- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Asthma and COPD are defined as different disease entities, but in practice patients often show features of both diseases making it challenging for primary care clinicians to establish a
correct diagnosis. We aimed to establish the added value of spirometry and more advanced lung function measurements to differentiate between asthma and COPD. A cross-sectional study in 10
Dutch general practices was performed. 532 subjects were extensively screened on respiratory symptoms and lung function. Two chest physicians assessed if asthma or COPD was present. Using
multivariable logistic regression analysis we assessed the ability of three scenarios (i.e. only patient history; diagnostics available to primary care; diagnostics available only to
secondary care) to differentiate between the two conditions. Receiver operator characteristics (ROC) curves and area under the curve (AUC) were calculated for each scenario, with the chest
physicians’ assessment as golden standard. Results showed that 84 subjects were diagnosed with asthma, 138 with COPD, and 310 with no chronic respiratory disease. In the scenario including
only patient history items, ROC characteristics of the model showed an AUC of 0.84 (95% CI 0.78–0.89) for differentiation between asthma and COPD. When adding diagnostics available to
primary care (i.e., pre- and postbronchodilator spirometry) AUC increased to 0.89 (95% CI 0.84–0.93; p = 0.020). When adding more advanced secondary care diagnostic tests AUC remained 0.89
(95% CI 0.85–0.94; p = 0.967). We conclude that primary care clinicians’ ability to differentiate between asthma and COPD is enhanced by spirometry testing. More advanced diagnostic tests
used in hospital care settings do not seem to provide a better overall diagnostic differentiation between asthma and COPD in primary care patients.
Asthma and chronic obstructive pulmonary disease (COPD) are both common chronic respiratory diseases affecting approximately 1 in 12 people worldwide1,2. The two conditions are defined as
different disease entities with unique pathophysiological mechanisms and characteristic clinical features1,2. The underlying pathophysiology in COPD is characterized predominantly by
neutrophilic inflammation, whereas in asthma the inflammatory pattern is mostly due to eosinophilic inflammation3. Asthma typically presents with intermittent respiratory symptoms caused by
airflow obstruction predominantly due to bronchial hyperresponsiveness4. Asthma is often presented at younger age as part of an atopic constitution, but can also be diagnosed in adulthood1.
In contrast, COPD is a slowly progressive lung disease with patients having persistent respiratory symptoms and airflow obstruction2. In high-income countries like the Netherlands COPD
usually presents in patients older than forty who are generally current or former smokers2. Patients with asthma or COPD are mostly diagnosed and managed by primary care clinicians.
Looking at the classic pathophysiological and clinical presentations, the distinction between asthma and COPD seems clear, but in clinical practice patients often show features of both
diseases5,6. These similarities make it difficult for clinicians to distinguish between asthma and COPD7, especially in older and more diverse patient populations encountered in primary
care8,9,10. However, differentiating between the two respiratory conditions is important as they have different pharmacotherapeutic regimens. In patients with asthma, inhaled corticosteroids
(ICS) are highly effective in reducing symptoms and reducing the risk of asthma-related mortality1. In contrast, patients with COPD respond poorly to ICS and are mainly treated with
(long-acting) bronchodilators to relieve symptoms2. In addition to this, misdiagnosing asthma for COPD could lead to serious health risks considering that monotherapy with long-acting
bronchodilators is contra-indicated in asthmatics since it increases the risk of severe exacerbation11,12,13. On the other hand, (unnecessary) treatment with ICS may cause pneumonia and
increased risk of osteoporosis14,15,16,17.
Thus, establishing a correct diagnosis is essential for optimal treatment of asthma and COPD, but this can be challenging for primary care clinicians. Supporting them in the diagnostic
process seems therefore essential, but this also depends on the availability of diagnostic tools. Although quality spirometry has shown to be feasible in primary care settings18 there is
substantial room for improvement of its use to accurately diagnose chronic respiratory diseases19,20. Thus, the first aim of our current study was to establish which patient characteristics
distinguish between patients diagnosed with asthma or COPD. The second and main aim was to establish the added value of spirometry and more advanced lung function measurements to
differentiate between these two chronic airways diseases.
In this observational multi-centre cross-sectional study, we compared patients diagnosed with asthma, patients diagnosed with COPD, and subjects without underlying chronic obstructive lung
conditions using data from a previous study, i.e., the Detection, Intervention and Monitoring of COPD’ (DIMCA) program21. This program was originally set up to improve early detection of
chronic airways disease in general practices. A random sample of 1,749 adult subjects (20–70 years) from ten general practices in The Netherlands were invited to participate21. At the start
of the program, patients with pre-existing asthma, COPD or another airway disease were excluded. In 2007, ten years after the start of the initial DIMCA program, all subjects (now aged 30–80
years) received an invitation for a comprehensive respiratory assessment consisting of extensive lung function measurements and a myriad of medical history questions22. A total of 532
subjects agreed to participate in this follow-up study. The results of the respiratory assessment of these subjects were submitted to two experienced chest physicians who assessed if a
chronic airways disease (i.e., COPD or asthma) was present or absent using a standardized protocol22 that was based on the international clinical guideline criteria that applied at the time
of the study (see below). The results of the chest physicians’ assessments were used as the golden standard in the current study.
The study was approved by the medical ethics review board CMO Regio Arnhem – Nijmegen (https://www.radboudumc.nl/over-het-radboudumc/kwaliteit-en-veiligheid/commissie-mensgebonden-onderzoek;
file number: 2002/028). Participants provided written informed consent to take part in the study.
Study participants were instructed to interrupt the use of any bronchodilators they might use for a specified number of hours before their visit to the pulmonary function laboratory. Lung
function testing involved pre- and postbronchodilator spirometry (both static and dynamic) and measurement of carbon monoxide diffusion capacity (DLCO) and bronchial hyperresponsiveness
(BHR)22. Aerosolized salbutamol 800 µg and/or ipratropium 160 µg were used as bronchodilators and were administered by volume spacer. Postbronchodilator forced expiratory volume in one
second (FEV1) was measured 15 min after salbutamol and 45 min after ipratropium. Bronchodilator reversibility was defined as an increase in FEV1 after bronchodilation by at least 12% and 200
mL. BHR was assessed by histamine challenge test and considered positive in case of a >20% drop in FEV1 at a provocative dose histamine of ≤8 mg/mL (PC20)1,23. All lung function tests were
conducted by certified lung function technicians in a hospital-based pulmonary function laboratory and were performed in accordance with the 1994 American Thoracic Society standards24.
Predicted normal lung function values for FEV1 were calculated using European Community for Coal and Steel reference values25. Following lung function testing, subjects were interviewed by
the lung function technician regarding respiratory symptoms, smoking behaviour, presence of allergies and eczema, respiratory problems triggered by environmental exposures, and family
history of COPD or asthma22.
Based on the results of the respiratory assessment the chest physicians assessed if a chronic airways disease (i.e., asthma or COPD) was present or absent using guideline criteria, their
expert knowledge, and their clinical expertise22. Study subjects were randomly assigned to the chest physicians in a 1:1 ratio. If a subject was diagnosed with a chronic airways disease by
the assigned chest physician the subject’s data was also presented to the other chest physician and a final joint diagnosis was established. To standardize the diagnostic process, a decision
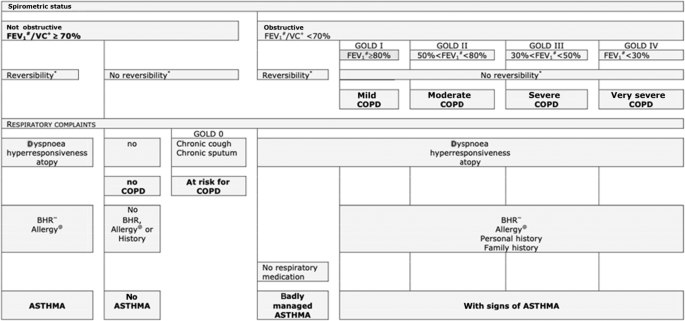
tree (Fig. 1) was created based on international clinical guideline criteria for diagnosing asthma (GINA guideline, 2007 update26) and COPD (GOLD guideline, 2006 update27) that applied at
the time, in co-operation with the two chest physicians. In case of uncertainty about the respiratory diagnosis the chest physicians could request additional diagnostic tests (i.e., allergy
skin testing, peak expiratory flow (PEF) monitoring) in order to maximize their diagnostic certainty22. Because the concept of asthma-COPD overlap (ACO) was introduced after the current
study was conducted, the chest physicians did not consider a diagnosis of ACO as a part of their assessment. They were instructed to, based on their systematic assessment of all diagnostic
information available, assign one single preferred diagnosis (i.e., either asthma or COPD) that fitted best according to their expert opinion. Figure 2 illustrates the spectrum of chronic
obstructive airways disease diagnoses and the parts of the spectrum on which the current study focuses. Strictly for the purpose of describing the study population and its diagnostic
subgroups (see Table 1) the Global Lung function Initiative (GLI) reference equations were applied at the time of the data analysis for the current paper28.
#Postbronchodilator forced expiratory volume. +Postbronchodilator vital capacity. *12% change in FEV1 (after bronchodilation), with a change of at least 200 mL. ~ Bronchial
hyperresponsiveness (positive at a provocative histamine concentration ≤ 8mg/mL). @Skin prick test.
The current study focusses on the parts to the left and right of the vertical dotted lines as indicated by the arrows. ACO asthma-COPD overlap, COPD chronic obstructive pulmonary disease.
In the present study, we categorized all items of the respiratory assessment in three subsections based on their availability in different healthcare settings, i.e., public health, primary
care, and secondary care (Table 2). Subsection 1 consists of items that are available in any public health or healthcare setting since they require no measurements or testing equipment but
only medical history questions (i.e., respiratory symptoms, smoking behaviour, body mass index (BMI)). Subsection 2 contains lung function test results that are available to primary care
clinicians (i.e., spirometry and reversibility testing) in countries with well-developed healthcare systems29,30,31. Finally, Subsection 3 contains results from more advanced diagnostic
tests as performed mainly in lung function laboratories in hospital care settings. These tests include measurement of static lung volumes, diffusion capacity, and histamine challenge
testing.
Demographic characteristics, clinical features and lung function values were univariately compared between the subgroups of patients diagnosed with asthma and COPD using independent t-tests
and Chi-square tests. The further analysis focussed on assessing the ability to differentiate between these chronic obstructive lung diseases in different healthcare settings. Since
physicians are not limited to asking a single medical history question or to conducting a single diagnostic test, we used multivariable logistic regression analysis to construct predictive
models based on the data of the subjects who were diagnosed with asthma or COPD by the chest physicians (i.e., the binary outcome measure for this analysis was to have a diagnosis of asthma
or a diagnosis of COPD). As described above, the items from the patient assessment were categorized in three subsections based on diagnostic availability and multivariable logistic
regression models were run for three ‘scenarios’ (Table 2). In the first scenario, we only used the medical history items from Subsection 1 in the model. In the second scenario, we added
diagnostic items available to primary care clinicians (i.e., Subsections 1 plus 2) to the model. In the third scenario, we added diagnostic items available to secondary care clinicians to
the model (i.e., Subsections 1 plus 2 plus 3). Only items with a p-value ≤0.20 in the univariate analysis were considered relevant as predictors and were included in the respective models.
In each scenario, the item with the highest p-value was manually removed from the model after which the logistic model was re-run (‘backward selection’). This step was repeated until only
variables with p-values






:max_bytes(150000):strip_icc():focal(999x0:1001x2)/donald-trump-jr-f820ece441d643ecb226cae9f46f61b7.jpg)