- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Various experimental analyses on hydrogen evolution, absorption, and cracking behaviors were conducted to gain a fundamental understanding of the hydrogen embrittlement of ultrastrong steel
sheets with galvanized (GI) and galvannealed (GA) coatings. The hydrogen evolution and absorption behaviors are controlled primarily by the potential differences between the coating and
exposed steel substrate, and the corrosion-induced damage pattern of the coating. The higher absorption rate of hydrogen was more pronounced in corroded GI-coated steel caused by the larger
cathodic polarization applied to the exposed substrate, and a more severe form of coating dissolution by aqueous corrosion in a 3.5% NaCl + 0.3% NH4SN solution. In contrast, the corrosive
species can only penetrate through the pre-existing cracks in the brittle Fe-Zn intermetallic phases composed of the GA coating, and the driving force for hydrogen evolution becomes smaller.
These result in significant differences in hydrogen penetration and cracking behaviors between the two coated ultrastrong steels.
With the growing environmental awareness and stricter regulations on emissions, the automotive industry is facing significant challenges. Among them, the application of lightweight materials
centered on ultra-high-strength steel sheets is considered one of the major technical issues1,2,3,4. Ultra-high-strength steel sheets with a tensile strength of >1500 MPa have been
developed and employed in some auto-parts. On the other hand, the higher strength of the steel sheet raises significantly a concern regarding hydrogen embrittlement and pre-mature cracking
failures1,4,5,6,7. When steel is exposed to atmospheric/aqueous environments during the service, hydrogen atoms can be generated by cathodic reduction8,9,10 or hydrolysis reactions10,11 on
the surface. Some of them are infused into the steel matrix and are trapped at metallurgical defects, leading to hydrogen embrittlement12,13.
Some auto-parts are coated with Zn-based alloys to provide an anti-corrosion function, and they have been employed under the name of galvanized steels with a hot-dip Zn coating (GI). For
additional improvements in weldability and paintability, a galvannealed (GA) coating, composed of several distinctive Zn–Fe intermetallic layers, is also used by annealing the GI coating at
500~600 °C14,15,16. The primary function of both coatings is based on galvanic protection of the underlying steel substrates from corrosion because the coatings have much lower
electrochemical potentials under most corrosive conditions17,18. On the other hand, the sacrificial dissolution characteristics of Zn and Zn–Fe coatings, providing an effective
anti-corrosion function, can be the primary driving force for hydrogen evolution on a steel surface. Theoretically, the exchange current density for hydrogen evolution reaction on Zn, and
the diffusion coefficient of hydrogen in Zn with a hexagonal close-packed (HCP) structure are much lower than the case of Fe7,19,20,21, and the danger of hydrogen infusion would not be
expected. Once the coatings are damaged locally, however, a galvanic couple between the coatings and steel substrate can be formed, and the applied large cathodic polarization on the exposed
steel substrate can promote hydrogen evolution. Nevertheless, the hydrogen evolution and absorption mechanism under a neutral aqueous corrosion system are unclear. Furthermore, the extent
and kinetics of the coating damage caused by the corrosion reaction can depend significantly on the composition and structure of the coatings. These suggest that the corrosion damage and
even the hydrogen evolution and penetration behaviors can be different in GI and GA coatings.
Therefore, this study examined the hydrogen evolution, absorption, and hydrogen-induced cracking behaviors of the GI and GA-coated steel sheets during the surface degradation of coatings in
a neutral aqueous environment, using a combination of electrochemical polarization, electrochemical permeation, and metallographic observation of coating dissolution. For a further
mechanistic approach, the polarization behaviors were analyzed by a numerical model based on the mixed potential theory. Moreover, the hydrogen absorption in the charging side of the
permeation cell was interpreted by galvanic current flow from the coating layer to the bare steel using a modified permeation apparatus.
The materials under investigation were GI and GA-coated ultra-high-strength steel sheets with a tensile strength of >1500 MPa. The chemical composition of the steel substrate, and variables
of the GI and GA processes are listed in Tables 1 and 2, respectively.
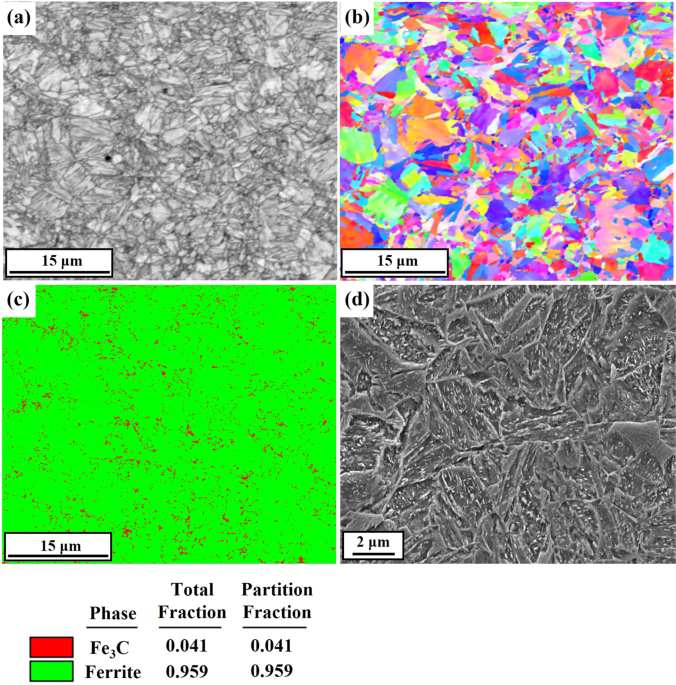
Figure 1 presents the microstructure of the cold-rolled and normalized (CR_N) steel sample without the coating. The microstructure consisted of the lath-type tempered martensite with
randomly oriented ferrite and fine Fe3C in small quantities (4.1%). The fine precipitates below 100 nm distributed uniformly throughout the matrix were shown in Fig. 2. The precipitates were
classified into two types according to their size. Fe3C and TiN are precipitates, 50–100 nm in size (Fig. 2b), and (Ti-Mo)C,N 250 HV32. Hence, the cracks can be more likely to occur in the
GA coating layer during the skin pass milling conducted after the galvanizing process. As shown in Fig. 3h, the δ phase in the GA coating layer has several pre-existing cracks. The surface
of pre-existing cracks can act as a high diffusion path for atomic hydrogen, resulting in an increase in the hydrogen diffusion kinetics and higher permeation flux.
Under no applied cathodic polarization condition, however, only GI-coated steel exhibits the abrupt increase in permeation current density in large quantities (Fig. 8b). This suggests that
hydrogen evolution and absorption were enhanced much more on the steel substrate with the corroded GI coating. Compared to a previous study33 that reported the level of hydrogen uptake at
the cut-edges or scratches in GI-coated steel, the permeation current density presented in this study appeared to be higher. The cause of this difference can be considered with respect to
the following two factors. The smaller surface area of the exposed steel in an advanced state of corrosion, as in this study, can provide less alkaline conditions, resulting in higher
hydrogen activity. The other is that using a recombination inhibitor (i.e., NH4SCN) in this study can further increase the hydrogen absorption in GI-coated steel. In the absence of a
recombination inhibitor, the permeation current density of GI was much lower, as shown in Supplementary Fig. 1. Nevertheless, the overall trend of permeation current density was similar.
Both cases showed an abrupt increase in current density after the long incubation times in the advanced state of corrosion. On the other hand, the permeation current density of GA in the
advanced state of corrosion in the absence of a recombination inhibitor was not measured in the preliminary test, which may be ascribed to the extremely low current level that is difficult
to measure using the permeation technique with the Devanathan-Stachurski (DS) cell. In contrast to the GI coating, much faster diffusion kinetics of hydrogen in smaller quantities was
obtained in GA-coated steel in the presence of NH4SCN (Fig. 8b). Under the corrosion condition, hydrogen penetration behaviors are controlled primarily by the corrosion-induced damage
pattern of the coating layer. In the case of GA-coated steel, δ and Γ intermetallic phases and the steel substrate are connected electrically, they corrode together. Specifically, the Γ
phase can dissolve prior to the δ phase due to the larger potential difference between the Γ phase and the substrate, as reported previously23. Therefore, it is expected that the driving
force for hydrogen evolution by the H2O reduction on GA-coated steel becomes smaller as corrosion proceeds (i.e., smaller cathodic overpotential applied by the galvanic couple between the Γ
phase and steel substrate). This can also be further validated by the experimental result (Fig. 13) that the galvanic current flow from each phase in GA coating, obtained by stripping
method, to bare steel, and the permeation flux of hydrogen reduced cathodically on the bare steel was decreased in the order of ζ, δ, and Γ, which is in accordance with the order of the
potential difference. The much smaller permeation flux maintained in the GA-coated steel samples, as presented in Fig. 8b and d, can be understood in this regard. On the other hand, both
electrochemical
analysis and metallographical observation indicated that much faster dissolution rate of the GI coating layer and local detachment of the coating layer by aqueous corrosion leaves a broader
area of the exposed substrate in GI-coated steel. Moreover, the comparatively larger cathodic polarization can be applied to the exposed substrate near the Zn layer (i.e., a higher hydrogen
evolution by H2O reduction), as evidenced by the higher galvanic current flow from the GI coating to bare steel (Fig. 8d). These result in the abrupt increase in permeation current density
in large quantities of GI-coated steel sample. Regarding the rapid decrease in permeation current densities, shown in Fig. 8d, there are several possible explanations. The quick initial
decrease after reaching the maximum current density is most likely due to an increase in pH on the steel surface via the reduction reactions and to the inhibitive effect by the Zn
cations34,35. The gradual decrease in current density after longer times in permeation could be resulted from both the decrease in the Zn coating present on the sacrificial anode used
polarizes the steel, and the formation of corrosion products on the corroding Zn coating. Based on the results and discussion given above, the mechanistic differences in the corrosion
damages and resulting hydrogen penetration behaviors between the two coated steel samples are illustrated schematically in Fig. 14.
a Galvanic corrosion current densities between the bare steel and stripped GA samples, obtained at respective potential steps (marked as a–c on Fig. 5b) in an electrochemical stripping test
in a 3.4 M NaCl + 0.3 M ZnSO4 solution. b Hydrogen permeation current densities measured in the detection side of bare steel as a result of a.
Compared to the corrosion behaviors of GA-coated steel, the preferential corrosion attacks along the grain boundary and much broader corrosion damage in the GI coating layer can lead to a
much larger quantity of hydrogen absorbed and permeated in the steel substrate, resulting in higher susceptibility to the hydrogen-assisted cracking failures. The higher susceptibility to
the intergranular cracking of GI-coated steel was evidenced experimentally by a four-point bent beam test conducted in a neutral aqueous environment. The hydrogen contents in as-galvanized
samples, measured by thermal desorption method, were quite small (