- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The need for improved functionalities is driving the search for more complicated multi-component materials. Despite the factorially increasing composition space, ordered compounds
with four or more species are rare. Here, we unveil the competition between the gain in enthalpy and entropy with increasing number of species by statistical analysis of the AFLOW data
repositories. A threshold in the number of species is found where entropy gain exceeds enthalpy gain. Beyond that, enthalpy can be neglected, and disorder—complete or partial—is unavoidable.
SIMILAR CONTENT BEING VIEWED BY OTHERS MULTIVARIATE ANALYSIS OF DISORDER IN METAL–ORGANIC FRAMEWORKS Article Open access 21 April 2022 THE CLUSTER DECOMPOSITION OF THE CONFIGURATIONAL
ENERGY OF MULTICOMPONENT ALLOYS Article Open access 19 July 2024 MULTIFUNCTIONAL HIGH-ENTROPY MATERIALS Article 30 September 2024 INTRODUCTION The formation of materials in the tangible
world, enjoying easy-life at room temperature and pressure, is governed by the Gibbs free energy. Enthalpy usually makes up a large part of it, and, for the last 2 decades, the search for
new materials has mostly focused on enthalpy optimization. A plethora of methods were proposed: e.g., cluster expansion,1 genetic-2 and combinatorial algorithms.3 In trying to cure the
Maddox curse4 (Maddox: “One of the continuing scandals in the physical sciences is that it remains in general impossible to predict the structure of even the simplest crystalline solids from
a knowledge of their chemical composition”), these approaches were all somewhat successful in predicting and discovering systems made up of few species. At least this was the impression:
polymorphs were still able to escape from the enthalpic jail! While the need for functionalities was driving the search for more complicated materials, something unexpected happened: the
discovery of high-entropy alloys.5,6 The ideal scenario of phase transitions—in which precursors constantly seek the most stable configuration—was shattered by the acceptance that disorder
is useful. Skeptics considered high-entropy alloys only a remake of solid-solutions. In the meantime, new families appeared: entropy-stabilized oxides, high-entropy carbides and borides.
Each carrying a panoply of unexpected properties. RESULTS AND DISCUSSION ARE ENTROPIC MATERIALS THE EXCEPTION? The question requires the disentanglement of the “enthalpy versus entropy”
dichotomy. Big-data statistical analysis gives the answer. Let us consider the AFLOW.org ab-initio materials repository7 and analyze its compounds’ energies with the associated tools.8,9 The
recursive formation enthalpy “gain” of an _N_-species ordered compound (called _N_-compound) with respect to combinations of {1, ⋯, _N_ − 1}-species ordered sub-components, Δ_H_[_N_|{1, ⋯,
_N_ − 1}], is defined as the energetic distance of the enthalpy of the _N_-compound, _H_[_N_], below the {1, ⋯, _N_ − 1} convex-hull hyper-surface _H_hull[{1, ⋯, _N_ − 1}] generated from its
{1, ⋯, _N_ − 1}-species components: Δ_H_[_N_|{1, ⋯, _N_ − 1}] ≡ _H_hull[{1, ⋯, _N_ − 1}] − _H_[_N_] if _H_[_N_] < _H_hull[{1, ⋯, _N_ − 1}] (for binary compounds, Δ_H_[2|1] is equivalent
to the usual formation enthalpy; for _N_ > 2, the formation enthalpies can be written as sums of the recursive gains) and zero otherwise (Δ_H_[_N_|{1, ⋯, _N_ − 1}] is conceptually similar
to the stability criterion _δ_sc described in ref. 9, except with all _N_-species entries removed from the pseudo-hull). Physically, Δ_H_[_N_|{1, ⋯, _N_ − 1}] represents the enthalpy gain
to create an ordered _N_-compound out of all the combinations of ordered {1, ⋯, _N_ − 1}-ones. Gain expectations can be obtained by the analysis of metal alloy phase diagrams “constructible”
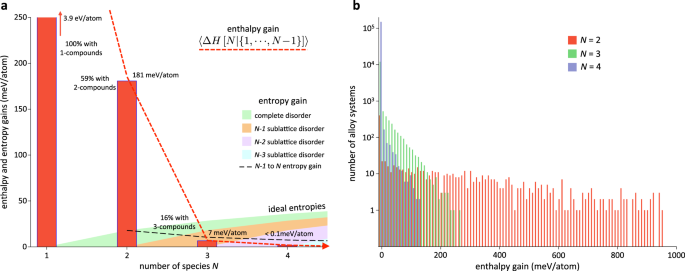
out of the AFLOW.org data. 〈Δ_H_〉 is shown in Fig. 1a for _N_ = 2, 3, 4, and as cohesive energy for _N_ = 1. FINDING NEW _N_-COMPOUNDS BECOMES DIFFICULT While 59% (588) of the binaries show
enthalpy gain by forming a total of 1995 2-compounds, only 16% (2237) of ternaries produce 3040 3-compounds and just 426 (0.3%) quaternaries form 4-compounds. Phase separation is
overwhelming. Contrary to common belief, the trend indicates that despite the combinatorially-growing number of potential configurations, finding accommodating prototypes becomes harder with
increasing _N_. ENTROPY TAKES OVER The surviving combinations of species forming new compounds become vulnerable with increasing _N_. The expectation of Δ_H_ is ~181 meV/atom for binaries.
It drastically reduces to ~7 meV/atom for ternaries, practically disappearing (<0.1 meV/atom) for quaternaries. 434 binary, 192 ternary but only 10 quaternary systems have a large
enthalpy gain (defined as exceeding 100 meV/atom): the frequency distributions of enthalpy gains for all systems are displayed in Fig. 1b. The numbers are compared to disorder promotion
where the ideal scenario is considered for simplicity.10 Figure 1a shows the ideal entropic contribution at room temperature for the fully (_N_) and partially disordered (_N_ − 1, _N_ − 2,
_N_ − 3) systems. Since the _N_ − 1 component systems could also feature disorder, i.e., the _N_ component system could phase-separate into multiple disordered phases, the log(_N_) − log(_N_
− 1) entropy gain is also plotted (black dashed line). Note that the latter already exceeds the enthalpy gain for _N_ = 3. The reducing probability of having _N_-compounds—also recursively
applying to sub-components—compounded with the drastically decreasing enthalpic gain and the monotonic entropic promotion, indicates that around the threshold _N_ ~ 4, entropy takes over the
stability of the system. BEYOND METALS The analysis focuses on the extensive AFLOW set of metallic compounds; a similar analysis for non-metals requires formation enthalpy corrections.11
Mixing different types of bonding can change the entropy threshold by adding additional enthalpic stabilization, leading to a shift of the onset of the entropic promotion (_N_ increases). It
is not a coincidence: entropy stabilized oxides were discovered with five metallic species mixed with oxygen,12 and high-entropy-carbides and borides were also synthesized with five
metallic species plus carbon13 or boron.14 There, oxygen, carbon, and boron are “spectator” species. The devil is in the details: reciprocal systems might be able to reduce the number of the
mixing species, thanks to the additional entropic stabilization of every equivalent sublattice. THE FUTURE Ramifications of the analysis are intriguing. Disordered systems can lead to
remarkable properties15,16,17—unexpected from the homologous crystalline counterparts—enabling revolutionary technologies. We just need to search for materials with a different mindset. (I)
The search for multi-component systems, performed with enthalpy optimization, is futile. With increasing _N_, the discovery probability decreases, and, even if an ordered material is found,
it would be overwhelmed by engulfing disorder. High-entropy promotion occurs at ~4 mixing species (~5+ with different bond types). (II) Sluggish kinetics at low temperatures can be a
blessing.18 Disorder is hard to cure, and high entropy solid-solutions synthesized at high-temperature can survive low-temperature phase separation13,18 while providing valuable
technological applications.15 (III) Maddox’s scandal was only an apparent curse. It was actually a blessing as it forced statistical analysis of the enthalpy versus entropy interplay.
Modeling can no longer ignore disorder—often neglected due to compelling difficulties. Advances in complex multi-component materials research will be driven by embracing disorder. It is
unavoidable. METHODS Calculations of the enthalpy gain are performed using the AFLOW-CHULL module, using the command:
$${\mathrm{aflow}}--\,{\mathrm{chull}}--\,{\mathrm{alloy}}={<}{\mathrm{system}}{>}--{\mathrm{print}}={\mathrm{json}},$$ where <system> is a list of the elements in the alloy
system. The enthalpy gain is contained in the JSON output file under the keyword _N_ +1_energy_gain. To enhance statistical significance, only metallic alloy systems composed of the elements
Ag, Al, Au, Ba, Be, Bi, Ca, Cd, Co, Cr, Cu, Fe, Ga, Hf, Hg, In, Ir, K, La, Li, Mg, Mn, Mo, Na, Nb, Ni, Os, Pb, Pd, Pt, Re, Rh, Ru, Sc, Sn, Sr, Ta, Tc, Ti, Tl, V, W, Y, Zn, Zr are considered
as AFLOW data is much richer in that material domain. A total of 990 _N_ = 2, 14,190 _N_ = 3, and 148,675 _N_ = 4 phase diagrams are generated, with 202,261, 974,808, and 432,840 ab-initio
entries respectively. DATA AVAILABILITY All the ab-initio data used in this analysis are freely available to the public as part of the AFLOW online repository and can be accessed through
AFLOW.org following the REST-API interface19 and AFLUX search language.8 The AFLOW source code is available for download at http://aflow.org under GNU GPL version 3. CHANGE HISTORY * _ 01
AUGUST 2019 In the original PDF version of this Article the command for the calculation of enthalpy in the Methods contained an error, introduced during copyediting. “<systemgt;” has now
been corrected to “<system>” in the PDF version. _ REFERENCES * de Fontaine, D. Cluster approach to order–disorder transformations in alloys. In _Solid State Physics_, Vol. 47 (eds
Ehrenreich, H. & Turnbull, D.) 33–176 (Academic Press, New York, 1994). * Bush, T. S., Catlow, C. R. A. & Battle, P. D. Evolutionary programming techniques for predicting inorganic
crystal structures. _J. Mater. Chem._ 5, 1269–1272 (1995). Article CAS Google Scholar * Curtarolo, S. et al. The high-throughput highway to computational materials design. _Nat. Mater._
12, 191–201 (2013). Article CAS Google Scholar * Maddox, J. Crystals from first principles. _Nature_ 335, 201 (1988). Article Google Scholar * Gao, M. C. Design of high-entropy alloys.
In _High-Entropy Alloys: Fundamentals and Applications_ (eds Gao, M. C. et al.) Ch. 11, 369–398 (Springer, Cham, Switzerland, 2016). * Widom, M. Frequency estimate for multicomponent
crystalline compounds. _J. Stat. Phys._ 167, 726–734 (2017). Article Google Scholar * Oses, C., Toher, C. & Curtarolo, S. Data-driven design of inorganic materials with the Automatic
FLOW framework for materials discovery. _MRS Bull._ 43, 670–675 (2018). * Rose, F. et al. AFLUX: The LUX materials search API for the AFLOW data repositories. _Comput. Mater. Sci._ 137,
362–370 (2017). * Oses, C. et al. AFLOW-CHULL: Cloud-oriented platform for autonomous phase stability analysis. _J. Chem. Inf. Model._ 58, 2477–2490 (2018). Article CAS Google Scholar *
McQuarrie, D. A. _Statistical Mechanics_. (Harper and Row, New York, 1976). Google Scholar * Friedrich, R. et al. Coordination corrected ab initio formation enthalpies. _npj Comput. Mater._
5, 59 (2019). Article Google Scholar * Rost, C. M. et al. Entropy-stabilized oxides. _Nat. Commun._ 6, 8485 (2015). Article CAS Google Scholar * Sarker, P., Harrington, T. et al.
High-entropy high-hardness metal carbides discovered by entropy descriptors. _Nat. Commun._ 9, 4980 (2018). * Gild, J. et al. High-entropy metal diborides: a new class of high-entropy
materials and a new type of ultrahigh temperature ceramics. _Sci. Rep._ 6, 37946 (2016). Article CAS Google Scholar * Gludovatz, B. et al. A fracture-resistant high-entropy alloy for
cryogenic applications. _Science_ 345, 1153–1158 (2014). Article CAS Google Scholar * Li, Z., Pradeep, K. G., Deng, Y., Raabe, D. & Tasan, C. C. Metastable high-entropy dual-phase
alloys overcome the strength-ductility trade-off. _Nature_ 534, 227–230 (2016). Article CAS Google Scholar * Braun, J. L. et al. Charge-induced disorder controls the thermal conductivity
of entropy-stabilized oxides. _Adv. Mater._ 30, 1805004 (2018). Article Google Scholar * Balluffi, R. W., Allen, S. M. & Carter, W. C. _Kinetics of Materials_. (John Wiley & Sons,
Hoboken, New Jersey, 2005). Book Google Scholar * Taylor, R. H. et al. A RESTful API for exchanging materials data in the AFLOWLIB.org consortium. _Comput. Mater. Sci._ 93, 178–192 (2014).
Article Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Ohad Levy for fruitful discussions. Research sponsored by DOD-ONR (N00014-15-1-2863, N00014-16-1-2326, and
N00014-17-1-2876). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Mechanical Engineering and Materials Science, Duke University, Durham, NC, 27708, USA Cormac Toher, Corey Oses,
David Hicks & Stefano Curtarolo Authors * Cormac Toher View author publications You can also search for this author inPubMed Google Scholar * Corey Oses View author publications You can
also search for this author inPubMed Google Scholar * David Hicks View author publications You can also search for this author inPubMed Google Scholar * Stefano Curtarolo View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.C. proposed the species-stabilization mechanism. C.T. and C.O. developed the recursive stability
codes. D.H. generated the library of _N_ = 4 compounds. All authors discussed the results and contributed to the writing of the article. CORRESPONDING AUTHOR Correspondence to Stefano
Curtarolo. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Toher, C., Oses, C., Hicks, D. _et al._ Unavoidable disorder and entropy in
multi-component systems. _npj Comput Mater_ 5, 69 (2019). https://doi.org/10.1038/s41524-019-0206-z Download citation * Received: 20 December 2018 * Accepted: 06 June 2019 * Published: 10
July 2019 * DOI: https://doi.org/10.1038/s41524-019-0206-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative