- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT To serve in a more aggressive chlorine (Cl−) ions-containing environment, a chlorine ions insensitivity Mg-Nd-Zr alloys is fabricated, exhibiting a stable corrosion resistance in
either 3.5wt.% or 10 wt.% NaCl solution. Unlike pure Mg who exhibit a negligible protection of the matrix, a distinct “hindering effect” of Cl− diffusion caused by doping elements within the
corrosion film was observed. Its underlying mechanism is demonstrated by X-ray photoelectron spectroscopy (XPS) and Density functional theory (DFT) analysis. The introducing with Nd and Zr
elements can effectively passivate vacancies and alter diffusion energy of chlorine ions. SIMILAR CONTENT BEING VIEWED BY OTHERS THE DEVELOPMENT OF Β PHASE MG–LI ALLOYS FOR ULTRALIGHT
CORROSION RESISTANT APPLICATIONS Article Open access 12 June 2020 CORROSION RESISTANCE AND BIOCOMPATIBILITY OF CARBON ION IMPLANTED AZ31B MAGNESIUM ALLOY Article Open access 25 October 2024
ELEMENTAL PARTITIONING AND CORROSION RESISTANCE OF NI–CR ALLOYS REVEALED BY ACCURATE AB-INITIO THERMODYNAMIC AND ELECTROCHEMICAL CALCULATIONS Article Open access 14 December 2023
INTRODUCTION Corrosion has become a significant concern in modern society due to the harsh service conditions that metallic alloys are subjected to. The presence of complex environmental
factors, such as destructive ions, external forces, cycling alternate wetting and drying conditions, microorganisms, etc., can accelerate corrosion reactions1,2,3,4,5. Surface coating and
alloying are the primary methods for enhancing the corrosion resistance of magnesium6,7. Zhang et al. significantly improve the corrosion resistance of AZ91D through conversion coating,
incorporating pre-precipitation sites to enhance its effectiveness. The accelerated formation process of this conversion coating contributes to the exceptional corrosion resistance exhibited
by this film8. However, due to the complexity involved in applying these coatings, practical implementation becomes challenging. Conversely, micro-alloying has been identified as an
efficient alternative with a simpler process for improving the corrosion resistance of Mg alloys9. Wang et al. propose the ‘Mg-LA-MA’ alloying strategy to achieve high corrosion resistance
in magnesium alloys. It has been observed that magnesium alloys with high corrosion resistance consist of a matrix element (Mg), a low-alloying element (LA), and a micro-alloying element
(MA). The LA and MA elements accelerate the formation process of the corrosion film and densify the corrosion barrier10. Despite micro-alloying exhibit great potential in enhancing corrosion
resistance of magnesium alloys, the corrosion resistance remains unsatisfactory, particularly in more aggressive environments such as those with elevated concentrations of harmful
ions11,12,13. Offensive ions, particularly chlorine ions (Cl−), have been proven to play a crucial role in corrosive environments and are among the most detrimental factors leading to severe
corrosion with localized attacks14,15. The deleterious effect of chlorine ions on alloys has been extensively investigated, and it has been demonstrated that the presence of chlorine ions
can significantly increase the corrosion rate by orders of magnitude16,17. Magnesium alloys exhibit highly susceptible to chloride-induced corrosion like stainless steel18,19,20. Plenty of
research has been conducted to demonstrate the role of chlorine ions in the corrosion process. The well-known point-defect models, void models, and adsorption mechanisms all emphasize the
importance of preventing invading by chlorine ions21,22,23,24. According to Hoar et al. view, Cl− is expected to penetrate the corrosion film and reach the interface between the film and
matrix. The introduction of Cl− results in external forces that cause the breakdown of corrosion film25. Burstein and Mattin suggest that a high concentration of Cl− at the interface may
result in the formation of metal chloride, leading to swelling of the corrosion film and pitting corrosion due to volume mismatch between compounds and metals26. The prevention of Cl−
accumulation at the interface between corrosion film and matrix is of central importance against Cl− attack. Corrosion film can avoid the connection between corrosive solution and matrix to
reduce the accumulation of chlorine ions. The hindrance of chlorine ions diffusion within the corrosion film must be considered when describing its chlorine ions-resistance ability.
Incorporating rare earth elements through alloying is considered an effective method for affect the diffusion process of ions within films27,28. Nevertheless, the clarifying diffusion
process in the corrosion film remains a tough challenge, owing to the low concentration, complicated composition of chemical state and high demand of spatial resolution. Fortunately, these
problems may be solved by the co-work of developed analyzing techniques. Time-of-flight secondary ion mass spectroscopy (ToF-SIMS) offers exceptional resolution, facilitating the
demonstration of ion diffusion in low concentrations29. X-ray photoelectron spectroscopy (XPS) can confirm chemical states and reveal the relationship between diffusion barriers and chemical
environments30. Auger electron spectroscopy (AES) excels depth profile investigations due to its high spatial resolution and efficient sputtering rate, enabling precise localization of the
film/metal interface31. In this study, we fabricated a Mg-Nd-Zr alloy, which exhibits a stable corrosion resistance in varying concentration of chlorine ions solution comparing to pure
magnesium (Mg). The diffusion process and the underling mechanism are investigated by several spectrum analysis techniques. The detailed atomic-scale difference in diffusion barrier is
investigated by Density functional theory (DFT) calculations. The hindering effect of corrosion film in Cl− diffusion is discussed, which may aid in the design of high-corrosion-resistant
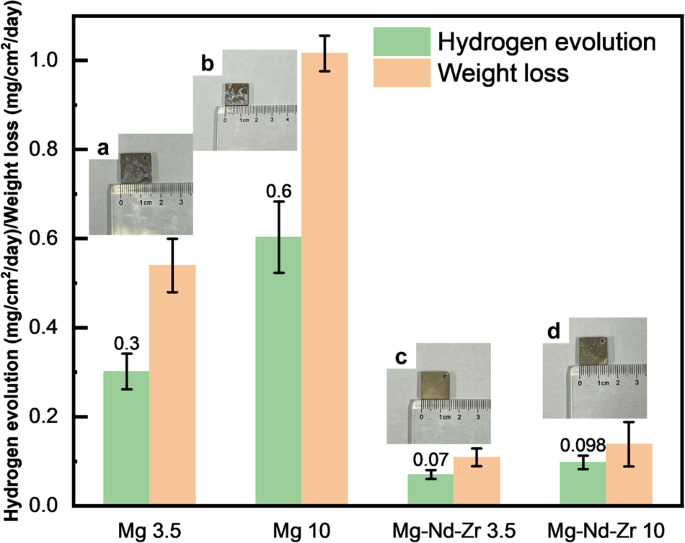
magnesium by impeding the diffusion of harmful ions. RESULTS AND DISCUSSION CORROSION PERFORMANCE IN 3.5/10 WT.% NACL SOLUTION Conventional corrosion tests, namely hydrogen evolution and
weight loss measurements, were employed to investigate the disparity in corrosion resistance among various magnesium alloys. The corrosion resistance of Mg-Nd-Zr in 3.5/10 wt.% NaCl solution
is comparable, as illustrated in Fig. 1, with only slight corrosion pits observed on the surface. In contrast, Mg exhibits more pronounced signs of corrosion in higher Cl− concentrations.
The morphology of Mg and Mg-Nd-Zr alloys in 3.5/10 wt.% NaCl solutions is depicted in Fig. 2 using Laser scanning confocal microscopy (LSCM), respectively. As illustrated in Fig. 2a, the
surface of Mg exhibits pronounced pitting corrosion when exposed to a 3.5 wt.% NaCl solution. With the increase of Cl− concentration, massive and larger corrosion pits are observed. The
higher concentration of chlorine ions, the enhanced susceptibility to pitting corrosion. As chlorine ions will lead to an invalidation of corrosion film due to the accumulation of stress
force32,33, broader and deeper corrosion pits occur. In contrast to Mg, the surface of the Mg-Nd-Zr alloy remains predominantly intact. Only minor pitting or crevices are discovered in
either 3.5 wt.% or 10 wt.% NaCl solutions, indicating its superior resistance against chlorine ions attack. The electrochemical tests of Mg and Mg-Nd-Zr are displayed in Fig. 3. The Open
circuit potential (OCP) reflects the surface changes during the corrosion process, relating to the formation of corrosion film34. As depicted in Fig. 3a, both Mg and Mg-Nd-Zr exhibit more
pronounced fluctuations during the immersion test in 10 wt.% NaCl solution, which can be attributed to the intensified corrosion process caused by higher chlorine ions concentration attack.
As the fluctuations in OCP representing of the formation and degradation of pitting35, Mg-Nd-Zr alloy performs relative smoothly fluctuations in OCP curves. As illustrated in Fig. 3b, the
Potentiodynamic polarization curves (PDP) demonstrate that both Mg and Mg-Nd-Zr exhibit a similar tendency. The essential parameters of PDP are presented in Table 1. For Mg, the corrosion
current density is approximately 2.3 × 10−5 A/cm2 in a 3.5 wt.% NaCl solution, and there is a significant acceleration of the corrosion rate to 7.2 × 10−5 A/cm2 in 10 wt% NaCl solution.
Conversely, only a slight increase in current density from 1.2 × 10−5 A/cm2 to 1.8 × 10−5 A/cm2 is observed in Mg-Nd-Zr alloy. Moreover, higher chlorine ion concentrations tend to accelerate
Hydrogen evolution reaction (HER)36,37, thus the cathodic branches of Mg and Mg-Nd-Zr both shift towards to a more noble direction in 3.5 wt.% and 10 wt.% NaCl solutions. However, comparing
to the significant shift of Mg, Mg-Nd-Zr performs a slight variation at cathodic branch. Moreover, in both 3.5 wt.% and 10 wt.% NaCl solutions, the Mg-Nd-Zr alloy exhibits quasi-passivation
behavior on the anodic branch, indicating a higher resistance to chlorine ions attack. The similar value of breakdown potential (Eb) implies that a protective corrosion film formed on the
surface. Furthermore, as shown in Fig. 3c, d, electrochemical impedance spectroscopy (EIS) was applied to investigate the corrosion behavior of Mg and Mg-Nd-Zr in different concentration of
chlorine ions solution. It is found that in both 3.5 wt.% and 10 wt.% NaCl solutions, Mg-Nd-Zr exhibits a relatively stable anti-corrosion ability. The dual-capacitance phenomenon is
observed in both spectra, which is considered to be indicative of significant corrosion resistance38,39. For Mg, a similar capacitance phenomenon can be observed in 3.5 wt.% NaCl solution
with smaller impedance modulus. However, when exposed to 10 wt.% NaCl solution, the capacitance zone transforms into an inductance zone due to its limited resistance against chlorine ions
attack, representing a significant deterioration of corrosion resistance40,41. The corrosion behavior of Mg exhibits a transition from capacitive to inductive loop. The inductive loop at low
frequency is related to the relaxation of ions or hydrogen bubbles at the interface42. On the other hand, Mg-Nd-Zr exhibits greater stability in response to changes in corrosion
environment, as evidenced by the absence of inductive loop in Nyquist diagram. Only a slight reduction of impedance modules is found. It implies that Mg-Nd-Zr exhibit an insensitivity to Cl−
corrosion. The fitting parameters of EIS are presented in Table 2. It is evident that the charge transfer resistance (Rct) of Mg exhibits a significant decrease, dropping from 834.9 Ω∙cm2
to 101.8 Ω∙cm2, representing an approximate reduction of 87.5% with the increasing of Cl− concentration. In the case of Mg-Nd-Zr alloy, only a slight decline in Rct (decreasing from 2011
Ω∙cm2 to 1425 Ω∙cm2) is observed, indicating the sustained resistance to Cl− attack. Additionally, Mg-Nd-Zr alloy demonstrates the presence of capacitive behavior with respective variation
values of 2347 Ω∙cm2 and 2236 Ω∙cm2 when immersed in 3.5 and 10 wt.% NaCl solution respectively. Thus, it is obvious that the Mg-Nd-Zr alloy exhibits superior corrosion resistance compared
to Mg when exposed to severer attack of chlorine ions. THE INVESTIGATION OF THE CORROSION FILM Time-of-flight secondary ion mass spectrometry (ToF-SIMS) depth profile analyses with positive
ion charge were conducted to identify the diffusion ability of chlorine ions within corrosion film on different Mg alloys. The observation areas are determined by Optical microscope (OM),
Scanning electron microscope (SEM) and the 3D view of ToF-SIMS. No significant devoid of signal are observed, implying no defects or cracks are present in the testing zone. Thereby, the
signal of chlorine ions mainly origin from the diffusion process within corrosion film. The depth profile of chlorine ions for various alloys, as revealed by ToF-SIMS, is presented in Fig.
4b. It demonstrates the diffusion behavior of Cl− on the upper region in corrosion film. Apparently, the concentration of chlorine ions on Mg-Nd-Zr decreases sharply to below 1 × 104
immediately. Conversely, the concentration of chlorine ions on Mg exhibits a relatively gradual decrease. Furthermore, the variation of Cl− intensity with sputtering time is represented by
differential form of \(\frac{{d\; intensity\; of\; chlorine\; ions}}{{d\; sputtering\; time}}\) in Fig. 4c. It indicates the variation rate of Cl− signal with the increasing of depth. The
derivative value of Mg-Nd-Zr alloy is significantly greater than that of Mg (in absolute terms), indicating a more pronounced hindering effect on the diffusion of chlorine ions within the
corrosion film, as evidenced by a faster reduction in the number of chlorine ions present. This suggests that diffusion in the Mg-Nd-Zr corrosion film is more difficult for chlorine ions. To
further clarify the diffusion process of chlorine ions, the deep profile imaging (cross section) was applied, and the results were displayed in Fig. 4d. The regions enriched with chlorine
ions exhibit a significantly larger area in the Mg corrosion film as compared to that of the Mg-Nd-Zr alloy. Furthermore, the depth profile imaging of chlorine ions penetration is
considerably greater within Mg corrosion film. Notably, an accumulation of chlorine ions is present at or near the surface which is believed to be responsible for initiating pitting
corrosion43,44. And its concentration gradually decreases with increasing depth, indicating poor hindrance to chlorine ions diffusion. In contrast, the diffusion of chlorine ions is rapidly
impeded and the diffusion depth nearly halves within Mg-Nd-Zr corrosion film, demonstrating a superior anti-chlorine ions ability. The 3D images presented in Fig. 5 further confirmed the
variation in chlorine ions prevention. At equivalent depths, Mg corrosion film exhibits higher concentrations of chlorine ions compared to Mg-Nd-Zr alloy, resulting a significant
accumulation of chlorine ions. The higher concentration of chlorine ions, the weaker hindering effect of corrosion film. The uniform distribution of Cl− concentration within the corrosion
film on Mg-Nd-Zr alloy suggests a reduced propensity for pitting. The results are consistent with the LSCM imaging in Fig. 2, where Mg-Nd-Zr exhibits predominantly uniform corrosion
characteristic rather than pitting corrosion. The X-ray photoelectron spectroscopy (XPS) survey scan of the corrosion film on Mg-Nd-Zr alloy was presented in Fig. 6a. The corrosion film
primarily consists of Mg and O elements, as revealed by survey scan analysis. The presence of Na and Cl signals can be attributed to residual NaCl within the corrosion film. Additionally,
the detection of Nd and Zr signals suggests the existence of these alloying elements. Notably, considering the co-localization of O KLL and Nd 3d signals, we further analyzed the Nd 4d
signal to determine its chemical state45,46,47. As evidenced by the detailed spectra of Mg and O shown in Fig. 6b, c, it can be confirmed that the composition of the corrosion film primarily
consists of Mg (OH)2 and MgO. Additionally, high-resolution XPS of Zr 3d and Nd 4d are presented in Fig. 6e, f. The Zr 3d XPS spectra display a typical Zr 3d\(\frac{5}{2}\) at 182.2 eV with
the Zr 3d\(\frac{3}{2}\) at 184.6 eV, which can be attributed to Zr4+. Similarly, the presence of Nd3+ 4d in 121.1 eV the corrosion film is confirmed by analysis of the Nd element. These
results confirm the presence of Zr and Nd elements in the corrosion film, which is believed to play a key role in hindering chlorine diffusion. Additionally, the presence of chlorine (Cl−)
ions is detected through XPS. Auger electron spectroscopy (AES) was incorporated for thorough investigation of the diffusion process of chlorine element from the surface of the corrosion
film to the interface. AES spectrum is depicted in Fig. 7 before and after sputtering. Distinct signals of O, Mg and C are detected. Immersing in a higher concentration of chlorine ions
solution results in a more pronounced Cl signal. The absent signal of Nd and Zr in AES surface spectrum might be blaming to its low content on surface of corrosion film. Nevertheless, for
the AES spectrum after sputtering, O signal tend to be absent with the appearance of Nd peaks48,49. It implies after test, the matrix of alloys is exposed. The depth profile by AES from the
surface to matrix record the variation of Mg, O, Cl, Nd and Zr in various concentration of chlorine ions solution in Fig. 8. It is seen that at the beginning, the fluctuation of Mg and O
fraction is small in both Mg and Mg-Nd-Zr. With the increasing of depth, Mg is significantly shifted accompanying by the reduction of O signal. The location of the interface is determined by
the characteristic phenomenon of weak signal of O and strong signal of Mg in Mg and Mg-Nd-Zr alloy, respectively in different corrosion solution50,51,52. Under the attack of different
concentration of chlorine ions, the thickness of corrosion film is different. In a 3.5 wt.% NaCl solution, the corrosion film thickness on Mg and Mg-Nd-Zr alloys is 530 nm (Fig. 8a) and 400
nm (Fig. 8c), respectively. However, in a 10 wt.% NaCl solution, there is a noticeable increase of the corrosion film thickness on Mg to 700 nm (Fig. 8b), whereas only a slight variation is
observed for Mg-Nd-Zr alloy. The variations of normalized intensity of Cl signal in Mg and Mg-Nd-Zr are displayed in Fig. 8e. Apparently, the variation of Cl signal within the corrosion film
is totally different in Mg and Mg-Nd-Zr alloy. As for Mg corrosion film, the variation of Cl signal is smooth. The fluctuation of Cl content versus the corrosion depth is small, indicating
the poor preventing ability of the corrosion film. Conversely, with regards to Mg-Nd-Zr, a significant reduction of Cl signal is observed in either 3.5 or 10 wt.% NaCl solution. The
normalized intensity of the Cl signal even decreased to more than one fifth in 3.5 wt.% NaCl solution, demonstrating the superior ability to prevent the invading of Cl. Although the
amplitude of decreasing become smaller with the increase of NaCl content, the Mg-Nd-Zr alloy shows much stronger enhancement of resistance to Cl compared with pure Mg. With such a minor Nd
and Zr doping, the impedance of corrosion film is significantly improved. MECHANISM OF INSENSITIVITY TO CHLORINE IONS ATTACK The up-mentioned study indicates that via alloying Nd and Zr, the
anti-ability of chlorine ions attack of Mg alloys is significantly improved. The ability to resist chlorine ions attack of Mg-Nd-Zr alloys may be attributed to changes in chemical states
within the corrosion film after introducing other elements. The refined spectra of O and Mg confirm the variation in chemical conditions after adding Nd and Zr elements into the corrosion
film. Based on the high-resolution XPS spectra of Mg 2p (Fig. 9a), it can be observed that the Mg 2p peak for Mg corrosion film is located at 49.42 eV with the blue line. In contrast, for
Mg-Nd-Zr, the Mg 2p peak shifts to a higher binding energy ranks 49.74 eV due to variations in chemical states for Mg2+. Analogously, the binding energies of oxygen are situated at 531.15 eV
in the case of Mg corrosion film, while a peak shift signal at 531.43 eV is detected for Mg-Nd-Zr corrosion film (Fig. 9b). The shift towards higher binding energy in the O 1 s peak
suggests that oxygen vacancies may be passivated following this introduction53. Meanwhile, the Mg 2p binding energies shifted to higher values, indicating strong chemical interactions
between the introduced elements and Mg. Furthermore, these differential binding interactions resulted in a charge transfer effect around the Mg2+ species, which might affect the diffusion
process of chlorine ions54. In short, the upward shift in binding energy of O 1 s and Mg 2p peaks indicate the changes in chemical environment. It could affect the diffusion process, thereby
contributing to its barrier properties against chlorine ions diffusion55,56. The inherent relationship between doping elements to diffusion process of chlorine element is demonstrated by
DFT. Figure 10a shows the top view of the computational model of Nd and Zr doped Mg (OH)2, with an Mg vacancy and two hydroxyl group vacancies neighboring to the Mg vacancy. The diffusion
behavior of chlorine element between the two neighboring hydroxyl vacancies has been studied in pure Mg (OH)2 and Nd and Zr doped Mg (OH)2. The structure is subsequently relaxed, and the
model with lower energy is chosen as the initial state. The transition state is then optimized using the climbing image nudged-elastic-band (CI-NEB) method57,58,59,60,61, the computational
details can refer to experiment section mentioned above. With the help of Lobster software package62,63,64, we calculated -pCOHP between Mg and chlorine element after introducing Nd and Zr
elements. Integrated COHP (ICOHP) represents the integral of -pCOHP below the Fermi level, and the more negative value corresponds to the stronger binding between atoms. Figure 10d show that
the average ICOHP between magnesium and chlorine elements in Mg-Nd-Zr corrosion film is more negative than in Mg corrosion film, indicating a stronger binding of chlorine element and Mg in
the corrosion film of Mg-Nd-Zr alloy. As illustrated in Fig. 10e, the chlorine diffusion barrier is 0.508 eV in Mg corrosion film and 0.640 eV in Mg-Nd-Zr corrosion film, respectively. The
diffusion barrier of Mg-Nd-Zr is much higher than that of Mg, thus the Cl− is more difficult to diffuse with Nd and Zr addition. Generally, the diffusion rate of chlorine ions at each
individual hop can be described in Arrhenius forms by transition state theory according to $$\varGamma ={v}^{* }{e}^{\left(\frac{-{\varDelta E}_{B}}{{kT}}\right)}$$ (1) where \({v}^{* }\) is
an effective vibrational frequency and \({\Delta E}_{B}\) represent the diffusion barrier defined as the energy difference between the activated state and the initial equilibrium state of
the hop65,66. Diffusion barrier is closely related to the structure and chemical composition of the diffusion condition. The presence of alloying elements in magnesium alloys can alter the
structure and chemical composition of the corrosion layer, so as to affect the diffusion process of the chlorine. The diffusion behavior of Cl− is closely related to the structure and
chemical state of corrosion film. The increase of diffusion barrier is mainly attributed to two factors in Mg-Nd-Zr alloy. On the one hand, the presence of alloying elements in magnesium
alloys can passivate the vacancies, decreasing the pathway of chlorine ions diffusion67. On the other hand, the adding of alloying elements could enhance the diffusion barrier of Cl−68.
These two factors work together to efficiently impede the diffusion of Cl− by enhancing the diffusion barrier of Cl−, as evidenced by up-mentioned results. The majority of magnesium alloys
demonstrate a pronounced sensitivity to Cl− due to its significant contribution to pitting corrosion69,70. The possibility of enhancing the resistance of Mg alloys to Cl− by incorporating
additional elements into the corrosion film through alloying has been experimentally demonstrated in this work. Therefore, it may be feasible to regulate the chemical states of the corrosion
film in order to obtain a more protective barrier against harmful ions. In general, the corrosion behavior, especially the Cl− diffusion behavior of Mg-Nd-Zr in solutions with varying
chlorine ions concentration is investigated. Through spectral analysis and DFT calculations, the “hindering effect” of the corrosion film in Mg-Nd-Zr alloy is demonstrated and explained. As
for Mg, the Cl− content at the surface and the interface is almost the same, which means the little protection of the corrosion film. In contrast, a sharp decrease of Cl− content with the
depth of corrosion film is observed in Mg-Nd-Zr alloy, implying the strong resistance to chlorine. The difference in Cl− diffusion behavior may generally be due to two factors, The
introducing with Nd and Zr elements can effectively passivate vacancies and alter diffusion energy of chlorine. METHODS MATERIAL PREPARATION AND COMPOSITIONAL ANALYSIS The Mg-Nd-Zr alloy is
fabricated via traditional casting method and make the commercial pure Mg as a reference. The chemical compositions of alloys were determined using an Inductive coupled plasma emission
spectrometer (ICP-OES, Thermo Fisher Scientific iCAP 7500, USA) for precise analysis in Table 3. ELECTROCHEMICAL TEST The corrosion resistance of Mg-Nd-Zr and Mg alloys was assessed using
conventional electrochemical techniques, including Potentialdynamic polarization (PDP), Electrochemical impedance spectroscopy (EIS), and Open circuit potential (OCP). All experiments were
conducted at room temperature in a in 3.5/10 wt.% NaCl solution utilizing a three-electrode system with an Ag/AgCl reference electrode and platinum electrode on an Autolab 302 N
electrochemical workstation, with each test repeated at least thrice. The specimen was cut to a diameter of 15 mm and a thickness of 5 mm. To eliminate the deleterious effects of
contamination, each surface was lightly ground with 2000 grit SiC emery paper followed by ethanol degreasing. Prior to conducting the PDP test, a 3600-second OCP test was performed to
evaluate the potential variation during immersion in NaCl solution. The PDP test commenced after a stabilization period at a scan rate of 0.1 mV/s, ranging from −5 mV vs open circuit
potential to 1.6 V vs reference electrode for the anodic branch and from 5 mV vs open circuit potential to −300 mV vs open circuit potential for the cathodic branch, respectively.
Additionally, following a 1-h stabilization period, electrochemical impedance spectroscopy (EIS) was conducted using a frequency range of 100 kHz to 0.01 Hz and an amplitude of 10 mV in
close proximity to the open circuit potential (OCP). CORROSION TEST Prior to the experiments, all specimens were polished using SiC paper with grit sizes of 800, 1200, 2400 and 4000, using
ethanol as a lubricant, the alloys were rinsed with ethanol and dried using compressed air. Specimens with dimensions of 15 * 15 * 3 mm were prepared via wire-electrode cutting from the
ingot. All samples were polished by different polish paste to obtain clean surface. Hydrogen collection and weight loss test were conducted in 3.5/10 NaCl wt.% solution for 7 days at room
temperature. After being immersed in 3.5/10% NaCl for 24 h, the corrosion products were removed using a dilute solution of chromium trioxide, silver nitrate, and barium nitrate followed by
cleaning with ethanol. The surface morphology of these alloys was then investigated using a Laser scanning confocal microscope (LSCM, KEYENCE VK-X3000, Japan). SPECTRUM ANALYSIS To control
the thickness of corrosion film, all samples were prepared for spectrum analysis in the size of 4 × 4 × 3 mm after immersion in 3.5%/10% NaCl for 1 h, respectively. Time-of-flight secondary
ion mass spectroscopy (ToF-SIMS) was performed using a ToF-SIMS instrument (ION TOF ToF SIMS 5-100, Germany) equipped with Bi Source analysis gun (LMIG, Liquid metal ion gun) and Argon
atomic cluster ion Gun (GCIB). The sputtering source is equipped with O source and Cs source. The Bi-LMIG was set in the positive ions mode using Bi+ ions with 30 keV, 45 deg and scanning
100 × 100 \(\mu {m}^{2}\) testing area to investigate chlorine ions (Cl−). Depth profiling and imaging was performed in the non-interlaced mode with 10 frame of analysis, 1 s of sputtering
and 0.5 s pausing per cycle while using and Cs ions at 1 keV and 0.3 nA were used. Both image and depth profile analyses were performed using the ION-ToF Surface Lab software (Version 6.3,
ION-ToF, GmbH, Münster, Germany). The total primary ion flux was kept below 1012 ions/cm2 to ensure static states for the surface imaging experiments. X-ray photoelectron spectroscopy (XPS,
Nexsa Thermo Fisher Scientific) was performed to clarify the composition of the corrosion film with an Al Kα X-ray source. The pass energy for recording the survey spectra and region spectra
was set at 160 eV and 20 eV, respectively. The sample area under investigation was selected as 700 × 300 μm. CASA-XPS software (version 2.3.18) was utilized to validate and evaluate the XPS
data, with the C1s signal serving as a calibration reference adjusted to 284.5 eV. Background subtraction (Shirley) was performed to calculate the region spectra. Auger electron
spectroscopy (AES) depth profiling was conducted using the PHI-700 ULVAC-PHI instrument from Japan to investigate the distribution of ions within the corrosion film at greater depths. The
experimental procedure involved scanning with an Ar+ gun, where the high voltage of the electron gun was set at 5 kV. The energy resolution was maintained at 1‰, while the incidence angle
was fixed at 30° with the vacuum degree in the analysis chamber exceeded 3.9 × 10 − 9 Torr. The sputtering rate is modified by SiO2 ranking 20 nm/min. DENSITY FUNCTIONAL THEORY (DFT) In
order to build the corrosion film model of Mg (OH)2 doped with alloying elements, the Mg(OH)2 unit cell is expanded into a 3 × 3 × 3 supercell. A magnesium atom and its adjacent two hydroxyl
groups are removed to simulate defects and diffusion channels in the corrosion film. Mg atoms around the defect are replaced by alloying elements. To construct the initial and final
structures, chloride ions are placed in place of the original hydroxyl groups, respectively. After optimization, three images are interpolated in the form of linear interpolation to be used
as a search for the reaction path. All Density functional theory (DFT) calculations were performed by using VASP code71. The inner cores of the atom were replaced by the frozen-core
approximations and the projector-augmented wave (PAW) method72 was applied to describe the electron−core interaction. The Perdew-Burke-Ernzerhof (PBE) functional within the generalized
gradient approximation (GGA)73 was used to model the exchange correlation energy. Spin-polarization effect was employed in all calculations. Geometry optimizations were performed by using
the conjugate gradient algorithm until all forces are smaller than 0.02 eV/Å. The self-consistent field (SCF) tolerance was set to 1 × 10−5 eV on total energy for the convergence criteria.
The k-point sampling of the Brillouin zone was obtained using a 3 × 3 × 2 grid for the repetitive unit by Gamma centered scheme. A Gaussian smearing with σ = 0.05 eV to the orbital
occupation is employed during structure relaxation and energy calculations. The transition state search is performed by the climbing image-nudged elastic band61,74 (CI-NEB) method. All pre-
and post-processing were done by VASPKIT75. DATA AVAILABILITY The data are available from the corresponding author on reasonable request. REFERENCES * Burstein, G. T., Liu, C., Souto, R. M.
& Vines, S. P. Origins of pitting corrosion. _Corros. Eng., Sci. Technol._ 39, 25–30 (2013). Article Google Scholar * Cao, F., Song, G.-L. & Atrens, A. Corrosion and passivation of
magnesium alloys. _Corros. Sci._ 111, 835–845 (2016). Article CAS Google Scholar * Esmaily, M. et al. Fundamentals and advances in magnesium alloy corrosion. _Prog. Mater. Sci._ 89,
92–193 (2017). Article CAS Google Scholar * Liu, L. J. & Schlesinger, M. Corrosion of magnesium and its alloys. _Corros. Sci._ 51, 1733–1737 (2009). Article CAS Google Scholar *
Song, G. & Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. _Adv. Eng. Mater._ 5, 837–858 (2003). Article CAS Google Scholar * Li, P. et al.
Enhancing corrosion resistance of magnesium alloys via combining green chicory extracts and metal cations as organic-inorganic composite inhibitor. _Corros. Commun._ 9, 44–56 (2023). Article
Google Scholar * Zhao, Y., Zhang, T., Xiong, H. & Wang, F. Bridge for the thermodynamics and kinetics of electrochemical corrosion: Modeling on dissolution, ionization, diffusion and
deposition in metal/solution interface. _Corros. Sci._ 191, 109763 (2021). Article CAS Google Scholar * Zhang, C. et al. Effects of nucleation pretreatment on corrosion resistance of
conversion coating on magnesium alloy Mg-10Gd-3Y-0.4Zr. _Corros. Commun._ 10, 69–79 (2023). Article Google Scholar * Yang, Y. et al. The effects of a corrosion product film on the
corrosion behavior of Mg-Al alloy with micro-alloying of yttrium in a chloride solution. _Corros. Commun._ 11, 12–22 (2023). Article Google Scholar * Wang, D., Zhou, P., Zhang, Y., Zhang,
T. & Wang, F. Bridge for the thermodynamics and kinetics of electrochemical corrosion: Designing of the high corrosion-resistant magnesium alloy. _Corros. Sci._ 222, 111428 (2023).
Article CAS Google Scholar * Song, Y., Han, E.-H., Shan, D., Yim, C. D. & You, B. S. The effect of Zn concentration on the corrosion behavior of Mg–xZn alloys. _Corros. Sci._ 65,
322–330 (2012). Article CAS Google Scholar * Sun, Y., Wang, R., Peng, C. & Cai, Z. Microstructure and corrosion behavior of as-extruded Mg-xLi-3Al-2Zn-0.2Zr alloys (x = 5, 8, 11
wt.%). _Corros. Sci_. 167 (2020). * Chen, Y., Ying, T., Yang, Y., Wang, J. & Zeng, X. Regulating corrosion resistance of Mg alloys via promoting precipitation with trace Zr alloying.
_Corros. Sci._ 216, 111106 (2023). Article CAS Google Scholar * Zhou, Y. T. et al. Atomic-scale decoration for improving the pitting corrosion resistance of austenitic stainless steels.
_Sci. Rep._ 4, 3604 (2014). Article CAS Google Scholar * Zanotto, F., Grassi, V., Balbo, A., Monticelli, C. & Zucchi, F. Stress corrosion cracking of LDX 2101® duplex stainless steel
in chloride solutions in the presence of thiosulphate. _Corros. Sci._ 80, 205–212 (2014). Article CAS Google Scholar * Tang, J., Shao, Y., Zhang, T., Meng, G. & Wang, F. Corrosion
behaviour of carbon steel in different concentrations of HCl solutions containing H2S at 90 °C. _Corros. Sci._ 53, 1715–1723 (2011). Article CAS Google Scholar * Simmons, J. W. Overview:
high-nitrogen alloying of stainless steels. _Mater. Sci. Eng.: A_ 207, 159–169 (1996). Article Google Scholar * Otani, K. & Sakairi, M. Effects of metal cations on corrosion of mild
steel in model fresh water. _Corros. Sci._ 111, 302–312 (2016). Article CAS Google Scholar * Cain, T. W., Glover, C. F. & Scully, J. R. The corrosion of solid solution Mg-Sn binary
alloys in NaCl solutions. _Electrochim. Acta_ 297, 564–575 (2019). Article CAS Google Scholar * Atrens, A. et al. Review of Mg alloy corrosion rates. _J. Magnes. Alloy._ 8, 989–998
(2020). Article CAS Google Scholar * Macdonald, D. D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. _Electrochim. Acta_ 56, 1761–1772
(2011). Article CAS Google Scholar * Li, Y., Macdonald, D. D., Yang, J., Qiu, J. & Wang, S. Point defect model for the corrosion of steels in supercritical water: Part I, film growth
kinetics. _Corros. Sci_. 163 (2020). * Lin, L. F., Chao, C. Y. & Macdonald, D. D. A point defect model for anodic passive films: II. Chemical breakdown and pit initiation. _J.
Electrochem. Soc._ 128, 1194–1198 (2019). Article Google Scholar * Betova, I., Bojinov, M. & Tzvetkoff, T. Role of surface reactions in the transpassive dissolution of ferrous alloys
in concentrated H3PO4. _Appl. Surf. Sci._ 220, 273–287 (2003). Article CAS Google Scholar * Hoar, T. P., Mears, D. C. & Rothwell, G. P. The relationships between anodic passivity,
brightening and pitting. _Corros. Sci._ 5, 279–289 (1965). Article CAS Google Scholar * Burstein, G. T., Pistorius, P. C. & Mattin, S. P. The nucleation and growth of corrosion pits
on stainless steel. _Corros. Sci._ 35, 57–62 (1993). Article CAS Google Scholar * Li, Y. & Gao, Y. Carboxylic acid group-induced oxygen vacancy migration on an anatase (101) surface.
_Langmuir_ 34, 546–552 (2018). Article CAS Google Scholar * Zhang, J., Peng, W., Chen, Z., Chen, H. & Han, L. Effect of cerium doping in the TiO2 photoanode on the electron transport
of dye-sensitized solar cells. _J. Phys. Chem. C._ 116, 19182–19190 (2012). Article CAS Google Scholar * Seyeux, A. et al. ToF-SIMS imaging study of the early stages of corrosion in Al-Cu
thin films. _J. Electrochem. Soc._ 158, C165 (2011). Article CAS Google Scholar * Santamaria, M., Di Quarto, F., Zanna, S. & Marcus, P. Initial surface film on magnesium metal: a
characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). _Electrochim. Acta_ 53, 1314–1324 (2007). Article CAS Google Scholar * Sánchez-Amaya, J.
M., Blanco, G., Garcia-Garcia, F. J., Bethencourt, M. & Botana, F. J. XPS and AES analyses of cerium conversion coatings generated on AA5083 by thermal activation. _Surf. Coat. Technol._
213, 105–116 (2012). Article Google Scholar * Hinds, G. et al. Novel method for determination of pitting susceptibility in aggressive environments at elevated temperature and pressure.
_Corros. Sci._ 85, 33–41 (2014). Article CAS Google Scholar * Jiang, X., Nešić, S., Kinsella, B., Brown, B. & Young, D. Electrochemical investigation of the role of Cl−on localized
carbon dioxide corrosion behavior of mild steel. _Corrosion_ 69, 15–24 (2013). Article CAS Google Scholar * Li, J., Zhang, B., Wei, Q., Wang, N. & Hou, B. Electrochemical behavior of
Mg-Al-Zn-In alloy as anode materials in 3.5 wt.% NaCl solution. _Electrochim. Acta_ 238, 156–167 (2017). Article CAS Google Scholar * Budruk Abhijeet, S., Balasubramaniam, R. & Gupta,
M. Corrosion behaviour of Mg–Cu and Mg–Mo composites in 3.5% NaCl. _Corros. Sci._ 50, 2423–2428 (2008). Article CAS Google Scholar * Salleh, S. H., Thomas, S., Yuwono, J. A., Venkatesan,
K. & Birbilis, N. Enhanced hydrogen evolution on Mg (OH)2 covered Mg surfaces. _Electrochim. Acta_ 161, 144–152 (2015). Article CAS Google Scholar * Li, C. Q. et al. Composition and
microstructure dependent corrosion behaviour of Mg-Li alloys. _Electrochim. Acta_ 260, 55–64 (2018). Article CAS Google Scholar * Guo, Q., Du, K., Guo, X. & Wang, F. Electrochemical
impedance spectroscope analysis of microwave absorbing coatings on magnesium alloy in 3.5wt.% NaCl solution. _Electrochim. Acta_ 98, 190–198 (2013). Article CAS Google Scholar * Hou, Y.,
Zhou, P., Yu, B., Zhang, T. & Wang, F. The dual role of Mg2+ in the conversion bath during the treatment of magnesium alloys: The completing effect between heterogeneous nucleation and
crystal growth. _Electrochim. Acta_ 388, 138568 (2021). Article CAS Google Scholar * Birbilis, N., King, A. D., Thomas, S., Frankel, G. S. & Scully, J. R. Evidence for enhanced
catalytic activity of magnesium arising from anodic dissolution. _Electrochim. Acta_ 132, 277–283 (2014). Article CAS Google Scholar * Bland, L. G., Gusieva, K. & Scully, J. R. Effect
of crystallographic orientation on the corrosion of magnesium: comparison of film forming and bare crystal facets using electrochemical impedance and raman spectroscopy. _Electrochim. Acta_
227, 136–151 (2017). Article CAS Google Scholar * Shi, Z., Cao, F., Song, G.-L., Liu, M. & Atrens, A. Corrosion behaviour in salt spray and in 3.5% NaCl solution saturated with
Mg(OH)2 of as-cast and solution heat-treated binary Mg–RE alloys: RE=Ce, La, Nd, Y, Gd. _Corros. Sci._ 76, 98–118 (2013). Article CAS Google Scholar * Fu, C.-C., Torre, J. D., Willaime,
F., Bocquet, J.-L. & Barbu, A. Multiscale modelling of defect kinetics in irradiated iron. _Nat. Mater._ 4, 68–74 (2004). Article Google Scholar * Lin, L. F. A point defect model for
anodic passive films. _J. Electrochem. Soc._ 128, 1194 (1981). Article CAS Google Scholar * Li, C., Zhang, N. & Gao, P. Lessons learned: how to report XPS data incorrectly about
lead-halide perovskites. _Mater. Chem. Front_. (2023). * Malvankar, S. et al. Co-Doped SnO2 nanocrystals: XPS, Raman, and Magnetic Studies. _J. Electron. Mater._ 49, 1872–1880 (2019).
Article Google Scholar * Crist, B. V. _Handbook of Monochromatic XPS Spectra, 3 Volume Set_. (2000). * Sekine, T. _Handbook of Auger electron spectroscopy_. (1982). * Powell, C. J.
Recommended Auger-electron kinetic energies for 42 elemental solids. _J. Electron Spectrosc. Relat. Phenom._ 182, 11–18 (2010). Article CAS Google Scholar * Danaie, M., Asmussen, R. M.,
Jakupi, P., Shoesmith, D. W. & Botton, G. A. The role of aluminum distribution on the local corrosion resistance of the microstructure in a sand-cast AM50 alloy. _Corros. Sci._ 77,
151–163 (2013). Article CAS Google Scholar * Brady, M. P. et al. Film breakdown and nano-porous Mg(OH)2Formation from corrosion of magnesium alloys in salt solutions. _J. Electrochem.
Soc._ 162, C140–C149 (2015). Article CAS Google Scholar * Esmaily, M., Blücher, D. B., Svensson, J. E., Halvarsson, M. & Johansson, L. G. New insights into the corrosion of magnesium
alloys — The role of aluminum. _Scr. Materialia_ 115, 91–95 (2016). Article CAS Google Scholar * Wang, R. et al. Gadolinium-doped SnO2 electron transfer layer for highly efficient planar
perovskite solar cells. _J. Power Sources_ 544, 231870 (2022). Article CAS Google Scholar * Zhang, J. et al. Increasing the oxygen vacancy density on the TiO2 surface by La-Doping for
Dye-Sensitized Solar Cells. _J. Phys. Chem. C._ 114, 18396–18400 (2010). Article CAS Google Scholar * Jiang, Q. et al. Surface reaction for efficient and stable inverted perovskite solar
cells. _Nature_ 611, 278–283 (2022). Article CAS Google Scholar * Shen, Z. et al. Rational design of a Ni(3)N(0.85) electrocatalyst to accelerate polysulfide conversion in lithium-sulfur
batteries. _ACS Nano_ 14, 6673–6682 (2020). Article CAS Google Scholar * Chen, S. et al. Boosting sodium storage of Fe(1-x)S/MoS(2) composite via heterointerface engineering. _Nanomicro
Lett._ 11, 80 (2019). Google Scholar * Xiao, W. et al. Insight into fast Li diffusion in Li-excess spinel lithium manganese oxide. _J. Mater. Chem. A_ 6, 9893–9898 (2018). Article CAS
Google Scholar * Xiao, P., Sheppard, D., Rogal, J. & Henkelman, G. Solid-state dimer method for calculating solid-solid phase transitions. _J. Chem. Phys._ 140, 174104 (2014). Article
Google Scholar * Xu, L. & Henkelman, G. Adaptive kinetic Monte Carlo for first-principles accelerated dynamics. _J. Chem. Phys._ 129, 114104 (2008). Article Google Scholar * Sheppard,
D., Xiao, P., Chemelewski, W., Johnson, D. D. & Henkelman, G. A generalized solid-state nudged elastic band method. _J. Chem. Phys._ 136, 074103 (2012). Article Google Scholar *
Nelson, R. et al. LOBSTER: Local orbital projections, atomic charges, and chemical-bonding analysis from projector-augmented-wave-based density-functional theory. _J. Comput Chem._ 41,
1931–1940 (2020). Article CAS Google Scholar * Maintz, S., Deringer, V. L., Tchougreeff, A. L. & Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT.
_J. Comput Chem._ 37, 1030–1035 (2016). Article CAS Google Scholar * Maintz, S., Deringer, V. L., Tchougreeff, A. L. & Dronskowski, R. Analytic projection from plane-wave and PAW
wavefunctions and application to chemical-bonding analysis in solids. _J. Comput Chem._ 34, 2557–2567 (2013). Article CAS Google Scholar * Vineyard, G. H. Frequency factors and isotope
effects in solid state rate processes. _J. Phys. Chem. Solids_ 3, 121–127 (1957). Article CAS Google Scholar * Van der Ven, A. & Ceder, G. Lithium diffusion mechanisms in layered
intercalation compounds. _J. Power Sources_ 97-98, 529–531 (2001). Article Google Scholar * Luo, L. et al. Stabilization of 3D/2D perovskite heterostructures via inhibition of ion
diffusion by cross-linked polymers for solar cells with improved performance. _Nat. Energy_ 8, 294–303 (2023). Article CAS Google Scholar * Liu, H. et al. Oriented construction Cu3P and
Ni2P heterojunction to boost overall water splitting. _Chem. Eng. J._ 448, 137706 (2022). Article CAS Google Scholar * Soltis, J. Passivity breakdown, pit initiation and propagation of
pits in metallic materials – Review. _Corros. Sci._ 90, 5–22 (2015). Article CAS Google Scholar * King, A. D., Birbilis, N. & Scully, J. R. Accurate electrochemical measurement of
magnesium corrosion rates; a combined impedance, mass-loss and hydrogen collection study. _Electrochim. Acta_ 121, 394–406 (2014). Article CAS Google Scholar * Kresse & Furthmuller
Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. _Phys. Rev. B, Condens. Matter_ 54, 11169–11186 (1996). Article CAS Google Scholar *
Blöchl, P. E. Projector augmented-wave method. _Phys. Rev. B_ 50, 17953–17979 (1994). Article Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient
approximation made simple. _Phys. Rev. Lett._ 77, 3865–3868 (1996). Article CAS Google Scholar * Jónsson, H., Mills, G. & Jacobsen, K. W. In _Classical and quantum dynamics in
condensed phase simulations_ 385-404 (World Scientific, 1998). * Wang, V., Xu, N., Liu, J. C., Tang, G. & Geng, W.-T. VASPKIT: a user-friendly interface facilitating high-throughput
computing and analysis using VASP code. _Comput. Phys. Commun._ 267, 108033 (2021). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National
Natural Science Foundation of China (No. 52271008, No. 52127801), Funding from Aero Engine Cooporation of China (ZZCX-2022-020), the Space Utilization System of China Manned Space
Engineering (No. KJZ-YY-WCL04), Center of Hydrogen Science of Shanghai Jiao Tong University, Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515110289). AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * National Engineering Research Center of Light Alloy Net Forming, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai, 200240, P.R.
China Yuyang Chen, Xinchen Xu, Yiwen Chen, Tao Ying, Yangxin Li & Xiaoqin Zeng * School Key Laboratory for Marine Corrosion and Protection, Luoyang Ship Material Research Institute
(LSMRI), Xiamen, 361100, China Guodong Fan * State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240, P.R. China Tao Ying & Xiaoqin Zeng *
University of Michigan - Shanghai Jiao Tong University Joint Institute, Shanghai Jiao Tong University, 200240, Shanghai, P.R. China Hong Zhu * Department of Electrical and Computer
Engineering, University of Nebraska, Lincoln, NE, 68588, USA Wanting Sun * AECC Guiyang Engine Design Institute, Guiyang, Guizhou, 550081, P.R. China Yang Gao * Aviation Military
Representative Office of the Army Arament Department Aviation Military Representative Bureau in Shanghai, 200240, Shanghai, P.R. China Zongyang Yang & Weiwei Song Authors * Yuyang Chen
View author publications You can also search for this author inPubMed Google Scholar * Guodong Fan View author publications You can also search for this author inPubMed Google Scholar *
Xinchen Xu View author publications You can also search for this author inPubMed Google Scholar * Yiwen Chen View author publications You can also search for this author inPubMed Google
Scholar * Tao Ying View author publications You can also search for this author inPubMed Google Scholar * Yangxin Li View author publications You can also search for this author inPubMed
Google Scholar * Hong Zhu View author publications You can also search for this author inPubMed Google Scholar * Wanting Sun View author publications You can also search for this author
inPubMed Google Scholar * Yang Gao View author publications You can also search for this author inPubMed Google Scholar * Zongyang Yang View author publications You can also search for this
author inPubMed Google Scholar * Weiwei Song View author publications You can also search for this author inPubMed Google Scholar * Xiaoqin Zeng View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS Y.C.: Conceptualization, Methodology, Software, Formal analysis, Writing - Original draft, Writing - Review & Editing. Guodong Fan:
Formal analysis, Investigation, Writing - Review & Editing. X.X.: Methodology, Validation, Formal analysis, Investigation, Writing - Review & Editing. Y.C.: Validation,
Investigation, Writing - Review & Editing. T.Y.: Conceptualization, Formal analysis, Writing - Review & Editing, Supervision, Funding acquisition. Y.L.: Formal analysis, Writing -
Review & Editing, Funding acquisition. W.S.: Writing - Review & Editing, Funding acquisition. G.Y.: Formal analysis, Writing - Review & Editing. H.Z.: Resources, Formal analysis.
Z.Y.: Writing - Review & Editing, spectrum analysis suggestions. W.S.: Corrosion tests assistance. X.Z.: Resources, Supervision, Project administration. CORRESPONDING AUTHORS
Correspondence to Tao Ying or Xiaoqin Zeng. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no financial or non-financial competing interests. ADDITIONAL INFORMATION PUBLISHER’S
NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, Y., Fan, G., Xu, X. _et al._ Attain insensitivity to chlorine
ions in magnesium alloys by impeding the diffusion process. _npj Mater Degrad_ 8, 7 (2024). https://doi.org/10.1038/s41529-023-00423-9 Download citation * Received: 13 October 2023 *
Accepted: 26 December 2023 * Published: 13 January 2024 * DOI: https://doi.org/10.1038/s41529-023-00423-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative