- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Many biomedical materials used today for applications such as orthopedic, dental, and cardiovascular implants and devices are made of corrosion-resistant, ‘inert’, metallic
materials of the cobalt–chromium, titanium, and stainless steel alloy groups. This perspective focuses on the role of proteins in the degradation of these materials in a human body
environment. After adsorption, the proteins interact relatively slowly with the metal and metal surface oxide. A number of factors, including the individual body chemistry (especially the
presence of inflammatory cells producing oxidative species), determine whether the proteins can bind to metals in the surface oxide and whether the metal–protein conjugates can detach from
the surface. Metals in the forms of protein-bound metal ions or nanosized particles can also increase protein–protein interactions and aggregation, which can cause some health effects and
change the material degradation mechanism. While proteins in some short-term studies (<6 h) even decrease material degradation due to shielding effects and better lubrication, they may
increase degradation after longer time periods due to relatively slow binding, detachment, and combined corrosion processes. In-vitro material degradation studies of relatively
corrosion-resistant alloys for biomedical applications should therefore include long-term studies, complexing agents or proteins, and realistic oxidative environments simulating inflammatory
conditions. SIMILAR CONTENT BEING VIEWED BY OTHERS DO TITANIUM BIOMATERIALS GET IMMEDIATELY AND ENTIRELY REPASSIVATED? A PERSPECTIVE Article Open access 12 July 2022 INFLUENCE OF CASEIN ON
THE DEGRADATION PROCESS OF POLYLACTIDE-CASEIN COATINGS FOR RESORBABLE ALLOYS Article Open access 15 August 2024 AN ANTIBACTERIAL COATED POLYMER PREVENTS BIOFILM FORMATION AND
IMPLANT-ASSOCIATED INFECTION Article Open access 11 February 2021 INTRODUCTION Replacement of human organs, bones, and teeth is challenging even though engineers and physicians have attempt
to find ideal replacement materials for decades, if not centuries. Metals and alloys are used today for the replacement of, or functional parts for, orthopedic, dental, spinal,
cardiovascular, neurological, and gynecological implants, or for fracture fixation.1 In most of these applications, materials with the highest possible corrosion resistance, so-called inert
materials, are used today. For metallic materials these are mainly titanium, cobalt–chromium, and stainless steel alloys. These will be the focus of this perspective. Still, there exists no
material that is as good as the natural material in the human body, regarding all the requirements for mechanical properties, corrosion resistance, biocompatibility, hardness, density, and
toxic properties.2 It has been known that the human body is a very corrosive environment resulting in a large range of different and combined corrosion types. Those are influenced by wear,
fretting, crevices, fatigue, salts, and inappropriate geometry of implants.3,4,5,6 Recent findings highlight the importance of inflammatory cells, individual body chemistry, Fenton
chemistry, and electrosurgery.1,7,8,9,10 In all, it becomes increasingly clear that accelerated corrosion tests or immersion tests in simple solutions (simulating the ionic strength,
chloride content, and pH of physiological conditions) are insufficient to predict corrosion observed in-vivo.2 This perspective will focus on the role of proteins for material degradation of
‘inert’ metal and alloy degradation by providing some insights into surface and colloid chemistry, degradation kinetics, and metal–protein interactions that are crucial to understand and
design predictive and comparative corrosion and metal release experiments for biomedical metallic ‘inert’ materials. FAST AND SLOW KINETICS OF METAL–PROTEIN INTERACTIONS ADSORPTION Most
proteins adsorb relatively rapidly (seconds to hours) on metal and alloy surfaces in physiological environments of relatively high ionic strength, even when the protein and the surface are
similarly charged.11 The kinetics of adsorption, and the competition among different proteins, are highly influenced by the surface properties of the metal/alloy, such as surface energy,
charge, roughness, hydrophobicity, and thickness of its surface oxide.11,12,13,14 Protein properties influencing the adsorption are protein size, charge, hydrophobicity, and concentration;
solution properties important for adsorption inside the human body are, e.g., ionic strength, pH, shear rates, and agitation.15 Adsorption as the first step of metal–protein interaction is
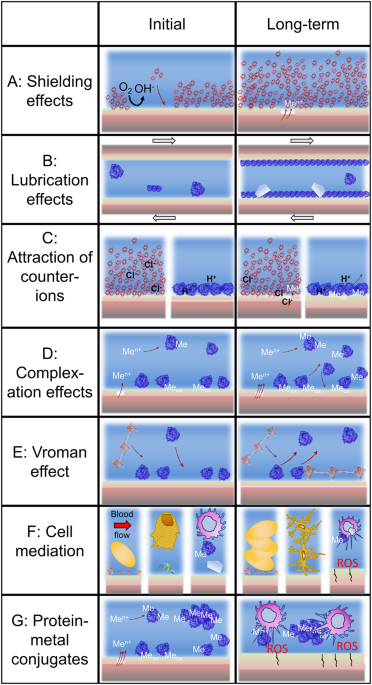
important for material degradation in several ways: (i) it may inhibit cathodic reactions and initial corrosion similar to a coating (Fig. 1a); (ii) it mediates cell adsorption16 (Fig. 1f);
and (iii) it can act as a lubricant for surfaces that are exposed to friction and fretting (Fig. 1b). Most of these effects decrease material degradation initially. Hence, this is the
conclusion of several, but not all, short-term (a few hours) studies.17,18 However, appearances can be deceiving. PROTEIN–METAL BINDING AND DETACHMENT Many proteins bind to metals at
specific binding sites and additionally by weak interactions like electrostatic interactions. Albumin was identified as the strongest metal binder of human blood proteins.19 This protein was
also found to induce the strongest metal release and corrosion among several other proteins investigated for stainless steel AISI 316L.13,20 Albumin is also present at relatively high
concentration in synovial (joint) fluid.21 Protein binding to metal atoms in metal oxides is however strongly dependent on the surface oxide properties and will only occur if the
protein–metal bond is stronger than metal-oxide or metal-hydroxide bonds or if those bonds are weakened by defects. Weakening of the metal-oxide bonds by metal–protein complexes is often a
very slow process and, in several cases, depends on the presence of other factors. If the metal–protein complex weakens the metal-oxide bonds, the metal–protein complex could eventually
detach from the surface oxide (an even slower process).12,13 This is facilitated by high driving forces for protein exchange (high concentration of proteins, agitation, high ionic strength),
which often are present in the human body environment. The Vroman effect,22 that is the replacement of a surface-bound protein by a protein with larger surface binding affinity (Fig. 1e),
also facilitates the detachment of metal–protein conjugates. Both metal–protein binding and metal–protein conjugate surface detachment are highly dependent upon a number of factors, such as,
the alloy composition and surface oxide composition, crystallinity, heterogeneity, and defect density. In contrast to corrosion in terms of metal oxidation, which is decreased for amorphous
oxides as compared to crystalline oxides,23,24 amorphous surface oxides increase the protein-induced dissolution due to an enhancement of the metal complexation and detachment24,25 (Fig.
1d). The reason is most probably a weaker metal-oxide bond (larger atomic distance) in amorphous as compared to crystalline oxides. The surface oxide of titanium alloys belongs to one of the
most chemically stable oxides. However, it is affected negatively by proteins in the presence of hydrogen peroxide or upon oxidation.26,27,28,29,30 Enhanced corrosion and metal release in
the presence of proteins have been observed after a few hours at the shortest (for stainless steel AISI 316L in phosphate buffered saline, pH 7.4)12 and can occur after significantly longer
times, e.g., after at least 24 h for a titanium–aluminum–vanadium alloy in the presence of hydrogen peroxide, which can form complexes with titanium in the surface oxide.27,30 Interestingly,
the metal release pattern in one of these harsh in-vitro studies combining proteins and hydrogen peroxide30 was similar to that observed in-vivo in rat tibia tissue.31 Hence, in certain
environments there is a transition from an initially beneficial effect of proteins to a detrimental effect for material degradation (Fig. 1). This transition occurs in most cases only after
several hours and might therefore be missed in the majority of short-term studies. A long-term (48 weeks) study investigating the metal release from a titanium–aluminum–vanadium alloy in rat
tibia tissue showed a stabilization or decrease of metals in tissue after about 6–24 weeks.31 Also, from the evidence that exists on metal oxide interactions with complexing agents,25,32
and from the similarity of complexing agents and proteins in terms of dissolution,12,33,34 it seems clear that at least some proteins can directly interact with the metal oxide (Fig. 1d),
which is different from what is claimed in Ref. 35. However, direct protein interactions with the metal (not the oxide), especially for defective oxides or under fretting conditions, are
more rapid (Fig. 1d) and can increase the dissolution/corrosion rate at non-passive conditions. This could be responsible for the increased nickel to chromium ratio20,36 in the release of
metals from stainless steel in the presence of albumin, as nickel metal is enriched beneath the surface oxide, which usually does not contain nickel.37 The attraction of counter-ions by
adsorbed proteins, that is, anions (such as chlorides) for net positively charged adsorbed proteins (e.g., lysozyme at pH 7.4) and cations (such as protons) for net negatively charged
adsorbed proteins (e.g., albumin), might further weaken the surface oxide and result in localized corrosion (Fig. 1c).20 METAL-INDUCED PROTEIN–PROTEIN INTERACTIONS Protein–metal interactions
do not only have consequences for material degradation but also directly for health and protein–protein interactions. Metal–protein conjugates can act as allergens, by either sensitizing
the immune system or eliciting cell-mediated immunological reactions (Fig. 1g).38 Further, metal ions or nanosized metal-containing particles can induce increased protein–protein
interactions, which result in aggregated protein particles (Fig. 1g).39,40,41 These protein particles can have negative health effects,42 or result in an increased coefficient of friction43
or inflammatory conditions, which may impact the material degradation negatively, e.g., by increased inflammatory (oxidative) conditions (Fig. 1g). Although proteins or protein layers act as
lubricants under friction or reduce the wear,44,45 it has been suggested that they retain abrasive particles and thereby increase the total wear (Fig. 1b).46 COMBINED MECHANISMS OF MATERIAL
DEGRADATION IN-VIVO It is clear from most recent literature within this field, mainly thanks to retrieval studies of implants observing ‘impossible’ corrosion types, that many combined
mechanisms of material degradation exist involving friction and fatigue-induced degradation, Fenton chemistry and highly oxidative environments, chemical dissolution, and crevice/pitting
corrosion (Fig. 2).1,2,5,6,10 Most of these combined mechanisms increase the extent of corrosion as compared to a single factor or type of mechanism. A recent study on particles released
from implant materials into tissues found that chromium in some particles in the tissues (for some patients) was oxidized to the hexavalent chromium form.47 This finding further strengthens
the view of the importance of individual human body chemistry and that inflammatory/infectious conditions due to oxidative species highly influence corrosion. This individual body response
depends on the extent and particle characteristics of released wear particles. It has been shown for a range of metallic materials and alloys that their wear particles cause increased
inflammatory or toxic responses, while their corresponding bulk materials are biocompatible.48 OUTLOOK This perspective highlights the importance of time, complexing agents, proteins, and
oxidative conditions (ideally in a combined way) for in-vitro material testing for biomedical applications. Protein-induced degradation includes slow chemical and combined processes, which
may require different experimental approaches than for traditional corrosion testing. The combined effect of protein- and hydrogen peroxide-induced degradation of titanium alloys should be
investigated further for other oxidative species and alloys. The development of new in-vitro material degradation study protocols for relatively corrosion-resistant alloys for biomedical
applications should also consider long-term studies, complexing agents (like citrate) or proteins (like albumin), and realistic oxidative environments simulating inflammatory conditions.
Worst case combinations of influencing factors should be used to simulate the complex environment in the human body. REFERENCES * Gilbert, J. Corrosion in the human body: metallic implants
in the complex body environment. _Corrosion_ 73, 1478–1495 (2017). Article Google Scholar * Milošev, I. From in vitro to retrieval studies of orthopedic implants. _Corrosion_ 73, 1496–1509
(2017). Article Google Scholar * Pound, B. G. Corrosion behavior of metallic materials in biomedical applications. II. Stainless steels and Co–Cr alloys. _Corros. Rev._ 32, 21–41 (2014).
Google Scholar * Pound, B. G. Corrosion behavior of metallic materials in biomedical applications. I. Ti and its alloys. _Corros. Rev._ 32, 1–20 (2014). Article Google Scholar * Teoh, S.
Fatigue of biomaterials: a review. _Int J. Fatigue._ 22, 825–837 (2000). Article Google Scholar * Maurer-Ertl, W. et al. Recall of the ASR XL head and hip resurfacing systems.
_Orthopedics_ 40, e340–e347 (2017). Article Google Scholar * Liu, Y. & Gilbert, J. L. The effect of simulated inflammatory conditions and Fenton chemistry on the electrochemistry of
CoCrMo alloy. _J. Biomed. Mater. Res. B_ 106, 209–220 (2018). Article Google Scholar * Kubacki, G. W., Sivan, S. & Gilbert, J. L. Electrosurgery induced damage to Ti-6Al-4V and CoCrMo
alloy surfaces in orthopedic implants in vivo and in vitro. _J. Arthroplast._ 32, 3533–3538 (2017). Article Google Scholar * Arnholt, C. M. et al. Corrosion damage and wear mechanisms in
long-term retrieved CoCr femoral components for total knee arthroplasty. _J. Arthroplast._ 31, 2900–2906 (2016). Article Google Scholar * Igual Muñoz, A., Schwiesau, J., Jolles, B. M.
& Mischler, S. In vivo electrochemical corrosion study of a CoCrMo biomedical alloy in human synovial fluids. _Acta Biomater._ 21, 228–236 (2015). Article Google Scholar *
Silva-Bermudez, P. & Rodil, S. E. An overview of protein adsorption on metal oxide coatings for biomedical implants. _Surf. Coat. Tech._ 233, 147–158 (2013). Article Google Scholar *
Hedberg, Y., Karlsson, M.-E., Blomberg, E., Odnevall Wallinder, I. & Hedberg, J. Correlation between surface physicochemical properties and the release of iron from stainless steel AISI
304 in biological media. _Colloid Surf. B_ 122, 216–222 (2014). Article Google Scholar * Hedberg, Y. et al. Interaction of albumin and fibrinogen with stainless steel—influence of
sequential exposure and protein aggregation on metal release and corrosion resistance. _Corrosion_ 73, 1423–1436 (2017). Article Google Scholar * Igual Muñoz, A. & Mischler, S.
Electrochemical quartz crystal microbalance and X-ray photoelectron spectroscopy study of cathodic reactions in bovine serum albumin containing solutions on a physical vapour
deposition–CoCrMo biomedical alloy. _Electrochim. Acta_ 180, 96–103 (2015). Article Google Scholar * Norde, W. Driving forces for protein adsorption at solid surfaces. _Macromol. Symp._
103, 5–18 (1996). Article Google Scholar * Wilson, C. J., Clegg, R. E., Leavesley, D. I. & Pearcy, M. J. Mediation of biomaterial–cell interactions by adsorbed proteins: a review.
_Tissue Eng._ 11, 1–18 (2005). Article Google Scholar * Cortizo, M. C., de Mele, M. F. L. & Cortizo, A. M. Metallic dental material biocompatibility in osteoblastlike cells. _Biol.
Trace Elem. Res._ 100, 151–168 (2004). Article Google Scholar * Koronfel, M. A. et al. Understanding the reactivity of CoCrMo-implant wear particles. _npj Mater. Degrad._ 2, 8 (2018).
Article Google Scholar * Merritt, K., Brown, S. & Sharkey, N. The binding of metal salts and corrosion products to cells and proteins in vitro. _J. Biomed. Mater. Res._ 18, 1005–1015
(1984). Article Google Scholar * Hedberg, Y. et al. Surface–protein interactions on different stainless steel grades—effects of protein adsorption, surface changes and metal release. _J.
Mater. Sci. Mater. M._ 24, 1015–1033 (2013). Article Google Scholar * Oates, K. M., Krause, W. E., Jones, R. L. & Colby, R. H. Rheopexy of synovial fluid and protein aggregation. _J.
R. Soc. Interface_ 3, 167–174 (2006). Article Google Scholar * Hirsh, S. L. et al. The Vroman effect: competitive protein exchange with dynamic multilayer protein aggregates. _Colloid
Surf. B_ 103, 395–404 (2013). Article Google Scholar * Shih, C.-C., Lin, S.-J., Chung, K.-H., Chen, Y.-L. & Su, Y.-Y. Increased corrosion resistance of stent materials by converting
current surface film of polycrystalline oxide into amorphous oxide. _J. Biomed. Mater. Res._ 52, 323–332 (2000). Article Google Scholar * Hedberg, Y. & Midander, K. Size matters:
mechanism of metal release from 316L stainless steel particles is governed by size-dependent properties of the surface oxide. _Mater. Lett._ 122, 223–226 (2014). Article Google Scholar *
Carbonaro, R. F., Gray, B. N., Whitehead, C. F. & Stone, A. T. Carboxylate-containing chelating agent interactions with amorphous chromium hydroxide: adsorption and dissolution.
_Geochim. Cosmochim. Ac._ 72, 3241–3257 (2008). Article Google Scholar * Khan, M., Williams, R. L. & Williams, D. F. The corrosion behaviour of Ti–6Al–4V, Ti–6Al–7Nb and Ti–13Nb–13Zr
in protein solutions. _Biomaterials_ 20, 631–637 (1999). Article Google Scholar * Zhang, Y. et al. Time-dependent enhanced corrosion of Ti6Al4V in the presence of H2O2 and albumin. _Sci.
Rep._ 8, 3185 (2018). Article Google Scholar * Wang, J. L. et al. A closer look at the in vitro electrochemical characterisation of titanium alloys for biomedical applications using
in-situ methods. _Acta Biomater._ 54, 469–478 (2017). Article Google Scholar * Yu, F., Addison, O. & Davenport, A. J. A synergistic effect of albumin and H2O2 accelerates corrosion of
Ti6Al4V. _Acta Biomater._ 26, 355–365 (2015). Article Google Scholar * Hedberg, Y. S., Žnidaršič, M., Herting, G., Milošev, I. & Odnevall Wallinder, I. Mechanistic insight on the
combined effect of albumin and hydrogen peroxide on surface oxide composition and extent of metal release from Ti6Al4V. _J. Biomed. Mater. Res. B_, in press
https://onlinelibrary.wiley.com/doi/abs/10.1002/jbm.b.34182. * Okazaki, Y. & Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. _Biomaterials_ 26, 11–21
(2005). Article Google Scholar * Schwertmann, U. Solubility and dissolution of iron oxides. _Plant Soil_ 130, 1–25 (1991). Article Google Scholar * Kocijan, A., Milošev, I. & Pihlar,
B. The influence of complexing agent and proteins on the corrosion of stainless steels and their metal components. _J. Mater. Sci. Mater. M._ 14, 69–77 (2003). Article Google Scholar *
Hedberg, Y., Mazinanian, N. & Odnevall Wallinder, I. Metal release from stainless steel powders and massive sheet—comparison and implication for risk assessment of alloys. _Env. Sci.
Process. Impact_ 15, 381–392 (2013). Article Google Scholar * Lewis, A. C., Kilburn, M. R., Papageorgiou, I., Allen, G. C. & Case, C. P. Effect of synovial fluid, phosphate-buffered
saline solution, and water on the dissolution and corrosion properties of CoCrMo alloys as used in orthopedic implants. _J. Biomed. Mater. Res. A_ 73A, 456–467 (2005). Article Google
Scholar * Merritt, K. & Brown, S. A. Effect of proteins and pH on fretting corrosion and metal ion release. _J. Biomed. Mater. Res._ 22, 111–120 (1988). Article Google Scholar *
Hedberg, Y. S. & Odnevall Wallinder, I. Metal release from stainless steel in biological environments: a review. _Biointerphases_ 11, 018901-1–018901-17 (2016). Article Google Scholar
* Thyssen, J. P. & Chen, J. (Eds.) Metal Allergy—From Dermatitis to Implant and Device Failure. (Springer International Publishing, Cham, 2018). * Yang, J. & Black, J. Competitive
binding of chromium, cobalt and nickel to serum proteins. _Biomaterials_ 15, 262–268 (1994). Article Google Scholar * Tyagi, A. K. et al. IgG particle formation during filling pump
operation: a case study of heterogeneous nucleation on stainless steel nanoparticles. _J. Pharm. Sci._ 98, 94–104 (2009). Article Google Scholar * Thompson, C. et al. Impact of magnetic
stirring on stainless steel integrity: effect on biopharmaceutical processing. _J. Pharm. Sci._ 106, 3280–3286 (2017). Article Google Scholar * Rosenberg, A. S. Effects of protein
aggregates: an immunologic perspective. _AAPS J._ 8, E501–E507 (2006). Article Google Scholar * Hedberg, Y. S. et al. Can cobalt(II) and chromium(III) ions released from joint prostheses
influence the friction coefficient? _ACS Biomater. Sci. Eng._ 1, 617–620 (2015). Article Google Scholar * Liao, Y. et al. Graphitic tribological layers in metal-on-metal hip replacements.
_Science_ 334, 1687–1690 (2011). Article Google Scholar * Espallargas, N., Fischer, A., Igual Muñoz, A., Mischler, S. & Wimmer, M. A. In-situ generated tribomaterial in metal/metal
contacts: current understanding and future implications for implants. _Biotribology_ 10, 42–50 (2017). Article Google Scholar * Arenas, M., Conde, A., De Frutos, A. & de Damborenea, J.
Electrochemical noise measurements of AISI 316L during wear in simulated physiological media. _Corros. Eng. Sci. Techn._ 49, 656–660 (2014). Article Google Scholar * Swiatkowska, I. et
al. Synchrotron analysis of human organ tissue exposed to implant material. _J. Trace Elem. Med. Bio._ 46, 128–137 (2018). Article Google Scholar * Gibon, E. et al. The biological response
to orthopaedic implants for joint replacement: Part I: metals. _J. Biomed. Mater. Res. B_ 105, 2162–2173 (2017). Article Google Scholar * Jackson, S. P. The growing complexity of platelet
aggregation. _Blood_ 109, 5087 (2007). Article Google Scholar Download references ACKNOWLEDGEMENTS The authors acknowledge Swedish Research Council for financial support, and Dr. J.
Hedberg and Prof. B. Lyne for reading and commenting the manuscript. This work was supported by the Swedish Research Council (Grant no. 2015-04177). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Division of Surface and Corrosion Science, Department of Chemistry, School of Engineering Sciences in Chemistry, Biotechnology and Health, KTH Royal Institute of Technology,
Drottning Kristinas väg 51, SE-10044, Stockholm, Sweden Yolanda S. Hedberg Authors * Yolanda S. Hedberg View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS Y.S.H. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Yolanda S. Hedberg. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hedberg, Y.S. Role of
proteins in the degradation of relatively inert alloys in the human body. _npj Mater Degrad_ 2, 26 (2018). https://doi.org/10.1038/s41529-018-0049-y Download citation * Received: 03 June
2018 * Revised: 23 July 2018 * Accepted: 20 August 2018 * Published: 07 September 2018 * DOI: https://doi.org/10.1038/s41529-018-0049-y SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative







