K. A.
et al. Fabrication of 3D biomimetic microfluidic networks in hydrogels. _Adv. Healthc. Mater._ 5, 2153–2160 (2016). CAS PubMed PubMed Central Google Scholar * Arakawa, C. K., Badeau, B.
A., Zheng, Y. & DeForest, C. A. Multicellular vascularized engineered tissues through user-programmable biomaterial photodegradation. _Adv. Mat._ 29, 1703156 (2017). Google Scholar *
Grigoryan, B. et al. Functional intravascular topologies and multivascular networks within biocompatible hydrogels. _Science_ 364, 458–464 (2019). CAS PubMed PubMed Central Google Scholar
* Golden, A. P. & Tien, J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. _Lab Chip_ 7, 720–725 (2007). CAS PubMed Google Scholar * Miller, J.
S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. _Nat. Mater._ 11, 768–774 (2012). CAS PubMed PubMed Central Google Scholar *
Bégin-Drolet, A. et al. Design of a 3D printer head for additive manufacturing of sugar glass for tissue engineering applications. _Addit. Manuf._ 15, 29–39 (2017). Google Scholar * Gelber,
M. K., Hurst, G., Comi, T. J. & Bhargava, R. Model-guided design and characterization of a high-precision 3D printing process for carbohydrate glass. _Addit. Manuf._ 22, 38–50 (2018).
CAS Google Scholar * Homan, K. A. et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. _Sci. Rep._ 6, 34845 (2016). CAS PubMed PubMed Central Google Scholar
* Kolesky, D. B. et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. _Adv. Mater._ 26, 3124–3130 (2014). CAS PubMed Google Scholar * Kolesky, D. B., Homan,
K. A., Skylar-Scott, M. A. & Lewis, J. A. Three-dimensional bioprinting of thick vascularized tissues. _Proc. Natl Acad. Sci. USA_ 113, 3179–3184 (2016). CAS PubMed PubMed Central
Google Scholar * Skylar-Scott, M. A. et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. _Sci. Adv._ 5, eaaw2459 (2019). CAS PubMed
PubMed Central Google Scholar * Wu, W., Deconinck, A. & Lewis, J. A. Omnidirectional printing of 3D microvascular networks. _Adv. Mater._ 23, H178–H183 (2011). CAS PubMed Google
Scholar * Song, K. H., Highley, C. B., Rouff, A. & Burdick, J. A. Complex 3D-printed microchannels within cell-degradable hydrogels. _Adv. Funct. Mater._ 28, 1801331 (2018). Google
Scholar * Pimentel, C. R. et al. Three-dimensional fabrication of thick and densely populated soft constructs with complex and actively perfused channel network. _Acta Biomater._ 65,
174–184 (2018). Google Scholar * Kinstlinger, I. S. et al. Open-source selective laser sintering (OpenSLS) of nylon and biocompatible polycaprolactone. _PLoS ONE_ 11, e0147399 (2016).
PubMed PubMed Central Google Scholar * Roszelle, B. N. et al. Flow diverter effect on cerebral aneurysm hemodynamics: An in vitro comparison of telescoping stents and the Pipeline.
_Neuroradiology_ 55, 751–758 (2013). PubMed Google Scholar * Saggiomo, V. & Velders, A. H. Simple 3D printed scaffold-removal method for the fabrication of intricate microfluidic
devices. _Adv. Sci._ 2, 1500125 (2015). Google Scholar * Nguyen, L. H. et al. Vascularized bone tissue engineering: approaches for potential improvement. _Tissue Eng. Part B_ 18, 363–382
(2012). CAS Google Scholar * Nguyen, Q. T., Hwang, Y., Chen, A. C., Varghese, S. & Sah, R. L. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels.
_Biomaterials_ 33, 6682–6690 (2012). CAS PubMed PubMed Central Google Scholar * Partlow, B. P. et al. Highly tunable elastomeric silk biomaterials. _Adv. Funct. Mater._ 24, 4615–4624
(2014). CAS PubMed PubMed Central Google Scholar * Mooney, R., Tawil, B. & Mahoney, M. Specific fibrinogen and thrombin concentrations promote neuronal rather than glial growth when
primary neural cells are seeded within plasma-derived fibrin gels. _Tissue Eng. Part A_ 16, 1607–1619 (2010). CAS PubMed Google Scholar * Duong, H., Wu, B. & Tawil, B. Modulation of
3D fibrin matrix stiffness by intrinsic fibrinogen–thrombin compositions and by extrinsic cellular activity. _Tissue Eng. Part A_ 15, 1865–1876 (2009). CAS PubMed PubMed Central Google
Scholar * Subhash, G., Liu, Q., Moore, D. F., Ifju, P. G. & Haile, M. A. Concentration dependence of tensile behavior in agarose gel using digital image correlation. _Exp. Mech._ 51,
255–262 (2011). CAS Google Scholar * Feugier, F. G., Mochizuki, A. & Iwasa, Y. Self-organization of the vascular system in plant leaves: inter-dependent dynamics of auxin flux and
carrier proteins. _J. Theor. Biol._ 236, 366–375 (2005). CAS PubMed Google Scholar * Fujita, H. & Mochizuki, A. The origin of the diversity of leaf venation pattern. _Dev. Dyn._ 235,
2710–2721 (2006). PubMed Google Scholar * Runions, A., Lane, B. & Prusinkiewicz, P. Modeling trees with a space colonization algorithm. In _Proc. 3rd Eurographics Conference on Natural
Phenomena_ (Eds Ebert, D. & Mérillou, S.) 63–70 (Eurographics Association, 2007). * Murray, C. D. The physiological principle of minimum work applied to the angle of branching of
arteries. _J. Gen. Physiol._ 9, 835–841 (1926). CAS PubMed PubMed Central Google Scholar * Miguel, A. F. Dendritic design as an archetype for growth patterns in nature: fractal and
constructal views. _Front. Phys._ 2, 9 (2014). Google Scholar * Moon, J. J. et al. Biomimetic hydrogels with pro-angiogenic properties. _Biomaterials_ 31, 3840–3847 (2010). CAS PubMed
PubMed Central Google Scholar * Calderon, G. et al. Tubulogenesis of co-cultured human iPS-derived endothelial cells and human mesenchymal stem cells in fibrin and gelatin methacrylate
gels. _Biomater. Sci._ 5, 1652–1660 (2017). CAS PubMed Google Scholar * Eskin, S. G., Ives, C., McIntire, L. & Navarro, L. Response of cultured endothelial cells to steady flow.
_Microvasc. Res._ 28, 87–94 (1984). CAS PubMed Google Scholar * Yang, P. J. & Temenoff, J. S. Engineering orthopedic tissue interfaces. _Tissue Eng. Part B_ 15, 127–141 (2009). CAS
Google Scholar * Eckes, B. et al. Fibroblast-matrix interactions in wound healing and fibrosis. _Matrix Biol._ 19, 325–332 (2000). CAS PubMed Google Scholar * Lu, P., Weaver, V. M. &
Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. _J. Cell Biol._ 196, 395–406 (2012). CAS PubMed PubMed Central Google Scholar * Radisic, M. et al. Oxygen
gradients correlate with cell density and cell viability in engineered cardiac tissue. _Biotechnol. Bioeng._ 93, 332–343 (2006). CAS PubMed Google Scholar * Tocchio, A. et al. Versatile
fabrication of vascularizable scaffolds for large tissue engineering in bioreactor. _Biomaterials_ 45, 124–131 (2015). CAS PubMed Google Scholar * Tsang, V. L. et al. Fabrication of 3D
hepatic tissues by additive photopatterning of cellular hydrogels. _FASEB J._ 21, 790–801 (2007). CAS Google Scholar * Krogh, A. The number and distribution of capillaries in muscles with
calculations of the oxygen pressure head necessary for supplying the tissue. _J. Physiol._ 52, 409–415 (1919). CAS PubMed PubMed Central Google Scholar * Lewis, M. C., MacArthur, B. D.,
Malda, J., Pettet, G. & Please, C. P. Heterogeneous proliferation within engineered cartilaginous tissue: the role of oxygen tension. _Biotechnol. Bioeng._ 91, 607–615 (2005). CAS
PubMed Google Scholar * Demol, J., Lambrechts, D., Geris, L., Schrooten, J. & Van Oosterwyck, H. Towards a quantitative understanding of oxygen tension and cell density evolution in
fibrin hydrogels. _Biomaterials_ 32, 107–118 (2011). CAS PubMed Google Scholar * Gu, W. Y., Yao, H., Huang, C. Y. & Cheung, H. S. New insight into deformation-dependent hydraulic
permeability of gels and cartilage, and dynamic behavior of agarose gels in confined compression. _J. Biomech._ 36, 593–598 (2003). CAS PubMed Google Scholar * Chuppa, S. et al. Fermentor
temperature as a tool for control of high-density perfusion cultures of mammalian cells. _Biotechnol. Bioeng._ 55, 328–338 (1997). CAS PubMed Google Scholar * Ducommun, P., Ruffieux, P.
A., Kadouri, A., Von Stockar, U. & Marison, I. W. Monitoring of temperature effects on animal cell metabolism in a packed bed process. _Biotechnol. Bioeng._ 77, 838–842 (2002). CAS
PubMed Google Scholar * Jorjani, P. & Ozturk, S. S. Effects of cell density and temperature on oxygen consumption rate for different mammalian cell lines. _Biotechnol. Bioeng._ 64,
349–356 (1999). CAS PubMed Google Scholar * Xiang, C. et al. Long-term functional maintenance of primary human hepatocytes in vitro. _Science_ 364, 399–402 (2019). CAS PubMed Google
Scholar * Bhatia, S. N., Balis, U. J., Yarmush, M. L. & Toner, M. Effect of cell–cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal
cells. _FASEB J._ 13, 1883–1900 (1999). CAS PubMed Google Scholar * Stevens, K. R. et al. InVERT molding for scalable control of tissue microarchitecture. _Nat. Commun._ 4, 1847 (2013).
CAS PubMed Google Scholar * Stevens, K. R. et al. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. _Sci. Transl. Med._ 9, eaah5505 (2017).
PubMed PubMed Central Google Scholar * Khetani, S. R. & Bhatia, S. N. Microscale culture of human liver cells for drug development. _Nat. Biotechnol._ 26, 120–126 (2008). CAS PubMed
Google Scholar * Bhatia, S. N., Underhill, G. H., Zaret, K. S. & Fox, I. J. Cell and tissue engineering for liver disease. _Sci. Transl. Med._ 6, 245sr2 (2014). PubMed PubMed Central
Google Scholar * Rafii, S., Butler, J. M. & Ding, B.-S. Angiocrine functions of organ-specific endothelial cells. _Nature_ 529, 316–325 (2016). CAS PubMed PubMed Central Google
Scholar * Baranski, J. D. et al. Geometric control of vascular networks to enhance engineered tissue integration and function. _Proc. Natl Acad. Sci USA_ 110, 7586–7591 (2013). CAS PubMed
PubMed Central Google Scholar * Mirabella, T. et al. 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. _Nat. Biomed. Eng._ 1, 0083 (2017). CAS PubMed PubMed
Central Google Scholar * Lindström, N. O. et al. Conserved and divergent molecular and anatomic features of human and mouse nephron patterning. _J. Am. Soc. Nephrol._ 29, 825–840 (2018).
PubMed PubMed Central Google Scholar * Bosco, D. et al. Unique arrangement of α- and β-cells in human islets of Langerhans. 59, 1202–1210 (2010). * Wang, X.-N. et al. A three-dimensional
atlas of human dermal leukocytes, lymphatics, and blood vessels. _J. Invest. Dermatol._ 134, 965–974 (2014). CAS PubMed Google Scholar * Kang, H.-W. et al. A 3D bioprinting system to
produce human-scale tissue constructs with structural integrity. _Nat. Biotechnol._ 34, 312–319 (2016). CAS PubMed Google Scholar * Shapiro, A. J. et al. Islet transplantation in seven
patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regime. _N. Engl. J. Med._ 343, 230–238 (2000). CAS PubMed Google Scholar * Iansante, V., Mitry, R.
R., Filippi, C., Fitzpatrick, E. & Dhawan, A. Human hepatocyte transplantation for liver disease: current status and future perspectives. _Pediatr. Res._ 83, 232–240 (2018). CAS PubMed
Google Scholar * Parker Ponder, K. et al. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. _Proc. Natl Acad. Sci. USA_ 88,
1217–1221 (1991). Google Scholar * Truslow, J. G. & Tien, J. Perfusion systems that minimize vascular volume fraction in engineered tissues. _Biomicrofluidics_ 5, 022201 (2011). PubMed
Central Google Scholar * Ronellenfitsch, H. & Katifori, E. Global optimization, local adaptation, and the role of growth in distribution networks. _Phys. Rev. Lett._ 117, 138301
(2016). PubMed Google Scholar * Freeman, R. Measuring the flow properties of consolidated, conditioned and aerated powders—a comparative study using a powder rheometer and a rotational
shear cell. _Powder Technol._ 174, 25–33 (2007). CAS Google Scholar * Thadavirul, N., Pavasant, P. & Supaphol, P. Development of polycaprolactone porous scaffolds by combining solvent
casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. _J. Biomed. Mater. Res. A_ 102, 3379–3392 (2013). PubMed Google Scholar * Miller, J. S. et al.
Bioactive hydrogels made from step-growth derived PEG-peptide macromers. _Biomaterials_ 31, 3736–3743 (2010). CAS PubMed PubMed Central Google Scholar * Rockwood, D. N. et al. Materials
fabrication from _Bombyx mori_ silk fibroin. _Nat. Protoc._ 6, 1612–1631 (2011). CAS PubMed Google Scholar * Li, W., Germain, R. N. & Gerner, M. Y. Multiplex, quantitative cellular
analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D)._Proc. Natl Acad. Sci. USA_ 114, E7321–E7330 (2017). CAS PubMed PubMed Central Google Scholar * Thielicke, W.
& Stamhuis, E. J. PIVlab—towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB. _J. Open Res. Softw._ 2, e30 (2014). Google Scholar * Thielicke,
W. _The Flapping Flight of Birds—Analysis and Application_. PhD thesis, Rijksuniversiteit Groningen (2014). * Thielicke, W. & Stamhuis, E. J. PIVlab—time-resolved digital particle image
velocimetry tool for MATLAB https://doi.org/10.6084/M9.FIGSHARE.1092508.V5 (2014). * Cheng, N.-S. Formula for the viscosity of a glycerol–water mixture. _Ind. Eng. Chem. Res._ 47, 3285–3288
(2008). CAS Google Scholar * Volk, A. & Kähler, C. J. Density model for aqueous glycerol solutions. _Exp. Fluids_ 59, 75 (2018). Google Scholar * van de Loosdrecht, A. A., Beelen, R.
H., Ossenkoppele, G. J., Broekhoven, M. G. & Langenhuijsen, M. M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells
from cell lines and patients with acute myeloid leukemia. _J. Immunol. Methods_ 174, 311–320 (1994). PubMed Google Scholar * Ahrens, J., Geveci, B. & Law, C. in _The Visualization
Handbook_ (eds Hansen, C. D. & Johnson, C. R.) 717–731 (2005). Download references ACKNOWLEDGEMENTS We thank A. Bastian, A. Ta and T. Schmidt for assistance with OpenSLS hardware and
firmware; E. Watson and A. Mikos for assistance with mechanical testing; J. Wagner, P. Desai and C. F. Higgs for assistance with powder rheology; D. De Santos for technical assistance with
carbohydrate SLS; D. Kaplan and W. Stoppel for providing silk fibroin; D. L. Gibbons for providing the 344SQ lung adenocarcinoma cell line; and C. Fortin for help with hepatocyte isolations.
This work was supported in part by a Medical Research Grant from the Robert J. Kleberg Jr and Helen C. Kleberg Foundation (J.S.M.), National Institutes of Health (grants HL134510 and
DK115461 (K.-D.B.)), the Texas Hepatocellular Carcinoma Consortium (THCCC) (CPRIT RP150587 (K.-D.B.)), National Insitutes of Health grant DP2HL137188 (K.R.S.) and National Insitutes of
Health NIBIB Cardiovascular Training grant (T32EB001650 (S.H.S.)). I.S.K. acknowledges support by an F31 National Research Service Award (NRSA) from the National Institutes of Health
(HL140905). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Bioengineering, Rice University, Houston, TX, USA Ian S. Kinstlinger, Gisele A. Calderon, Karen Vasquez Ruiz, David R. Yalacki, Palvasha R. Deme, Kevin D. Janson, Daniel W.
Sazer, Saarang S. Panchavati & Jordan S. Miller * Department of Bioengineering, University of Washington, Seattle, WA, USA Sarah H. Saxton, Fredrik Johansson & Kelly R. Stevens *
Nervous System, Palenville, NY, USA Jessica E. Rosenkrantz & Jesse D. Louis-Rosenberg * Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX, USA
Karl-Dimiter Bissig * Department of Pathology, University of Washington, Seattle, WA, USA Kelly R. Stevens Authors * Ian S. Kinstlinger View author publications You can also search for this
author inPubMed Google Scholar * Sarah H. Saxton View author publications You can also search for this author inPubMed Google Scholar * Gisele A. Calderon View author publications You can
also search for this author inPubMed Google Scholar * Karen Vasquez Ruiz View author publications You can also search for this author inPubMed Google Scholar * David R. Yalacki View author
publications You can also search for this author inPubMed Google Scholar * Palvasha R. Deme View author publications You can also search for this author inPubMed Google Scholar * Jessica E.
Rosenkrantz View author publications You can also search for this author inPubMed Google Scholar * Jesse D. Louis-Rosenberg View author publications You can also search for this author
inPubMed Google Scholar * Fredrik Johansson View author publications You can also search for this author inPubMed Google Scholar * Kevin D. Janson View author publications You can also
search for this author inPubMed Google Scholar * Daniel W. Sazer View author publications You can also search for this author inPubMed Google Scholar * Saarang S. Panchavati View author
publications You can also search for this author inPubMed Google Scholar * Karl-Dimiter Bissig View author publications You can also search for this author inPubMed Google Scholar * Kelly R.
Stevens View author publications You can also search for this author inPubMed Google Scholar * Jordan S. Miller View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS I.S.K. and J.S.M. conceived and initiated the project and wrote the manuscript. I.S.K., S.H.S., G.A.C., K.V.R., D.R.Y., P.R.D., K.D.J. and F.J. designed and performed
experiments. I.S.K., S.H.S. and G.A.C. acquired and analysed imaging data. J.E.R., J.D.L.-R. and S.S.P. developed generative design algorithms. D.W.S. synthesized materials. K.-D.B., K.R.S.
and J.S.M. supervised the project. CORRESPONDING AUTHOR Correspondence to Jordan S. Miller. ETHICS DECLARATIONS COMPETING INTERESTS J.S.M. is a co-founder and holds an equity stake in
Volumetric, Inc. J.E.R. and J.D.L.-R. are co-founders and hold equity stakes in Nervous System, Inc. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Methods. REPORTING SUMMARY SUPPLEMENTARY VIDEO 1
Annotated video recording of one layer of carbohydrate SLS. SUPPLEMENTARY VIDEO 2 Mutual-tree-attraction algorithm for the generative design of dendritic networks. SUPPLEMENTARY VIDEO 3
Fluorescent bead perfusion through a whole planar dendritic network. SUPPLEMENTARY VIDEO 4 Magnified view of fluorescent bead perfusion through the centre of a planar dendritic network.
SUPPLEMENTARY VIDEO 5 Perfusion of dendritic architectures at a high volumetric flow rate. SUPPLEMENTARY VIDEO 6 Animated volume rendering of a region in an endothelialized planar dendritic
network. SUPPLEMENTARY VIDEO 7 Rotating rendering of a volumetric μCT scan of a dendritic carbohydrate template. SUPPLEMENTARY VIDEO 8 Fly-through rendering of a volumetric μCT scan of a
dendritic carbohydrate template. SUPPLEMENTARY VIDEO 9 Fly-through sequence of MTT staining in sections from a cell-laden gel with dendritic architecture. SUPPLEMENTARY VIDEO 10 Fly-through
sequence of nuclear staining in sections from a cell-laden gel with dendritic architecture. SUPPLEMENTARY VIDEO 11 Fly-through sequence of processed MTT staining images from a cell-laden gel
with dendritic architecture. SUPPLEMENTARY VIDEO 12 Rotating rendering of a volumetrically reconstructed MTT signal in a cell-laden gel with dendritic architecture. SUPPLEMENTARY VIDEO 13
Computational fluid-dynamics simulation of perfusion through a 3D dendritic architecture. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kinstlinger,
I.S., Saxton, S.H., Calderon, G.A. _et al._ Generation of model tissues with dendritic vascular networks via sacrificial laser-sintered carbohydrate templates. _Nat Biomed Eng_ 4, 916–932
(2020). https://doi.org/10.1038/s41551-020-0566-1 Download citation * Received: 27 September 2018 * Accepted: 01 May 2020 * Published: 29 June 2020 * Issue Date: September 2020 * DOI:
https://doi.org/10.1038/s41551-020-0566-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative