- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Transcranial direct current stimulation (tDCS) is a promising noninvasive intervention for Parkinson’s disease (PD). However, studies of its motor and cognitive effect have produced mixed
results. We conducted a systematic review including 38 studies and meta-analysis of 12 randomized sham-controlled trials with 263 PD patients. No significant differences were found between
active and sham tDCS in motor function (UPDRS-III: SMD = –0.14, p = 0.74), gait (SMD = 0.10, p = 0.513), attention and working memory (SMD = 0.24, p = 0.13), executive function (SMD = 0.03,
p = 0.854), and memory and learning (SMD: −0.07, p = 0.758). The prediction intervals indicated substantial heterogeneity among studies. Meta-regression showed small positive effects in
younger PD patients with milder symptoms. These findings are preliminary but suggest tDCS may benefit some PD patients while being neutral or harmful to others.
Parkinson’s disease (PD) is a progressive brain disease characterized by motor and non-motor symptoms1,2,3. In addition to motor symptoms such as tremor, bradykinesia, and rigidity,
non-motor symptoms such as autonomic dysfunction and cognitive deficits significantly affect a patient’s quality of life and even prognosis, in particular the cases of dementia4,5. Similar
to motor symptoms, cognitive non-motor symptoms vary individually, ranging from mild cognitive impairment to dementia, and may potentially be predicted by genetic or clinical features6.
Although dopaminergic medications are available that can help to alleviate motor symptoms in the initial stages of PD, these medications become less effective with illness progression and
carry risk for serious adverse effects, such as motor complications and hallucinations7. Furthermore, no interventions are available that can improve the cognitive symptoms of PD or halt
further cognitive decline. As a result, extensive research is underway to explore new or more advanced therapeutic interventions for PD8.
One promising intervention is noninvasive brain stimulation, which includes transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tES; encompassing both direct
current, tDCS, and alternating current, tACS)9. TMS, first introduced by Barker et al. in 1985, employs pulsed magnetic fields to stimulate specific cortical regions, thereby modulating
neural activity and influencing brain function10. The evolution of this technology led to the development of repetitive TMS (rTMS), which allows for extended treatment sessions and has
significantly enhanced the therapeutic potential of TMS in modulating brain activity11. With tES, patients receive weak (1–2 mA) electric currents aimed at alleviating clinical symptoms by
directly modulating brain activity. Although its precise mechanism of action remains elusive, pre and clinical improvements following tES have been attributed to effects on cell membrane
potentials, neurotrophic signaling, and long-term potentiation/depression-type neuroplasticity12. At a macroscopic level, the clinical benefits of tDCS have been linked to its effects on
cortical excitability13, while tACS has been associated with modulating brain oscillatory dynamics14, and both improving large-scale motor and cognitive network functioning via
corticostriatal circuit15. Compared to TMS, tES is relatively inexpensive, painless, easy to administer, and portable9.
Over the past 15 years, several randomized clinical trials (RCTs) and meta-analytic reviews have been published on motor and cognitive outcomes in patients with PD following tDCS delivery.
However, the results of different meta-analytic reviews have been mixed, probably in part due to use of different study inclusion criteria and data analytic approach. For example, some
meta-analytic reviews16,17 report that tDCS given to PD patients produced a significant improvement in overall motor function, as indexed by a reduced score on the Unified Parkinson’s
Disease Ranking Scale (UPDRS)-III, but other meta-analyzes report no significant differences18,19. On the other hand, meta-analytical reviews of cognitive outcomes of tDCS seem to have
yielded somewhat more consistent results, including a small but significant improvement on tests of working memory and attention, which was evident in not only PD patients but also patients
with other neurological or psychiatric disorders20.
It appears, thus, that tDCS may exert beneficial effects on cognitive functioning in patients with PD while having no or only minor effects on motor symptoms19. However, such a conclusion
would be based primarily on point effect size estimates and corresponding confidence intervals (CIs) and p-values, which do not provide the range of true tDCS effects expected to be observed
in similar studies and patients, such as in a next study or in a comparable real-life setting21,22. In contrast, the prediction interval (PI) conveys this information. Unfortunately, the PI
has not yet been utilized to capture variation in the true effect size across different populations of studies and patients included in the meta-analysis. Thus, the PI could disclose large
between-study heterogeneity of tDCS intervention effects, which may range from beneficial effects to zero effect in some populations and, perhaps, even effects in the opposite direction of
the summary point effect size estimate (i.e., detrimental effects) in other populations22.
In this context, we conducted a systematic review and meta-analysis of studies that utilized tDCS intervention for symptom relief in patients with PD. Our aim was to provide a better
understanding of the strength and heterogeneity of the effects of tDCS on patients’ motor and cognitive functioning, as measured by established clinical symptom rating scales or standardized
motor and cognitive tests.
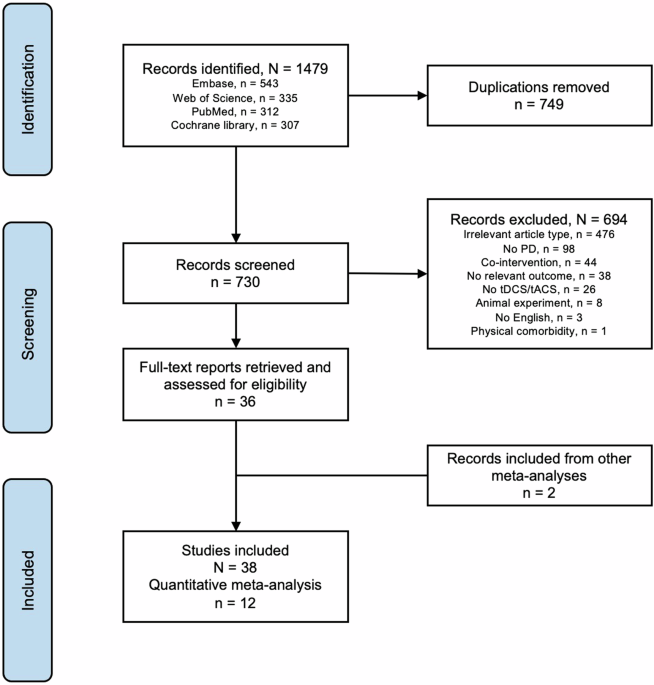
From the databases, we identified 1479 records (operators in Supplementary). After removing 749 duplicates, we retained 730 records for screening and selection (Fig. 1). Thirty-eight studies
examined tDCS effects in a total of 754 patients with PD (Table 1). Thirty-four of these studies were RCTs and 4 were non-RCTs, including observational studies.
Flow diagram depicting search, identification, exclusion, and inclusion of studies for review and meta-analysis.
Twelve of the 38 studies were included in the meta-analysis (7 using a parallel group and 5 a cross-over design), encompassing a total of 263 patients with PD [mean age: 65 years, range:
47–74; mean illness duration: 8.3 years, range: 0.5 to 10 years; mean illness severity/disease stage measured by the Hoehn and Yahr (H-Y) scale: mean 2.2, range: 1.5–2.5]. One study measured
working memory in the “off” medication status, two studies regarding motor function examined both “on” and “off” medication status, and the rest were performed with patients “on” medication
(Table 1). Motor outcome measures in the 12 RCTs mainly included overall motor function as assessed by the UPDRS-III (n = 5), and gait and balance (n = 5). Cognitive outcome measures
primarily fell into the domains of attention and working memory (n = 6), executive function (n = 4), and memory and learning (n = 2). Outcomes were measured immediately after one or more
sessions of tDCS, with some studies also including follow-up assessments a few weeks or months later (Table 1).
Most studies showed a low risk of bias in the randomization process (D1) and deviations from intended interventions (D2). There was more variability in the risk of bias due to missing
outcome data (D3), with several studies showing some concerns or high risk. The measurement of outcomes (D4) was mostly at low risk, while the selection of reported results (D5) presented
some concerns across multiple studies.
The highest levels of bias were observed in the domains of missing outcome data and measurement of outcomes, with several studies showing high or some concerns in these areas. The details of
the risk of bias of all the included studies were available in Fig. 2a and summarized in Fig. 2b.
a “Traffic light” plots of the domain-level judgements for each individual result. b Weighted bar plots of the distribution of risk-of-bias judgements within each bias domain.
Figure 3 presents forest plots illustrating the individual and weighted average effect size estimates for the outcome measures of overall motor function (UPDRS-III score) (panel a) and gait
and balance (panel b).
a Outcome measures of overall motor function (UPDRS-III score). b Outcome measures of gait and balance.
For the UPDRS-III score, Hedge’s g pooled across 5 studies [77 patients after active tDCS, 76 patients after sham tDCS; target: premotor-M1 area + DLPFC (k = 1), M1 + DLPFC (k = 1), M1 + SMA
(k = 1), M1 alone and cerebellum alone (k = 1), M1 alone (k = 1)] reached a value of −0.14 (95% CI: −1.23 to 0.95) (Fig. 3a). This result indicates that the mean UPDRS-III score tended to
be slightly higher (toward motor impairment) after active tDCS compared to sham tDCS, but the mean difference was not statistically significant (t = −0.36, df = 4, p = 0.737). However,
considerable effect size heterogeneity was evident (tau-squared = 0.64, I-squared = 0.84), along with a significant Q-test statistic (chi-square = 25.93, df = 4, p
:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)



