- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Renal interstitial fibrosis (RIF) is a fundamental pathological feature of chronic kidney disease (CKD). However, toxicity and poor renal enrichment of fibrosis inhibitors limit their
further applications. In this study, a platform for CKD therapy is developed using superparamagnetic iron oxide nanoparticles (SPION) decorated mesenchymal stem cells derived extracellular
vesicles with carboxyl terminus of Hsc70-interacting protein (CHIP) high expression (SPION-EVs) to achieve higher renal-targeting antifibrotic therapeutic effect. SPION-EVs selectively
accumulate at the injury renal sites under an external magnetic field. Moreover, SPION-EVs deliver CHIP to induce Smad2/3 degradation in renal tubular cells which alleviates Smad2/3
activation-mediated fibrosis-like changes and collagen deposition. The extracellular vesicle engineering technology provides a potential nanoplatform for RIF therapy through CHIP-mediated
Smad2/3 degradation.
With the continuous improvement of the global economy, the prevalence of chronic kidney disease (CKD) continues to elevate which has emerged as a severe public health problem, ≈15% of people
suffer from CKD1,2. CKD is typically characterized by renal interstitial fibrosis and excessive extracellular matrix deposition contributing to the progressive loss of renal function3.
Unfortunately, existing effective clinical treatments only alleviate the process of renal fibrosis but do not complete the cure for the disease4. Therefore, developing a method for
anti-renal fibrosis has very important clinical significance5.
Numerous studies have reported that Smad2/3 positively regulates cell fibrosis-like change and inflammation in renal interstitial fibrosis6,7. In addition, expression of Smad2/3 in the
nucleus can aggregates in the renal tubular cell and accelerate CKD progression8,9. Therefore, the key to anti-fibrosis is to regulate the expression of Smad2/3 protein. A recent study
revealed that carboxyl terminus of Hsp70 interacting protein (CHIP) as an E3 ubiquitin ligase, may directly participate in regulating the stability of Smad2/3 protein and inhibiting
tumorigenesis10,11. This finding suggests that increasing the activity of CHIP in the renal tubular cells to promote the clearance of Smad2/3 may be important for RIF therapy. However, CHIP
ligase was susceptible to biochemical and physical instability, which may induce to inhibit Smad2/3 activity weakness and lead to decrease the therapeutic efficacy12. Moreover, the low
enrichment in the renal for CHIP-mediated ubiquitin degradation significantly limits their research and the future clinical transformation applications for RIF therapy13. The key to solving
problems is to improve CHIP activity and selectively rich in the injured kidney tissue to promote Smad2/3 ubiquitination and degradation.
Extracellular vesicles (EVs) such as microvesicles, are double membrane microparticles (60–1000 nm in size) secreted by cells in a constitutive or inducible manner14,15. The released EVs
naturally function as intercellular messengers by selecting transporting nucleic acids and proteins to distal or nearby recipient cells16. A large number of studies have shown the potential
of using EVs as mighty and feasible nanocarriers for drug delivery vector in various situations, from tumor therapy to gene regeneration therapy17,18. Compared with current delivery systems,
EVs have a unique advantage in the natural origin, which enables them to escape phagocytosis, extend the half-life of therapeutic agents, and low immunogenicity19. In addition, magnetic
targeted therapy has become a novel research hotspot in recent years, and magnetic nanoparticles have small size and magnetic guided properties20, and targeted migration of tissue damage
sites under the action of magnetic fields has broad application prospects21. Engineered EVs have shown great potential as a promising therapeutic platform and have attracted considerable
attention in tissue regeneration22. EVs could be engineered to overexpress various proteins in targeting tissues and active signals pathway for the regulation of fibrosis like cells23.
Therefore, we use superparamagnetic iron oxide nanoparticles to modify engineering EVs loaded CHIP delivery system to enhance anti-fibrosis therapeutic effect for the effectively treatment
of CKD.
Here, we provide the valid method for SPION decorated CHIP high-expressing MSC-EVs in CKD renal fibrosis treatment. We found that SPION-EVs-CHIP showed great ability to target injury renal
in unilateral ureteral obstruction (UUO) rat. More importantly, SPION-EVs-CHIP significantly reversed collagen deposition by inducing Smad2/3 ubiquitination and degradation of renal tubular
cells and inhibiting tubular damage-mediated inflammatory responses as compared to MSC-EVs. Our EV-engineering technology demonstrated that SPION-EVs with CHIP overexpression provide a
potential platform for effective renal interstitial fibrosis therapy by inducing Smad2/3 degradation.
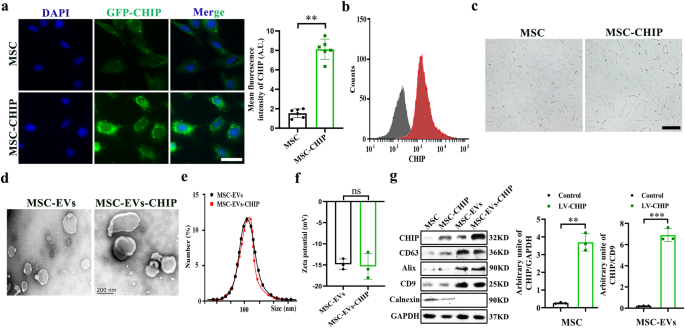
Umbilical derived MSCs with high purity were isolated from rat according to previous protocols (Supplementary Fig. 1). We next infected MSCs with lentivirus encoding the CHIP gene and
selected with puromycin to obtain CHIP high-expressing MSCs, and confocal graph shows that CHIP is mainly expressed in the cytoplasm (Fig. 1a, b). The morphology of MSCs was not affected by
high CHIP expression levels (Fig. 1c), lipogenesis and osteogenesis experiment slightly induced stem-cell differentiation compared to non-transfected MSCs (Supplementary Fig. 2). Then MSCs
derived EVs were then isolated and purified according to previously described protocols. Transmission electron microscopy (TEM) imaging shows then cup shaped vesicle morphology of
MSC-EVs-CHIP (Fig. 1d). And the average particle diameter of MSC-EVs-CHIP measured by dynamic light scattering (DLS) is 108 ± 10.6 nm (Fig. 1e). The zeta potential of MSC-EVs-CHIP was ≈−15
mV (Fig. 1f). Finally, we confirmed the expression of CHIP in MSCs-EVs by western blotting assay. The results showed that CHIP was also highly expressed in engineered EVs containing
EVs-associated proteins, such as CD63, CD9, Alix and Calnexin was expressed negatively (Fig. 1g), and GFP signals was also found in EVs-CHIP. These results suggested that the genetically
engineered MSC-EVs with high CHIP expression were successfully prepared.
a MSCs were transfected with lentivirus encoding the CHIP gene. The expression of CHIP (green) with GFP-tagged in MSCs was determined by immunofluorescence (left). Nuclei were stained with
DAPI (Blue). Scale bar: 50 µm. Representative quantification of the mean fluorescence intensity (MFI) of CHIP (n = 3) (right). b Flow cytometry analysis of CHIP expression in MSCs (left) and
representative quantification of the mean fluorescence intensity (MFI) of CHIP (n = 3) (right). c Morphology of MSCs with lentivirus transfection or non-transfection. Scale bar: 50 µm. d
Representative transmission electron microscope (TEM) images of MSC-EVs and MSC-EVs- CHIP. Scale bar: 100 nm. e The size distribution of MSC-EVs and MSC-EVs-CHIP was measured by dynamic
light scattering (DLS). f Surface zeta potential of MSC-EVs and MSC-EVs-CHIP (n = 3). g The expression of CHIP, CD73, CD63, and CD9 in MSCs and MSC-EVs with lentivirus was determined by
western blotting (left). Relative values of CHIP/GAPDH and CHIP/CD9 stipe gray (n = 3) (right). Data are represented as the mean ± standard error of the mean (SEM). Statistical significance
was calculated by a student’s t test. ns non-significance, **P